Abstract
Rationale
Atypical antipsychotic drugs are classically associated with lower propensity to extrapyramidal symptoms (EPS) and hyperprolactinemia than typical antipsychotic drugs. It has not been clarified why some atypical antipsychotic drugs, such as amisulpride, induce prolactin plasma concentration (PRL) elevation, but little EPS. Previous studies have found an association between striatal D2/D3 receptor occupancy and PRL in typical antipsychotic treated patients suggesting that PRL is a marker of central D2/D3 receptors blockade.
Objective
We have evaluated the relationship between PRL and central (striatum, temporal cortex and thalamus) D2/D3 receptor occupancy in amisulpride treated schizophrenic patients.
Methods
Single photon emission tomography (SPET) and [123I]-epidepride were used to determine D2/D3 receptor occupancy in eight amisulpride treated patients. PRL was measured concurrently with the scans.
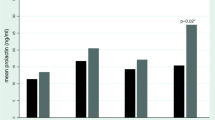
Results
The mean PRL was 1166 (range 499–1892 mIU/l) for a mean amisulpride dose of 406 mg/day (range 150–600 mg/day). Amisulpride plasma concentration and central D2/D3 receptor occupancy were positively correlated (r=0.83–0.89, df=4, P<0.05). No significant correlations were observed between PRL and amisulpride (daily dose or plasma concentration, P>0.05), or between PRL and central D2/D3 receptor occupancy (P>0.05).
Conclusions
Our findings show that amisulpride-induced hyperprolactinemia is uncoupled from central D2/D3 receptor occupancy. Amisulpride has poor blood–brain barrier penetration and reaches much higher concentration at the pituitary, which is outside the blood–brain barrier. Higher D2/D3 receptor occupancy at the pituitary gland than at central regions is a possible explanation for amisulpride PRL elevation with low EPS. Further studies evaluating pituitary D2/D3 receptor occupancy in vivo are necessary to confirm this hypothesis.


Similar content being viewed by others
References
Addington D, Addington J, Maticka-Tyndale E, Joyce J (1992) Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res 6:201–208
Alcohol Drug Abuse, and Mental Health Administration (1974) Abnormal involuntary movement scale (AIMS). Department of Health, Education and Welfare, Washington D.C.
Barnes TR (1989) A rating scale for drug-induced akathisia. Br J Psychiatry 154:672–676
Bressan RA, Erlandsson K, Jones HM, Mulligan R, Flanagan RJ, Ell PJ, Pilowsky LS (2003a) Is regionally selective D2/D3 dopamine occupancy sufficient for atypical antipsychotic effect? An in vivo quantitative [123I]epidepride SPET study of amisulpride-treated patients. Am J Psychiatry 160:1413–1420
Bressan RA, Erlandsson K, Jones HM, Mulligan RS, Ell PJ, Pilowsky LS (2003b) Optimizing limbic selective D2/D3 receptor occupancy by risperidone: a [123I]-epidepride SPET study. J Clin Psychopharmacol 23:5–14
Brown WA, Laughren TP (1981) Tolerance to the prolactin-elevating effect of neuroleptics. Psychiatry Res 5:317–322
Erlandsson K, Bressan RA, Mulligan RS, Ell PJ, Cunningham VJ, Pilowsky LS (2003) Analysis of D2 dopamine receptor occupancy with quantitative SPET using the high-affinity ligand [123I]epidepride: resolving conflicting findings. Neuroimage 19:1205–1214
Flores CM, Hulihan-Giblin BA, Hornby PJ, Lumpkin MD, Kellar KJ (1992) Partial characterization of a neurotransmitter pathway regulating the in vivo release of prolactin. Neuroendocrinology 55:519–528
Folstein M, Folstein F, McHugh P (1975) Mini mental state—a practical method for grading the cognitive state of patients for the clinicians. J Psychiatr Res 12:189–198
Gruen PH, Sachar EJ, Langer G, Altman N, Leifer M, Frantz A, Halpern FS (1978) Prolactin responses to neuroleptics in normal and schizophrenic subjects. Arch Gen Psychiatry 35:108–116
Grunder G, Wetzel H, Schlosser R, Anghelescu I, Hillert A, Lange K, Hiemke C, Benkert O (1999) Neuroendocrine response to antipsychotics: effects of drug type and gender. Biol Psychiatry 45:89–97
Igarashi Y, Higuchi T, Toyoshima R, Noguchi T, Moroji T (1985) Tolerance to prolactin secretion in the long-term treatment with neuroleptics in schizophrenia. Adv Biochem Psychopharmacol 40:95–98
Invitti C, Danesi L, Dubini A, Cavagnini F (1998) Neuroendocrine effects of chronic administration of sodium valproate in epileptic patients. Acta Endocrinol 118:381–388
Jaber M, Robinson SW, Missale C, Caron MG (1996) Dopamine receptors and brain function. Neuropharmacology 35:1503–1519
Kapur S, Zipursky R, Jones C, Remington G, Houle S (2000) Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry 157:514–520
Kapur S, Langlois X, Vinken P, Megens AA, de Coster R, Andrews JS (2002) The differential effects of atypical antipsychotics on prolactin elevation are explained by their differential blood-brain disposition: a pharmacological analysis in rats. J Pharmacol Exp Ther 302:1129–1134
Kay SR, Fiszbein A, Opler LA (1987) The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276
Kuruvilla A, Peedicayil J, Srikrishna G, Kuruvilla K, Kanagasabapathy AS (1992) A study of serum prolactin levels in schizophrenia: comparison of males and females. Clin Exp Pharmacol Physiol 19:603–606
Lammertsma AA, Hume SP (1996) Simplified reference tissue model for PET receptor studies. Neuroimage 4:153–158
Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, Frackowiak RSJ (1996) Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab 16:42–52
Laughren TP, Brown WA, Williams BW (1979) Serum prolactin and clinical state during neuroleptic treatment and withdrawal. Am J Psychiatry 136:108–110
Leucht S, Pitschel-Walz G, Engel RR, Kissling W (2002) Amisulpride, an unusual “atypical” antipsychotic: a meta-analysis of randomized controlled trials. Am J Psychiatry 159:180–190
Martinot JL, Paillere-Martinot ML, Poirier MF, Dao-Castellana MH, Loc’h C, Maziere B (1996) In vivo characteristics of dopamine D2 receptor occupancy by amisulpride in schizophrenia. Psychopharmacology 124:154–158
Melkersson KI, Hulting AL, Rane AJ (2001) Dose requirement and prolactin elevation of antipsychotics in male and female patients with schizophrenia or related psychoses. Br J Clin Pharmacol 51:317–324
Meltzer HY (1985) Long-term effects of neuroleptic drugs on the neuroendocrine system. Adv Biochem Psychopharmacol 40:59–68
Meltzer HY, Fang VS (1976) The effect of neuroleptics on serum prolactin in schizophrenic patients. Arch Gen Psychiatry 33:279–286
Mizuchi A, Kitagawa N, Miyachi Y (1983) Regional distribution of sultopride and sulpiride in rat brain measured by radioimmunoassay. Psychopharmacology 81:195–198
Monteleone P, Maj M, Ariano MG, Iovino M, Fiorenza L, Steardo L (1998) Prolactin response to sodium valproate in schizophrenics with and without tardive dyskinesia. Psychopharmacology 96:223–226
Naber D, Fischer H, Ackenheil M (1979) Effect of long-term neuroleptic treatment on dopamine tuberoinfundibular system: development of tolerance? Commun Psychopharmacol 3:59–65
Nördstrom AL, Farde L (1998) Plasma prolactin and central D2 receptor occupancy in antipsychotic drug-treated patients. J Clin Psychopharmacol 18:305–310
Nördstrom AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C et al. (1993) Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry 33:227–235
Overall J, Gorham D (1962) The brief psychiatric rating scale. Psychol Rep 10:799–812
Protais P, Hermier C, Costentin J (1985) The discriminant dopamine antagonist property of benzamides is observed at various times after their systemic or intracerebroventricular administration. Neuropharmacology 24:861–867
Rao ML, Brown WA (1987) Stability of serum neuroleptic and prolactin concentrations during short-term and long-term treatment of schizophrenic patients. Psychopharmacology 93:237–242
Rivera JL, Lal S, Ettigi P, Hontela S, Muller HF, Friesen HG (1976) Effect of acute and chronic neuroleptic therapy on serum prolactin levels in men and women of different age groups. Clin Endocrinol (Oxf) 5:273–282
Schlosser R, Grunder G, Anghelescu I, Hillert A, Ewald-Grunder S, Hiemke C et al. (2002) Long-term effects of the substituted benzamide derivative amisulpride on baseline and stimulated prolactin levels. Neuropsychobiology 46:33–40
Simpson GM, Angus JW (1970) A rating scale for extrapyramidal side effects. Acta Psychiatr Scand 212:11–19
Talairach J, Tourneaux P (1988) Co-planar stereotactic atlas of the human brain. Thieme, New York
Xiberas X, Martinot JL, Mallet L, Artiges E, Canal M, Loc’h C, Maziere B, Paillere-Martinot ML (2001) In vivo extrastriatal and striatal D2 dopamine receptor blockade by amisulpride in schizophrenia. J Clin Psychopharmacol 21:207–214
Acknowledgements
R.A. Bressan was supported by a charitable educational grant from Sanofi Synthelabo during the data collection. K. Erlandsson is supported on a UK MRC Senior clinical research fellowship. L.S. Pilowsky is a UK Medical Research Council (MRC) Senior Clinical Research Fellow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bressan, R.A., Erlandsson, K., Spencer, E.P. et al. Prolactinemia is uncoupled from central D2/D3 dopamine receptor occupancy in amisulpride treated patients. Psychopharmacology 175, 367–373 (2004). https://doi.org/10.1007/s00213-004-1826-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-004-1826-6