Abstract
Rationale
The C57BL/6J (C57) and DBA/2J (DBA) mice are the most common genotypes used to identify chromosomal regions and neurochemical mechanisms of interest in opioid addiction. Unfortunately, outside of the oral two-bottle choice procedure, limited and sometimes controversial evidence is available for determining their relative sensitivity to the rewarding effects of morphine.
Objectives
The purpose of this study was to utilize classically accepted models of drug abuse liability to determine relative susceptibility to the rewarding effects of morphine.
Methods
The ability of morphine or amphetamine to potentiate lateral hypothalamic brain stimulation and intravenous morphine self-administration (across three doses in a fixed ratio schedule and at the highest dose in progressive ratio schedules) was investigated in both genotypes.
Results
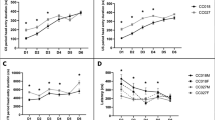
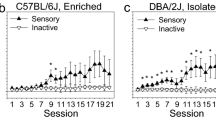
In both measures, C57 and DBA mice differed dramatically in their response to morphine. Morphine potentiated rewarding stimulation in the C57 mice but antagonized it in the DBA mice. Consistent with these findings, intravenous morphine did not serve as a positive reinforcer in DBA mice under conditions that were effective in the C57 mice using a fixed ratio schedule and failed to sustain levels of responding sufficient to maintain a constant rate of drug intake under a progressive ratio schedule. In contrast, amphetamine potentiated the rewarding effects of brain stimulation similarly in the two genotypes.
Conclusions
These findings provide strong evidence that morphine is rewarding in the C57 genotype and not in the DBA genotype. Understanding their relative susceptibility is important given the prominence of these genotypes in candidate gene identification and gene mapping.







Similar content being viewed by others
References
Alexander RC, Heydt D, Ferraro TN, Vogel W, Berrettini WH (1996) Further evidence for a quantitative trait locus on murine chromosome 10 controlling morphine preference in inbred mice. Psychiatr Genet 6:29–31
Ambrosio E, Goldberg SR, Elmer GI (1995) Behavior genetic analysis of innate locomotor activity and acquisition of morphine self-administration behavior. Behav Pharmacol 6:99–106
Belknap JK, Crabbe JC, Riggan J, O’Toole LA (1993) Voluntary consumption of morphine in 15 inbred mouse strains. Psychopharmacology (Berl) 112:352–358
Belknap JK, Mogil JS, Helms ML, Richards SP, O’Toole LA, Bergeson SE, Buck KJ (1995) Localization to chromosome 10 of a locus influencing morphine analgesia in crosses derived from C57BL/6 and DBA/2 strains. Life Sci 57:L117–L124
Benaliouad F, Kapur S, Rompre PP (2007) Blockade of 5-HT2a receptors reduces haloperidol-induced attenuation of reward. Neuropsychopharmacology 32:551–561
Berrettini WH, Alexander R, Ferraro TN, Vogel WH (1994a) A study of oral morphine preference in inbred mouse strains. Psychiatr Genet 4:81–86
Berrettini WH, Ferraro TN, Alexander RC, Buchberg AM, Vogel WH (1994b) Quantitative trait loci mapping of three loci controlling morphine preference using inbred mouse strains. Nat Genet 7:54–58
Cabib S, Orsini C, Le Moal M, Piazza PV (2000) Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science 289:463–465
Carlezon WA Jr, Chartoff EH (2007) Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2:2987–2995
Carlezon WA Jr, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM (2006) Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther 316:440–447
Cazala P, Cardo B (1977) Hypothalamic self-stimulation and operant activity in the mottled mutant mouse. Brain Res Bull 2:163–167
Cazala P, Guenet JL (1979) Effects of the albino gene on self-stimulation behavior in the lateral hypothalamus in the mouse. Physiol Behav 22:7–9
Cazala P, Cazals Y, Cardo B (1974) Hypothalamic self-stimulation in three inbred strains of mice. Brain Res 81:159–167
Cunningham CL, Niehus DR, Malott DH, Prather LK (1992) Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 107:385–393
D’Este L, Casini A, Puglisi-Allegra S, Cabib S, Renda TG (2007) Comparative immunohistochemical study of the dopaminergic systems in two inbred mouse strains (C57BL/6J and DBA/2J). J Chem Neuroanat 33:67–74
Davis CM, Roma PG, Dominguez JM, Riley AL (2007) Morphine-induced place conditioning in Fischer and Lewis rats: acquisition and dose–response in a fully biased procedure. Pharmacol Biochem Behav 86:516–523
Doyle GA, Furlong PJ, Schwebel CL, Smith GG, Lohoff FW, Buono RJ, Berrettini WH, Ferraro TN (2008) Fine mapping of a major QTL influencing morphine preference in C57BL/6 and DBA/2 mice using congenic strains. Neuropsychopharmacology 33:2801–2809
Dunnett CW, Horn M, Vollandt R (2001) Sample size determination in step-down and step-up multiple tests for comparing treatments with a control. J Stat Plan Infer 97:367–384
Eiler WJ 2nd, Woods JE 2nd, Masters J, McKay PF, Hardy L 3 rd, Goergen JJ, Mensah-Zoe B, Cook JB, Johnson NJ, June HL (2005) Brain stimulation reward performance and sucrose maintained behaviors in alcohol-preferring and -nonpreferring rats. Alcohol Clin Exp Res 29:571–583
Eiler WJ 2nd, Masters J, McKay PF, Hardy L 3 rd, Goergen J, Mensah-Zoe B, Seyoum R, Cook J, Johnson N, Neal-Beliveau B, June HL (2006) Amphetamine lowers brain stimulation reward (BSR) threshold in alcohol-preferring (P) and -nonpreferring (NP) rats: regulation by D-sub-1 and D-sub-2 receptors in the nucleus accumbens. Exp Clin Psychopharmacol 14:361–376
Eiler WJ 2nd, Hardy L 3rd, Goergen J, Seyoum R, Mensah-Zoe B, June HL (2007) Responding for brain stimulation reward in the bed nucleus of the stria terminalis in alcohol-preferring rats following alcohol and amphetamine pretreatments. Synapse 61:912–924
Elmer GI, Mathura CB, Goldberg SR (1993) Genetic factors in conditioned tolerance to the analgesic effects of etonitazene. Pharmacol Biochem Behav 45:251–253
Elmer GI, Evans JL, Ladenheim B, Epstein CJ, Cadet JL (1995a) Transgenic superoxide dismutase mice differ in opioid-induced analgesia. Eur J Pharmacol 283:227–232
Elmer GI, Pieper JO, Goldberg SR, George FR (1995b) Opioid operant self-administration, analgesia, stimulation and respiratory depression in µ-deficient mice. Psychopharmacology 117:23–31
Elmer GI, Evans JL, Goldberg SR, Epstein CJ, Cadet JL (1996) Transgenic superoxide dismutase mice: Increased mesolimbic micro-opioid receptors results in greater opioid-induced stimulation and opioid-reinforced behavior. Behav Pharmacol 7:628–639
Elmer GI, Pieper JO, Negus SS, Woods JH (1998) Genetic variance in nociception and its relationship to the potency of morphine-induced analgesia in thermal and chemical tests. Pain 75:129–140
Elmer GI, Pieper JO, Rubinstein M, Low MJ, Grandy DK, Wise RA (2002) Failure of intravenous morphine to serve as an effective instrumental reinforcer in dopamine D2 receptor knock-out mice. J Neurosci 22:RC224
Elmer GI, Pieper JO, Levy J, Rubinstein M, Low MJ, Grandy DK, Wise RA (2005) Brain stimulation and morphine reward deficits in dopamine D2 receptor-deficient mice. Psychopharmacology (Berl) 182:33–44
Ferraro TN, Golden GT, Smith GG, Martin JF, Schwebel CL, Doyle GA, Buono RJ, Berrettini WH (2005) Confirmation of a major QTL influencing oral morphine intake in C57 and DBA mice using reciprocal congenic strains. Neuropsychopharmacology 30:742–746
Ford CP, Mark GP, Williams JT (2006) Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci 26:2788–2797
Gallistel CR (1987) Determining the quantitative characteristics of a reward pathway. In: Church RM, Commons ML, Stellar JR, Wagner AR (eds) Biological Determinants of Reinforcement. Lawrence Erlbaum Associates, Hillsdale, pp 1–30
Gallistel CR, Freyd G (1987) Quantitative determination of the effects of catecholaminergic agonists and antagonists on the rewarding efficacy of brain stimulation. Pharmacol Biochem Behav 26:731–741
Garrigues AM, Cazala P (1983) Central catecholamine metabolism and hypothalamic self-stimulation behaviour in two inbred strains of mice. Brain Res 265:265–271
Grabus SD, Martin BR, Brown SE, Damaj MI (2006) Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology (Berl) 184:456–463
Grahame NJ, Cunningham CL (1995) Genetic differences in intravenous cocaine self-administration between C57BL/6J and DBA/2J mice. Psychopharmacology (Berl) 122:281–291
Gregory SG, Sekhon M, Schein J, Zhao S, Osoegawa K, Scott CE, Evans RS, Burridge PW, Cox TV, Fox CA, Hutton RD, Mullenger IR, Phillips KJ, Smith J, Stalker J, Threadgold GJ, Birney E, Wylie K, Chinwalla A, Wallis J, Hillier L, Carter J, Gaige T, Jaeger S, Kremitzki C, Layman D, Maas J, McGrane R, Mead K, Walker R, Jones S, Smith M, Asano J, Bosdet I, Chan S, Chittaranjan S, Chiu R, Fjell C, Fuhrmann D, Girn N, Gray C, Guin R, Hsiao L, Krzywinski M, Kutsche R, Lee SS, Mathewson C, McLeavy C, Messervier S, Ness S, Pandoh P, Prabhu AL, Saeedi P, Smailus D, Spence L, Stott J, Taylor S, Terpstra W, Tsai M, Vardy J, Wye N, Yang G, Shatsman S, Ayodeji B, Geer K, Tsegaye G, Shvartsbeyn A, Gebregeorgis E, Krol M, Russell D, Overton L, Malek JA, Holmes M, Heaney M, Shetty J, Feldblyum T, Nierman WC, Catanese JJ, Hubbard T, Waterston RH, Rogers J, de Jong PJ, Fraser CM, Marra M, McPherson JD, Bentley DR (2002) A physical map of the mouse genome. Nature 418:743–750
Grice DE, Reenila I, Mannisto PT, Brooks AI, Smith GG, Golden GT, Buxbaum JD, Berrettini WH (2007) Transcriptional profiling of C57 and DBA strains of mice in the absence and presence of morphine. BMC Genomics 8:76
Henry DJ, Wise RA, Rompre PP, White FJ (1992) Acute depolarization block of A10 dopamine neurons: interactions of morphine with dopamine antagonists. Brain Res 596:231–237
Hitzemann R, Hitzemann B, Rivera S, Gatley J, Thanos P, Shou LL, Williams RW (2003) Dopamine D2 receptor binding, Drd2 expression and the number of dopamine neurons in the BXD recombinant inbred series: genetic relationships to alcohol and other drug associated phenotypes. Alcohol Clin Exp Res 27:1–11
Horowitz GP, Whitney G, Smith JC, Stephan FK (1977) Morphine ingestion: genetic control in mice. Psychopharmacology (Berl) 52:119–122
Jamensky NT, Gianoulakis C (1997) Content of dynorphins and kappa-opioid receptors in distinct brain regions of C57BL/6 and DBA/2 mice. Alcohol Clin Exp Res 21:1455–1464
Keith BJ, Franklin KBJ, Paxinos G (2008) The mouse brain in stereotaxic coordinates. Elsevier Academic Press, Boston
Korostynski M, Piechota M, Kaminska D, Solecki W, Przewlocki R (2007) Morphine effects on striatal transcriptome in mice. Genome Biol 8:R128
Meliska CJ, Bartke A, McGlacken G, Jensen RA (1995) Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav 50:619–626
Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ (1998) Familial transmission of substance use disorders. Arch Gen Psychiatry 55:973–979
Miliaressis E, Rompre PP, Laviolette P, Philippe L, Coulombe D (1986) The curve-shift paradigm in self-stimulation. Physiol Behav 37:85–91
Mittleman G, Van Brunt CL, Matthews DB (2003) Schedule-induced ethanol self-administration in DBA/2J and C57BL/6J mice. Alcohol Clin Exp Res 27:918–925
Okazaki Y, Furuno M, Kasukawa T, Adachi J, Bono H, Kondo S, Nikaido I, Osato N, Saito R, Suzuki H, Yamanaka I, Kiyosawa H, Yagi K, Tomaru Y, Hasegawa Y, Nogami A, Schonbach C, Gojobori T, Baldarelli R, Hill DP, Bult C, Hume DA, Quackenbush J, Schriml LM, Kanapin A, Matsuda H, Batalov S, Beisel KW, Blake JA, Bradt D, Brusic V, Chothia C, Corbani LE, Cousins S, Dalla E, Dragani TA, Fletcher CF, Forrest A, Frazer KS, Gaasterland T, Gariboldi M, Gissi C, Godzik A, Gough J, Grimmond S, Gustincich S, Hirokawa N, Jackson IJ, Jarvis ED, Kanai A, Kawaji H, Kawasawa Y, Kedzierski RM, King BL, Konagaya A, Kurochkin IV, Lee Y, Lenhard B, Lyons PA, Maglott DR, Maltais L, Marchionni L, McKenzie L, Miki H, Nagashima T, Numata K, Okido T, Pavan WJ, Pertea G, Pesole G, Petrovsky N, Pillai R, Pontius JU, Qi D, Ramachandran S, Ravasi T, Reed JC, Reed DJ, Reid J, Ring BZ, Ringwald M, Sandelin A, Schneider C, Semple CA, Setou M, Shimada K, Sultana R, Takenaka Y, Taylor MS, Teasdale RD, Tomita M, Verardo R, Wagner L, Wahlestedt C, Wang Y, Watanabe Y, Wells C, Wilming LG, Wynshaw-Boris A, Yanagisawa M, Yang I, Yang L, Yuan Z, Zavolan M, Zhu Y, Zimmer A, Carninci P, Hayatsu N, Hirozane-Kishikawa T, Konno H, Nakamura M, Sakazume N, Sato K, Shiraki T, Waki K, Kawai J, Aizawa K, Arakawa T, Fukuda S, Hara A, Hashizume W, Imotani K, Ishii Y, Itoh M, Kagawa I, Miyazaki A, Sakai K, Sasaki D, Shibata K, Shinagawa A, Yasunishi A, Yoshino M, Waterston R, Lander ES, Rogers J, Birney E, Hayashizaki Y (2002) Analysis of the mouse transcriptome based on functional annotation of 60,770 full-length cDNAs. Nature 420:563–573
Orsini C, Buchini F, Piazza PV, Puglisi-Allegra S, Cabib S (2004) Susceptibility to amphetamine-induced place preference is predicted by locomotor response to novelty and amphetamine in the mouse. Psychopharmacology (Berl) 172:264–270
Orsini C, Bonito-Oliva A, Conversi D, Cabib S (2005) Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology (Berl) 181:327–336
Ranaldi R, Bauco P, McCormick S, Cools AR, Wise RA (2001) Equal sensitivity to cocaine reward in addiction-prone and addiction-resistant rat genotypes. Behav Pharmacol 12:527–534
Risinger FO, Brown MM, Doan AM, Oakes RA (1998) Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol Clin Exp Res 22:677–684
Robinson SF, Marks MJ, Collins AC (1996) Inbred mouse strains vary in oral self-selection of nicotine. Psychopharmacology (Berl) 124:332–339
Rocha BA, Odom LA, Barron BA, Ator R, Wild SA, Forster MJ (1998) Differential responsiveness to cocaine in C57BL/6J and DBA/2J mice. Psychopharmacology (Berl) 138:82–88
Rompre PP, Wise RA (1989) Opioid–neuroleptic interaction in brainstem self-stimulation. Brain Res 477:144–151
Sanchez-Cardoso P, Higuera-Matas A, Martin S, del Olmo N, Miguens M, Garcia-Lecumberri C, Ambrosio E (2007) Modulation of the endogenous opioid system after morphine self-administration and during its extinction: a study in Lewis and Fischer 344 rats. Neuropharmacology 52:931–948
Semenova S, Kuzmin A, Zvartau E (1995) Strain differences in the analgesic and reinforcing action of morphine in mice. Pharmacol Biochem Behav 50:17–21
Sora I, Elmer G, Funada M, Pieper J, Li XF, Hall FS, Uhl GR (2001) Mu opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo mu receptor reserve. Neuropsychopharmacology 25:41–54
Stolerman IP, Naylor C, Elmer GI, Goldberg SR (1999) Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology (Berl) 141:297–306
Suzuki T, George FR, Meisch RA (1992) Etonitazene delivered orally serves as a reinforcer for Lewis but not Fischer 344 rats. Pharmacol Biochem Behav 42:579–586
Tapocik JD, Letwin N, Mayo CL, Frank B, Luu T, Achinike O, House C, Williams R, Elmer GI, Lee NH (2009) Identification of candidate genes and gene networks specifically associated with analgesic tolerance to morphine. J Neurosci 29:5295–5307
Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA Jr (2004) Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 172:463–470
Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L (1996) Genetic influences on DSM-III-R drug abuse and dependence: a study of 3, 372 twin pairs. Am J Med Genet 67:473–477
Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, Goldberg J, True W, Lin N, Toomey R, Eaves L (1998) Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch Gen Psychiatry 55:967–972
Tsuang MT, Bar JL, Harley RM, Lyons MJ (2001) The Harvard twin study of substance abuse: what we have learned. Harv Rev Psychiatry 9:267–279
van der Veen R, Piazza PV, Deroche-Gamonet V (2007) Gene–environment interactions in vulnerability to cocaine intravenous self-administration: a brief social experience affects intake in DBA/2J but not in C57BL/6J mice. Psychopharmacology (Berl) 193:179–186
van der Veen R, Koehl M, Abrous DN, de Kloet ER, Piazza PV, Deroche-Gamonet V (2008) Maternal environment influences cocaine intake in adulthood in a genotype-dependent manner. PLoS ONE 3:e2245
Ventura R, Alcaro A, Mandolesi L, Puglisi-Allegra S (2004) In vivo evidence that genetic background controls impulse-dependent dopamine release induced by amphetamine in the nucleus accumbens. J Neurochem 89:494–502
Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigo R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M, Karlsson EK, Karolchik D, Kasprzyk A, Kawai J, Keibler E, Kells C, Kent WJ, Kirby A, Kolbe DL, Korf I, Kucherlapati RS, Kulbokas EJ, Kulp D, Landers T, Leger JP, Leonard S, Letunic I, Levine R, Li J, Li M, Lloyd C, Lucas S, Ma B, Maglott DR, Mardis ER, Matthews L, Mauceli E, Mayer JH, McCarthy M, McCombie WR, McLaren S, McLay K, McPherson JD, Meldrim J, Meredith B, Mesirov JP, Miller W, Miner TL, Mongin E, Montgomery KT, Morgan M, Mott R, Mullikin JC, Muzny DM, Nash WE, Nelson JO, Nhan MN, Nicol R, Ning Z, Nusbaum C, O’Connor MJ, Okazaki Y, Oliver K, Overton-Larty E, Pachter L, Parra G, Pepin KH, Peterson J, Pevzner P, Plumb R, Pohl CS, Poliakov A, Ponce TC, Ponting CP, Potter S, Quail M, Reymond A, Roe BA, Roskin KM, Rubin EM, Rust AG, Santos R, Sapojnikov V, Schultz B, Schultz J, Schwartz MS, Schwartz S, Scott C, Seaman S, Searle S, Sharpe T, Sheridan A, Shownkeen R, Sims S, Singer JB, Slater G, Smit A, Smith DR, Spencer B, Stabenau A, Stange-Thomann N, Sugnet C, Suyama M, Tesler G, Thompson J, Torrents D, Trevaskis E, Tromp J, Ucla C, Ureta-Vidal A, Vinson JP, Von Niederhausern AC, Wade CM, Wall M, Weber RJ, Weiss RB, Wendl MC, West AP, Wetterstrand K, Wheeler R, Whelan S, Wierzbowski J, Willey D, Williams S, Wilson RK, Winter E, Worley KC, Wyman D, Yang S, Yang SP, Zdobnov EM, Zody MC, Lander ES (2002) Initial sequencing and comparative analysis of the mouse genome. Nature 420:520–562
Winkler A, Spanagel R (1998) Differences in the kappa opioid receptor mRNA content in distinct brain regions of two inbred mice strains. NeuroReport 9:1459–1464
Wise RA (1978) Neuroleptic attenuation of intracranial self-stimulation: reward or performance deficits? Life Sci 22:535–542
Wise RA (2008) Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res 14:169–183
Wise RA, Rompre PP (1989) Brain dopamine and reward. Annu Rev Psychol 40:191–225
Woods JE 2nd, McKay PF, Masters J, Seyoum R, Chen A, La Duff L, Lewis MJ, June HL (2003) Differential responding for brain stimulation reward and sucrose in high-alcohol-drinking (HAD) and low-alcohol-drinking (LAD) rats. Alcohol Clin Exp Res 27:926–936
Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA (2008) Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol 42:149–160
Zacharko RM, Lalonde GT, Kasian M, Anisman H (1987) Strain-specific effects of inescapable shock on intracranial self-stimulation from the nucleus accumbens. Brain Res 426:164–168
Zacharko RM, Gilmore W, MacNeil G, Kasian M, Anisman H (1990) Stressor induced variations of intracranial self-stimulation from the mesocortex in several strains of mice. Brain Res 533:353–357
Acknowledgment
This work was supported by the Intramural Research Program/NIDA, NIDA/MPRC NS001, HHSN271200599091C and in part by NIDA grant DA19813.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elmer, G.I., Pieper, J.O., Hamilton, L.R. et al. Qualitative differences between C57BL/6J and DBA/2J mice in morphine potentiation of brain stimulation reward and intravenous self-administration. Psychopharmacology 208, 309–321 (2010). https://doi.org/10.1007/s00213-009-1732-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-009-1732-z