Abstract
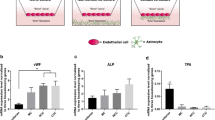
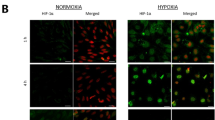
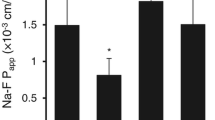
Confluent cell monolayers of brain capillary endothelial cells (BCEC) are used widely as an in vitro cell culture model of the blood–brain barrier. The present study describes the influence of cell-culture conditions on tight junctions, filamentous-actin cytoskeleton, and expression of ATP-binding cassette (ABC) transporters in primary cell cultures of porcine BCEC. Astrocyte as well as C6 glioma-conditioned cell culture medium was used in combination with retinoic acid, dexamethasone, cyclic adenosine monophosphate (cAMP) analogs, or 1,25-dihydroxyvitamin D3. It was shown that C6-conditioned medium led to a reorganization of filamentous actin and to an improved staining of zonula occludens-associated protein-1 (ZO-1). Further optimization of these culture conditions was achieved with cAMP analogs and dexamethasone. Retinoic acid, as well as 1,25-dihydroxyvitamin D3, did not improve cellular tight junctions as judged by filamentous actin, ZO-1 rearrangement, and transcellular electrical resistance (TER) measurements. However, these morphological changes did not influence the paracellular permeability of the extracellular marker sucrose. Expression of ABC transporters such as P-glycoprotein, multidrug resistance-associated protein-1( MRP1), and MRP2 were compared by measuring messenger RNA (mRNA) levels in whole-brain tissue, isolated brain capillaries, and cultured cells. In freshly isolated BCEC, mRNA levels of MRP2 and P-glycoprotein dropped by two- to sevenfold, respectively, whereas MRP1 mRNA levels were slightly increased. During cell culture, mRNA levels of MRP1 and MRP2 decreased by up to fivefold, while P-glycoprotein levels remained constant. These results were unaltered by different cell-culture conditions. In conclusion, the present study suggests that paracellular permeability, as well as mRNA expression of the studied ABC transporters in primary cultures, of porcine BCEC are insensitive toward changes in cell-culture conditions.





Similar content being viewed by others
Abbreviations
- ACEM:
-
Astrocyte-conditioned endothelial medium
- BCEC:
-
Brain capillary endothelial cells
- C6CEM:
-
C6-conditioned endothelial medium
- TER:
-
Transcellular electrical resistance
References
Abbott NJ, Revest PA, Romero IA (1992) Astrocyte-endothelial interaction: physiology and pathology. Neuropathol Appl Neurobiol 18:424–433
Allt G, Lawrenson JG (2001) Pericytes: cell biology and pathology. Cells Tissues Organs 169:1–11
Bouillon R, Okamura WH, Norman AW (1995) Structure–function relationships in the vitamin D endocrine system. Endocr Rev 16:200–257
Childs S, Ling V (1996) Duplication and evolution of the P-glycoprotein genes in pig. Biochim Biophys Acta 1307:205–212
Declèves X, Regina A, Laplanche JL et al. (2000) Functional expression of P-glycoprotein and multidrug resistance-associated protein (Mrp1) in primary cultures of rat astrocytes. J Neurosci Res 60:594–601
Dehouck MP, Dehouck B, Schluep C, Lemaire M, Cecchelli R (1995) Drug transport to the brain: comparison between in vitro and in vivo models of the blood–brain barrier. Eur J Pharm Sci 3:357–365
Durieu-Trautmann O, Federici C, Creminon C et al. (1993) Nitric oxide and endothelin secretion by brain microvessel endothelial cells: regulation by cyclic nucleotides. J Cell Physiol 155:104–111
El Hafny B, Chappey O, Piciotti M, Debray M, Boval B, Roux F (1997) Modulation of P-glycoprotein activity by glial factors and retinoic acid in an immortalized rat brain microvessel endothelial cell line. Neurosci Lett 236:107–111
Franke H, Galla HJ, Beuckmann CT (1999) An improved low-permeability in vitro-model of the blood–brain barrier: transport studies on retinoids, sucrose, haloperidol, caffeine and mannitol. Brain Res 818:65–71
Fricker G, Nobmann S, Miller DS (2002) Permeability of porcine blood brain barrier to somatostatin analogues. Br J Pharmacol 135:1308–1314
Gaillard PJ, Sandt IC van der, Voorwinden LH et al. (2000) Astrocytes increase the functional expression of P-glycoprotein in an in vitro model of the blood–brain barrier Pharm Res 17:1198–1205
Golden PL, Pardridge WM (2000) Brain microvascular P-glycoprotein and a revised model of multidrug resistance in brain. Cell Mol Neurobiol 20:165–181
Grabb PA, Gilbert MR (1995) Neoplastic and pharmacological influence on the permeability of an in vitro blood–brain barrier. J Neurosurg 82:1053–1058
Gumbiner B, Simons K (1986) A functional assay for proteins involved in establishing an epithelial occluding barrier: identification of a uvomorulin-like polypeptide. J Cell Biol 102:457–468
Gutmann H, Török M, Fricker G et al. (1999) Modulation of multidrug resistance protein expression in porcine brain capillary endothelial cells in vitro. Drug Metab Dispos 27:937–941
Hayashi Y, Nomura M, Yamagishi S et al. (1997) Induction of various blood–brain barrier properties in non-neural endothelial cells by close apposition to cocultured astrocytes. Glia 19:13–26
Huai-Yun H, Secrest DT, Mark KS et al. (1998) Expression of multidrug resistance-associated protein (MRP) in brain microvessel endothelial cells. Biochem Biophys Res Commun 243:816–820
Huwyler J, Drewe J, Klusemann C, Fricker G (1996) Evidence for P-glycoprotein-modulated penetration of morphine-6-glucuronide into brain capillary endothelium. Br J Pharmacol 118:1879–1885
Huwyler J, Fricker G, Török M, Schneider M, Drewe J (1997) Transport of clonidine across cultured brain microvessel endothelial cells. J Pharmacol Exp Ther 282:81–85
Janzer RC, Raff MC (1987) Astrocytes induce blood–brain barrier properties in endothelial cells. Nature 325:253–257
Klein I, Sarkadi B, Varadi A (1999) An inventory of the human ABC proteins. Biochim Biophys Acta 1461:237–262
Korn J, Christ B, Kurz H (2002) Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J Comp Neurol 442:78–88
Lechardeur D, Schwartz B, Paulin D, Scherman D (1995) Induction of blood–brain barrier differentiation in a rat brain-derived endothelial cell line. Exp Cell Res 220:161–170
Lillien LE, Sendtner M, Rohrer H, Hughes SM, Raff MC (1988) Type-2 astrocyte development in rat brain cultures is initiated by a CNTF-like protein produced by type-1 astrocytes. Neuron 1:485–494
Löscher W, Potschka H (2002) Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J Pharmacol Exp Ther 301:7–14
Meyer J, Rauh J, Galla HJ (1991) The susceptibility of cerebral endothelial cells to astroglial induction of blood–brain barrier enzymes depends on their proliferative state. J Neurochem 57:1971–1977
Miller DS, Nobmann SN, Gutmann H et al. (2000) Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol 58:1357–1367
Miller DS, Graeff C, Droulle L, Fricker S, Fricker G (2002) Xenobiotic efflux pumps in isolated fish brain capillaries. Am J Physiol Regul Integr Comp Physiol 282:191–198
Pardridge WM (1993) Brain drug delivery and blood–brain barrier transport. Drug Delivery 1:83–101
Pardridge WM, Eisenberg J, Yamada T (1985) Rapid sequestration and degradation of somatostatin analogues by isolated brain microvessels. J Neurochem 44:1178–1184
Pardridge WM, Triguero D, Yang J, Cancilla PA (1990) Comparison of in vitro and in vivo models of drug transcytosis through the blood–brain barrier. J Pharmacol Exp Ther 253:884–891
Ramsauer M, Krause D, Dermietzel R (2002) Angiogenesis of the blood–brain barrier in vitro and the function of cerebral pericytes. FASEB J 16:1274–1276
Raub TJ (1996) Signal transduction and glial cell modulation of cultured brain microvessel endothelial cell tight junctions. Am J Physiol Cell Physiol 271:495–503
Raub TJ, Kuentzel SL, Sawada GA (1992) Permeability of bovine brain microvessel endothelial cells in vitro: barrier tightening by a factor released from astroglioma cells. Exp Cell Res 199:330–340
Reese DH, Fiorentino GJ, Claflin AJ, Malinin TI, Politano VA (1981) Rapid induction of alkaline phosphatase activity by retinoic acid. Biochem Biophys Res Commun 102:315–321
Regina A, Koman A, Piciotti M et al. (1998) Mrp1 multidrug resistance-associated protein and P-glycoprotein expression in rat brain microvessel endothelial cells. J Neurochem 71:705–715
Rist RJ, Romero IA, Chan MW et al. (1997) F-actin cytoskeleton and sucrose permeability of immortalised rat brain microvascular endothelial cell monolayers: effects of cyclic AMP and astrocytic factors. Brain Res 768:10–18
Rubin LL, Staddon JM (1999) The cell biology of the blood–brain barrier. Annu Rev Neurosci 22:11–28
Rubin LL, Hall DE, Porter S et al. (1991) A cell culture model of the blood–brain barrier. J Cell Biol 115:1725–1735
Schinkel AH, Wagenaar E, Mol CA, Deemter L van (1996) P-glycoprotein in the blood–brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest 97:2517–2524
Staddon JM, Rubin LL (1996) Cell adhesion, cell junctions and the blood–brain barrier. Curr Opin Neurobiol 6:622–627
Stewart PA, Wiley MJ (1981) Developing nervous tissue induces formation of blood–brain barrier characteristics in invading endothelial cells: a study using quail-chick transplantation chimeras. Dev Biol 84:183–192
Takakura Y, Audus KL, Borchardt RT (1991) Blood–brain barrier: transport studies in isolated brain capillaries and in cultured brain endothelial cells. Adv Pharmacol 22:137–165
Török M, Huwyler J, Drewe J, Gutmann H, Fricker G (1998) Transport of the β-lactam antibiotic benzylpenicillin and the dipeptide glycylsarcosine by brain capillary endothelial cells in vitro. Drug Metab Dispos 26:1144–1148
Tsao MS, Batist G (1988) Induction of γ-glutamyl transpeptidase activity by all-trans retinoic acid in cultured rat liver epithelial cells. Biochem Biophys Res Commun 157:1039–1045
Zhang Y, Han H, Elmquist WF, Miller DW (2000) Expression of various multidrug resistance-associated protein (MRP) homologues in brain microvessel endothelial cells. Brain Res 876:148–153
Zonta M, Angulo MC, Gobbo S. (2003) Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6:43–50
Acknowledgements
This work was supported by the Swiss National Science Foundation (grant 32-052918.97). We thank U. Behrens for excellent technical assistance. We would also like to thank S. Brill and Dr. P. Roberts for careful revision of this manuscript
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Török, M., Huwyler, J., Gutmann, H. et al. Modulation of transendothelial permeability and expression of ATP-binding cassette transporters in cultured brain capillary endothelial cells by astrocytic factors and cell-culture conditions. Exp Brain Res 153, 356–365 (2003). https://doi.org/10.1007/s00221-003-1620-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1620-4