Abstract
Rationale
Compared with the use of classic receptor ligands, antisense oligonucleotides (ASO) targeted at specific central nervous system receptors are an effective alternative in experiments designed to examine the behavioral role of such systems.
Objectives
The nociception/orphaninFQ (N/OFQ) system has been implicated in mediating endocrine function, feeding, stress, pain, anxiety, and the rewarding effects of drugs of abuse. The objective of the current study was to examine whether long-term ASO-induced downregulation of N/OFQ’s receptor (NOP) produced changes in endocrine, anxiety, nociception and ethanol’s (EtOH’s) locomotor activating properties.
Methods
Male Long Evans rats were implanted with osmotic mini-pumps containing ASO for the NOP receptor. ASO was chronically infused for 26 days and, during this time, multiple behavioral and physiological measurements were conducted.
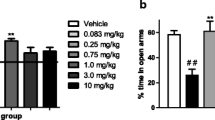
Results
ASO infusion significantly reduced expression of the NOP receptor in brain, confirmed by significant reductions of OFQ-stimulated [35S]-GTPγS binding in the paraventricular nucleus, prefrontal cortex, and septum. Behavioral changes were observed in ASO-treated animals including higher body temperature, increased water intake, decreased corticosterone (CORT) levels, decreased grooming in the open field, increased tail-flick latency, shorter durations on the open arms of the elevated plus maze, and heightened locomotor activity following EtOH.
Conclusions
These behavioral, physiological and endocrine changes are relatively consistent with previous findings with agonists and antagonists for the NOP receptor and, taken together, suggest that ASO-induced downregulation of the NOP receptor is an effective method for studying the N/OFQ system.


Similar content being viewed by others
References
Blakley GG, Pohorecky LA, Benjamin D (2001) Bidirectional changes in ethanol consumption in rats with site specific antisense down-regulation of 5-hydroxytryptamine2A receptors in brain. J Pharmacol Exp Ther 299:277–289
Bytner B, Huang Y-H, Yu L-C, Lundeberg T, Nylander I, Rosen A (2001) Nociception/orphanin FQ into the rat periqueductal gray decreases the withdrawal latency to heat and loading, an effect reversed by (Nphe1)nociception(1–13)NH2. Brain Res 922:118–124
Calo G, Geurrini R, Bigoni R, Rizzi A, Marzola G, Okawa H, Bianchi C, Lambert DG, Salvadori S, Regoli D (2000) Characterization of (Nphe1)nociception(1–13)NH2, a new selective nociception receptor antagonist. Br J Pharmacol 129:1183–1193
Chen X, McClatchy DB, Geller EB, Liu-Chen L-Y, Tallarida RJ, Adler MW (2001) Possible mechanism of hypothermia induced by intracereberoventricular injection of orphanin FQ/nociception. Brain Res 904:252–258
Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M (1998) Effect of Nociceptin on alcohol intake in ethanol-preferring rats. Psychopharmacology 141:220–224
Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M (2000) Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur J Pharmacol 404:153–159
Ciccocioppo R, Polidori C, Antonelli L, Salvadori S, Guerrini R, Massi M (2002) Pharmacological characterization of the nociceptin receptor which mediates reduction of alcohol drinking in rats. Peptides 23:117–125
Devine DP, Reinscheeid RK, Monsma FJ, Civelli O, Akil H (1996) The novel peptide orphanin FQ fails to produce conditioned place preference or aversion. Brain Res 727:225–229
Devine DP, Watson SJ, Akil H (2001) Nociceptio/orphanin FQ regulates neuroendocrine function of the limbic-hypothalamic–pituitary adrenal axis. Neuroscience 102:541–553
Florin S, Meunier JC, Costentin (2001a) Autoradiographic localization of [3H]nociceptin binding sites in the rat brain. Brain Res 880:11–16
Florin S, Meunier JC, Costentin (2001b) Corrigendum to: autoradiographic localization of [3H]nociceptin binding sites in the rat brain. Brain Res 910:208–209
Griebel G, Perrault G, Sanger DJ (1999) Orphanin FQ, a novel neuropeptide with anti-stress-like activity. Brain Res 836:221–224
Hawes BE, Fried S, Xiaorui Y, Weig B, Graziano M (1998) Nociceptin (NOP4) and μ-opioid receptors mediate mitogen-activated protein kinase activation in CHO cells through a Gi-coupled signaling pathway: evidence for distinct mechanisms of agonist-mediated desensitization. J Neurochem 17:1024–1033
Herz A (1997) Endogenous opioid systems and alcohol addiction. Psychopharmacology 129:99–111
Higgins GA, Grottick AJ, Ballard TM, Richards JG, Messer J, Takeshime H, Pauly-Evers M, Jenck F, Adam G, Wichmann J (2001) Influence of the selective NOP4 receptor agonist, Ro 64–6198, on rodent neurological function. Neuropharmacology 41:97–107
Jenck F, Moreau J-L, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Nothacker H-P, Civelli O (1997) Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc Natl Acad Sci U S A 94:14854–14858
Jenck F, Quagazzai AM, Pauley-Evers M, Moreau J-L (2000a) Orphanin FQ: role in behavioral fear responses and vulnerability to stress? Mol Psychiatry 5:572–564
Jenck F, Wichmann J, Dautzenberg FM, Moreau J-L, Quagazzai AM, Martin JR, Lundstrom K, Cesura AM, Polli SM, Roever S, Kolczewski S, Adam G, Kilpatrick G (2000b) A synthetic agonist at the orphanin FQ/nociception receptor NOP4: anxiolytic profile in the rat. Proc Natl Acad Sci U S A 97:4938–4943
Leventhal L, Mathis JP, Rossi GC, Pasternak GW, Bodnar RJ (1998) Orphanin opioid receptor antisense probes block orphanin FQ-induced hyperphagia. Eur J Pharmacol 349: R1–R3
Lindholm S, Ploj K, Frank J, Nylander I (2002) Nociception/orphanin FQ tissue concentration in the rat brain. Effects of repeated ethanol administration at various post-treatment intervals. Prog Neuropsychopharmacol Biol Psychiatry 26:303–306
Malin DH, Lake JR, Moon WD, Moy D, Montellano AL, Moy E, Campbell TD, Bell MV, Bryant D, Harrison LM, Grandy DK (2000) Nociception/orphanin FQ (N/OFQ) induces a quasi-morphine abstinence syndrome in the rat. Psychopharmacology 151:344–350
Massi M, Panocka I, Angeletti S, Ciccocioppo R, Polidori C (1999) Nociception (NC) attenuates ethanol intake and ethanol induced conditioned place preference (CPP) in alcohol-preferring rats. Brain Res 848:A41–42
Meunier J-C, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour J-L, Guillemot J-C, Ferrara P, Montsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J (1995) Isolation and structure of the endogenous agonist of opioid receptor-like NOP4 receptor. Nature 377:532–535
Mogil JS, Pasternak GW (2001) The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev 53:381–415
Mollereau C, Parmentier M, Mailleux P, Butour J-L, Moisand C, Chalon P, Caput D, Vassart G, Meunier J-C (1994) NOP4, a novel member of the opioid receptor family: cloning, functional expression and localization. FEBS Lett 341:33–38
Moran TD, Abdulla FA, Smith PA (2000) Cellular neurophysiological actions of nociception/orphanin FQ. Peptides 21:969–976
Murphy NP, Lee YL, Maidment NT (1999) Orphanin FQ/nociception blocks acquisistion of morphine place preference. Brain Res 832:168–170
Narayanan S, Maidment NT (1999) Orphanin FQ and behavioral sensitization to cocaine. Pharmacol Biochem Behav 63:271–277
Paxinos G, Watson C (1986) The rat brain, stereotaxic coordinates, 2nd edn. Academic Press Limited, San Diego, California
Pellow S, File SE (1986) Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav 24:525–529
Pohorecky LA, Patel V, Roberst P (1989) Effects of ethanol in an open field apparatus: modification by U50488H and WIN 44441–3. Physiol Behav 45:273–287
Pohorecky LA, Skiandos A, Zhang X, Rice K, Benjamin D (1999) Effects of chronic social stress on δ-opioid receptor function in the rat. J Pharmacol Exp Ther 290:196–206
Reinscheid RK, Nothacker H-P, Civelli O (1995) Orphanin FQ: a novel neuropeptide which is a natural ligand of an opioid-like G protein-coupled receptor. Science 270:792–794
Reinscheid RK, Higelin J, Henningsen RA, Monsma FJ Jr, Civelli O (1998) Structures that delineate orphanin FQ and dynorphin A pharmacological selectives. J Biol Chem 273:1490–1495
Shirasaka T, Miyahara S, Takasaki M, Kannan H (2001) Nociception/orphanin FQ and [Phe1 ψ(CH2-NH)Gly2]nociceptin(1–13)NH2 modulates the activity of hypothalamic paraventricular nucleus neurons in vitro. Brain Res 890:147–153
Siegfried B, Frischknecht H-F, De Souza RLN (1990) An ethological model for the study of activation and interaction of pain, memory and defense systems in the attacked mouse. Role of endogenous opioids. Neurosci Biobehav Rev 14:481–490
Sim LJ, Selley DE, Childers SR (1995) In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5’-[y-[35 S]thio]-triphosphate binding. Proc Natl Acad Sci U S A 92:7242–7246
Tanda G, Di Chiara G (1998) A dopamine-mu1 opioid link in the rat ventral tegmentum shared by palatable food (Fonzies) and non-psychostimulant drugs of abuse. Eur J Neurosci 10:1179–1187
Xiangping C, Ningshan X, Peng L, Wang JQ (1999) The nociception receptor-mediated inhibition of the rat rostral ventrolateral medulla neurons in vitro. Eur J Pharmacol 364:49–53
Xu X-J, Hao J-X, Weissenfield-Hallin Z (1996) Nociception of antinociception: potent spinal antinociceptive effect of orphanin FQ/nociception. Neuroreport 7:2092–2094
Yamada K, Nabeshima T (1995) Stress-induced behavioral responses and multiple opioid systems in the brain. Behav Brain Res 67:133–145
Yamamoto T, Nozaki- Taguchi N, Kimura S (1997) Analgesic effect of intrathecally administered nociception, an opioid receptor like1 receptor agonist, in the rat formalin test. Neuroscience 81:249–254
Yang Z-L, Zhang Y-Q, Wu G-W (2001) Distinct effect of orphanin FQ in nucleus raphe magnus and nucleus reticularis gigantocellularis on the rat tail flick reflex. Neurosci Lett 306:69–72
Acknowledgements
This research was funded by grant no. AAA10124, and additional support was provided by Rutgers University Center of Alcohol Studies. The authors with to thank Harry Suthord for his technical assistance in the endocrine analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Blakley, G.G., Pohorecky, L.A. & Benjamin, D. Behavioral and endocrine changes following antisense oligonucleotide-induced reduction in the rat NOP receptor. Psychopharmacology 171, 421–428 (2004). https://doi.org/10.1007/s00213-003-1597-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-003-1597-5