Summary
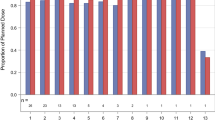
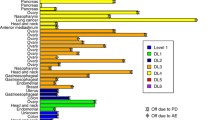
Purpose: GEM®231 is a second-generation antisense oligonucleotide targeting the mRNA of the R1α regulatory subunit of cAMP dependent protein kinase A. Preclinical studies have demonstrated synergistic antitumor activity when GEM®231 is combined with docetaxel. This trial assesses the safety of this combination. Experimental Design: Docetaxel was administered once every three weeks (one-cycle) at doses between 50–75 mg/m2. GEM®231 was administered twice weekly at 220 mg/m2 for 3 (schedule-A), or 2 (schedule-B) weeks. Results: Twenty patients with chemotherapy-refractory advanced cancer received a total of 39 cycles of therapy. Six patients in schedule-A received docetaxel 50 mg/m2, and 14 patients in schedule-B received docetaxel 50–75 mg/m2. In schedule-A, 2 of 6 patients developed cycle-1 dose limiting toxicity (DLT)-grade-3 fatigue or grade-3 serum transaminase elevation. In schedule-B, 1 of 4 patients developed cycle-1 DLT at the highest dose of docetaxel tested (75 mg/m2)—grade–3 febrile neutropenia. Subsequent dose escalations were not pursued since the overall incidence of grade-3 toxicities (including those that occurred after cycle 1) was 75%, and this dose was close to the single agent MTD of docetaxel. Grade-3 toxicities included fatigue (2 patients), transaminase elevation (4 patients), and altered mentation (1 patient). The mean post-infusion aPTT was significantly higher than the pre-infusion value [14.8 seconds; p<0.001]; however, there were no hemorrhagic episodes. Conclusions: The recommended dose for further development of the combination of docetaxel and GEM®231 is 75 mg/m2 and 220 mg/m2, respectively. It is important to administer GEM®231 twice weekly for 2 consecutive weeks followed by a one-week break.
Similar content being viewed by others
Abbreviations
- aPTT:
-
activated partial thromboplastin time
- ALT:
-
alanine aminotransferase
- AST:
-
aspartate aminotransferase
- ASO:
-
antisense oligonucleotide
- cAMP:
-
cyclic adenosine monophosphate
- DLT:
-
dose limiting toxicity
- MTD:
-
maximum tolerated dose
- PKA RIα:
-
regulatory subunit α of PKA-I
- RPTD:
-
recommended phase two dose
- ST:
-
serum transaminases
References
Agrawal S, Kandimalla ER: Antisense and siRNA as agonists of Toll-like receptors. Nat Biotechnol 22: 1533–1537, 2004
Vitravene Study Group: A randomized controlled clinical trial of intravitreous fomivirsen for treatment of newly diagnosed peripheral cytomegalovirus retinitis in patients with AIDS. Am J Ophthalmol 133: 467–474, 2002
Agrawal S, Jiang Z, Zhao Q, Shaw D, Cai Q, Roskey A, Channavajjala L, Saxinger C, Zhang R: Mixed-backbone oligonucleotides as second generation antisense oligonucleotides: in vitro and in vivo studies. Proc Natl Acad Sci USA 94: 2620–2625, 1997
Agrawal S, Kandimalla ER: Corrigenda: Antisense and siRNA as agonists of Toll-like receptors. Nat Biotechnol 23: 117, 2005
Rai KR, Moore JO, Boyd TE, Larratt LM, Golenkov AK, Koziner B, Chanan-Khan A, Bociek RG, Itri LM: Phase 3 Randomized Trial of Fludarabine/Cyclophosphamide Chemotherapy with or without Oblimersen Sodium (Bcl-2 Antisense; Genasense; G3139) for Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL). Proceedings of the 46th Annual Meeting of the American Society of Hematology, San Diego, CA, Abstract # 338, 2004
Millward MJ, Bedikian AY, Conry RM, Gore ME, Pehamberger HE, Sterry W, Pavlick AC, De Conti RC, Gordon D, Itri LM: Randomized multinational phase 3 trial of dacarbazine (DTIC) with or without Bcl-2 antisense (oblimersen sodium) in patients with advanced malignant melanoma: Analysis of long-term survival. Proceedings of the 40th Annual Meeting of the American Society of Clinical Oncology, New Orleans, LA, Abstract # 7505, 2004
Cho-Chung YS, Nesterova M, Pepe S, Lee GR, Noguchi K, Srivastava RK, Srivastava AR, Alper O, Park YG, Lee YN: Antisense DNA-targeting protein kinase A-RIA subunit: A novel approach to cancer treatment. Front Biosci 4: D898–907, 1999
Tortora G, Ciardiello F: Protein kinase A as target for novel integrated strategies of cancer therapy. Ann N Y Acad Sci 968: 139–147, 2002
Miller WR: Regulatory subunits of PKA and breast cancer. Ann N Y Acad Sci 968: 37–48, 2002
Miller WR, Hulme MJ, Bartlett JM, MacCallum J, Dixon JM: Changes in messenger RNA expression of protein kinase A regulatory subunit alpha in breast cancer patients treated with tamoxifen. Clin Cancer Res 3: 2399–2404, 1997
Amieuz PS, McKnight GS: The essential role of RI alpha in the maintenance of regulated PKA activity. Ann N Y Acad Sci. 968: 75–95, 2002.
Wang H, Hang J, Shi Z, Li M, Yu D, Kandimalla ER, Agrawal S, Zhang R: Antisense oligonucleotide targeted to RIalpha subunit of cAMP-dependent protein kinase (GEM231) enhances therapeutic effectiveness of cancer chemotherapeutic agent irinotecan in nude mice bearing human cancer xenografts: in vivo synergistic activity, pharmacokinetics and host toxicity. Int. J Oncol 21: 73–80, 2002
Cho YS, Kim MK, Tan L, Srivastava R, Agrawal S, Cho-Chung YS: Protein kinase A RIalpha antisense inhibition of PC3M prostate cancer cell growth: Bcl-2 hyperphosphorylation, Bax up-regulation, and Bad-hypophosphorylation. Clin Cancer Res 8: 607–614, 2002
Nesterova M, Cho-Chung YS: Oligonucleotide sequence-specific inhibition of gene expression, tumor growth inhibition, and modulation of cAMP signaling by an RNA-DNA hybrid antisense targeted to protein kinase A RIalpha subunit. Antisense Nucleic Acid Drug Dev 6: 423–433, 2000
Wang H, Cai Q, Zeng X, Yu D, Agrawal S, Zhang R: Antitumor activity and pharmacokinetics of a mixed-backbone antisense oligonucleotide targeted to the RIalpha subunit of protein kinase A after oral administration. Proc Natl Acad Sci USA 24: 13989–13994, 1999
Nesterova M, Cho-Chung YS: A single-injection protein kinase A-directed antisense treatment to inhibit tumour growth. Nat Med 1: 528–533, 1995
Tortora G, Caputo R, Damiano V, Bianco R, Pepe S, Bianco AR, Jiang Z, Agrawal S, Ciardiello F: Synergistic inhibition of human cancer cell growth by cytotoxic drugs and mixed backbone antisense oligonucleotide targeting protein kinase A. Proc Natl Acad Sci USA 94: 12586–12591, 1997
Chen HX, Marshall JL, Ness E, Martin RR, Dvorchik B, Rizvi N, Marquis J, McKinlay M, Dahut W, Hawkins MJ: A safety and pharmacokinetic study of a mixed-backbone oligonucleotide (GEM231) targeting the type I protein kinase A by two-hour infusions in patients with refractory solid tumors. Clin Cancer Res 4: 1259–1266, 2000
Goel S, Desai K, Bulgaru A, Fields A, Goldberg G, Agrawal S, Martin R, Grindel M, Mani S: A safety study of a mixed-backbone oligonucleotide (GEM231) targeting the type I regulatory subunit alpha of protein kinase A using a continuous infusion schedule in patients with refractory solid tumors. Clin Cancer Res 9: 4069–4076, 2003
Tortora G, Caputo R, Pomatico G, Pepe S, Bianco AR, Agrawal S, Mendelsohn J, Ciardiello F: Cooperative inhibitory effect of novel mixed backbone oligonucleotide targeting protein kinase A in combination with docetaxel and anti-epidermal growth factor-receptor antibody on human breast cancer cell growth. Clin Cancer Res 5: 875–881, 1999
Tortora G, Bianco R, Damiano V, Fontanini G, De Placido S, Bianco AR, Ciardiello F: Oral antisense that targets protein kinase A cooperates with taxol and inhibits tumor growth, angiogenesis, and growth factor production. Clin Cancer Res 6: 2506–2512, 2000
Agrawal S, Kandimalla ER, Yu D, Ball R, Lombardi G, Lucas T, Dexter DL, Hollister BA, Chen SF: GEM 231, a second-generation antisense agent complementary to protein kinase A RIalpha subunit, potentiates antitumor activity of irinotecan in human colon, pancreas, prostate and lung cancer xenografts. Int J Oncol 21: 65–72, 2002
Beebe SJ, Holloway R, Rannels SR, Corbin JD: Two classes of cAMP analogs which are selective for the two different cAMP-binding sites of type II protein kinase demonstrate synergism when added together to intact adipocytes. J Biol Chem 259: 3539–3547, 1984
Srivastava RK, Srivastava AR, Korsmeyer SJ, Nesterova M, Cho-Chung YS, Longo DL: Involvement of microtubules in the regulation of Bcl2 phosphorylation and apoptosis through cyclic AMP-dependent protein kinase. Mol Cell Biol 18: 3509–3517, 1998
Rohlff C, Glazer RI: Regulation of the MDR1 promoter by cyclic AMP-dependent protein kinase and transcription factor Sp1. Int J Oncol 12: 383–386, 1998
Shirakawa K, Takara K, Tanigawara Y, Aoyama N, Kasuga M, Komada F, Sakaeda T, Okumura K: Interaction of docetaxel (Taxotere) with human P-glycoprotein. Jpn J Cancer Res 90: 1380–1386, 1999
Goel S, Cho-Chung YS, Bulgaru A, Desai K, Nesterova M, Wang H, Li M, Martin R, McKinlay M, Mani S: Extracellular Protein Kinase A (ECPKA) assay in cancer patients treated with multiday continuous intravenous infusions (CIV) of GEM®231, a second-generation antisense oligonucleotide targeting the PKA RIα subunit. Proceedings of the 94th Annual Meeting of the American Association of Cancer Research, Washington D.C., 2003, abstract R 653.
Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC: Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 89: 1138–1147, 1997
Package insert. Docetaxel.
Bruno R, Riva A, Hille D, Lebecq A, Thomas L: Pharmacokinetic and pharmacodynamic properties of docetaxel: results of phase I and phase II trials. Am J Health Syst Pharm 54(24 Suppl 2): S16–19, 1997
Miller AB, Hoogstraten B, Staquet M, Winkler A: Reporting results of cancer treatment. Cancer 47: 207–214, 1981
Green S, Weiss GR: Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs 10: 239–253, 1992
Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J: Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18: 2095–2103, 2000
Graham MJ, Crooke ST, Monteith DK, Cooper SR, Lemonidis KM, Stecker KK, Martin MJ, Crooke RM: In vivo distribution and metabolism of a phosphorothioate oligonucleotide within rat liver after intravenous administration. J Pharmacol Exp Ther 286: 447–458, 1998
Chi KN, Gleave ME, Klasa R, Murray N, Bryce C, Lopes de Menezes DE, D'Aloisio S, Tolcher AW: A phase I dose-finding study of combined treatment with an antisense Bcl-2 oligonucleotide (Genasense) and mitoxantrone in patients with metastatic hormone-refractory prostate cancer. Clin Cancer Res 7: 3920–3927, 2001
Rudin CM, Otterson GA, Mauer AM, Villalona-Calero MA, Tomek R, Prange B, George CM, Szeto L, Vokes EE: A pilot trial of G3139, a bcl-2 antisense oligonucleotide, and paclitaxel in patients with chemorefractory small-cell lung cancer. Ann Oncol 13: 539–545, 2002
Adjei AA, Dy GK, Erlichman C, Reid JM, Sloan JA, Pitot HC, Alberts SR, Goldberg RM, Hanson LJ, Atherton PJ, Watanabe T, Geary RS, Holmlund J, Dorr FA: A phase I trial of ISIS 2503, an antisense inhibitor of H-ras, in combination with gemcitabine in patients with advanced cancer. Clin Cancer Res 9: 115–123, 2003
Marcucci G, Byrd JC, Dai G, Klisovic MI, Kourlas PJ, Young DC, Cataland SR, Fisher DB, Lucas D, Chan KK, Porcu P, Lin ZP, Farag SF, Frankel SR, Zwiebel JA, Kraut EH, Balcerzak SP, Bloomfield CD, Grever MR, Caligiuri MA: Phase 1 and pharmacodynamic studies of G3139, a Bcl-2 antisense oligonucleotide, in combination with chemotherapy in refractory or relapsed acute leukemia. Blood 101: 425–432, 2003
Mani S, Rudin CM, Kunkel K, Holmlund JT, Geary RS, Kindler HL, Dorr FA, Ratain MJ: Phase I clinical and pharmacokinetic study of protein kinase C-alpha antisense oligonucleotide ISIS 3521 administered in combination with 5-fluorouracil and leucovorin in patients with advanced cancer. Clin Cancer Res 8: 1042–1048, 2002
Rudin CM, Kozloff M, Hoffman PC, Edelman MJ, Karnauskas R, Tomek R, Szeto L, Vokes EE : Phase I Study of G3139, a bcl-2 Antisense Oligonucleotide, Combined With Carboplatin and Etoposide in Patients With Small-Cell Lung Cancer. J Clin Oncol 22: 1110–1117, 2004
Tolcher AW, Kuhn J, Schwartz G, Patnaik A, Hammond LA, Thompson I, Fingert H, Bushnell D, Malik S, Kreisberg J, Izbicka E, Smetzer L, Rowinsky EK: A phase I pharmacokinetic and biological correlative study of oblimersen sodium (genasense, G3139), an antisense oligonucleotide to the bcl-2 mRNA, and of docetaxel in patients with hormone-refractory prostate cancer. Clin Cancer Res 10: 5048–5057, 2004
Sheehan JP, Lan HC: Phosphorothioate oligonucleotides inhibit the intrinsic tenase complex. Blood 92: 1617–1625, 1998
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH: Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346: 92–98, 2002
Mani S, Gu Y, Wadler S, Fingert H: Antisense therapeutics in oncology: Points to consider in their clinical evaluation. Antisense Nucleic Acid Drug Dev 9: 543–547, 1999
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED: Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351: 1513–1520, 2004
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. TAX 327 Investigators: Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351: 1502–1512, 2004
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by grants from Hybridon, Inc. and Aventis Pharmaceuticals.
Rights and permissions
About this article
Cite this article
Goel, S., Desai, K., Macapinlac, M. et al. A phase I safety and dose escalation trial of docetaxel combined with GEM®231, a second generation antisense oligonucleotide targeting protein kinase A R1α in patients with advanced solid cancers. Invest New Drugs 24, 125–134 (2006). https://doi.org/10.1007/s10637-006-2378-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-006-2378-x