Abstract
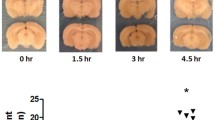
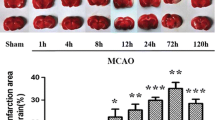
Blood–brain barrier (BBB) leakage plays a key role in cerebral ischemia–reperfusion injury. It is quite necessary to further explore the characteristic and mechanism of BBB leakage during stroke. We induced a focal cerebral ischemia model by transient middle cerebral artery occlusion in male rats for defining the time course of BBB permeability within 120 h following reperfusion and evaluate the specific role of tight junction (TJ) associated proteins claudin-5, occludin, and ZO-1 as well as protein kinase C delta (PKCδ) pathway in BBB leakage induced by reperfusion injury. We verified a bimodal increase in the permeability of the BBB following focal ischemia by Evans blue assay. Two peaks of BBB permeability appeared at 3 h and 72 h of reperfusion after 2 h focal ischemia, respectively. The leak at the endothelial cell was represented at the level of transmission electron microscopy. TTC staining results showed increased infarct size with time after cerebral ischemia reperfusion. The mRNA and protein expression levels of these three TJ associated proteins were significantly decreased compared with the sham-operated group within 120 h of reperfusion, corresponding to the time-dependent change of the biphasic pattern in BBB leakage. The redistribution of claudin-5, occludin, and ZO-1 in ischemia brain microvascular endothelial cells was observed at the same time points. In addition, Western blot assay revealed PKCδ level was also significantly increased in a similar biphasic pattern to above results within 120 h after cerebral ischemia–reperfusion. This study demonstrates the timing of TJ associated proteins claudin-5, occludin, and ZO-1 in light of BBB permeability associated with cerebral ischemia reperfusion, and suggests PKCδ pathway may participate in TJ barrier open and BBB leakage during reperfusion injury in a time-dependent manner.







Similar content being viewed by others
References
Angelow S, Zeni P, Höhn B, Galla HJ (2005) Phorbol ester induced short- and long-term permeabilization of the blood–CSF barrier in vitro. Brain Res 1063:168–179
Ayata C, Ropper AH (2002) Ischaemic brain oedema. J Clin Neurosci 9:113–124
Belayev L, Busto R, Zhao W, Ginsberg MD (1996) Quantitative evaluation of blood–brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res 739:88–96
Biegel D, Spencer DD, Pachter JS (1995) Isolation and culture of human brain microvessel endothelial cells for the study of blood–brain barrier properties in vitro. Brain Res 692:183–189
Bright R, Mochly-Rosen D (2005) The role of protein kinase C in cerebral ischemic and reperfusion injury. Stroke 36:2781–2790
Bright R, Raval AP, Dembner JM, Pérez-Pinzón MA, Steinberg GK, Yenari MA, Mochly-Rosen D (2004) Protein kinase C delta mediates cerebral reperfusion injury in vivo. J Neurosci 24:6880–6888
Brodie C, Blumberg PM (2003) Regulation of cell apoptosis by protein kinase C delta. Apoptosis 8:19–27
Chou WH, Messing RO (2008) Hypertensive encephalopathy and the blood–brain barrier: is δPKC a gatekeeper? J Clin Invest 118:17–20
Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, Lowell CA, Ferriero DM, Messing RO (2004) Neutrophil protein kinase Cdelta as a mediator of stroke–reperfusion injury. J Clin Invest 114:49–56
Date I, Takagi N, Takagi K, Tanonaka K, Funakoshi H, Matsumoto K, Nakamurab T, Takeo S (2006) Hepatocyte growth factor attenuates cerebral ischemia-induced increase in permeability of the blood–brain barrier and decreases in expression of tight junctional proteins in cerebral vessels. Neurosci Lett 407:141–145
Feldman GJ, Mullin JM, Ryan MP (2005) Occludin: structure, function and regulation. Adv Drug Deliv Rev 57:883–917
Glaser N (2009) Cerebral injury and cerebral edema in children with diabetic ketoacidosis: could cerebral ischemia and reperfusion injury be involved? Pediatr Diab 10:534–541
Gloor SM, Wachtel M, Bolliger MF, Ishihara H, Landmann R, Frei K (2001) Molecular and cellular permeability control at the blood–brain barrier. Brain Res 36:258–264
Honda M, Nakagawa S, Hayashi K, Kitagawa N, Tsutsumi K, Nagata I, Niwa M (2006) Adrenomedullin improves the blood–brain barrier function through the expression of claudin-5. Cell Mol Neurobiol 26:109–118
Huang ZG, Xue D, Preston E, Karbalai H, Buchan AM (1999) Biphasic opening of the blood–brain barrier following transient focal ischemia: effects of hypothermia. Can J Neurol Sci 26:298–304
Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, Sakata H, Goeders CE, Chan PH (2010) Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol 41:172–179
Kago T, Takagi N, Date I, Takenaga Y, Takagi K, Takeo S (2006) Cerebral ischemia enhances tyrosine phosphorylation of occludin in brain capillaries. Biochem Biophys Res Commun 339:1197–1203
Kim JH, Kim JH, Jun HO, Yu YS, Kim KW (2010a) Inhibition of protein kinase Cδ attenuates blood–retinal barrier breakdown in diabetic retinopathy. Am J Pathol 176:1517–1524
Kim YA, Park SL, Kim MY, Lee SH, Baik EJ, Moon CH, Jung YS (2010b) Role of PKCβII and PKCδ in blood–brain barrier permeability during aglycemic hypoxia. Neurosci Lett 468:254–258
Kuroiwa T, Ting P, Martinez H, Klatzo I (1985) The biphasic opening of the blood–brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol 68:122–129
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Majumder PK, Mishra NC, Sun X, Bharti A, Kharbanda S, Saxena S, Kufe D (2001) Targeting of protein kinase C delta to mitochondria in the oxidative stress response. Cell Growth Differ 12:465–470
Mark KS, Davis TP (2002) Cerebral microvascular changes in permeability and tight junctions induced by hypoxia–reoxygenation. Am J Physiol Heart Circ Physiol 282:H1485–H1494
Miettinen S, Roivainen R, Keinänen R, Hökfelt T, Koistinaho J (1996) Specific induction of protein kinase C delta subspecies after transient middle cerebral artery occlusion in the rat brain: inhibition by MK-801. J Neurosci 16:6236–6245
Nishimura Y, Ito T, Saavedra JM (2000) Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke 31:2478–2486
Piontek J, Winkler L, Wolburg H, Müller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE (2008) Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J 22:146–158
Rosenberg GA, Estrada EY, Dencoff JE (1998) Matrix metalloproteinases and TIMPs are associated with blood–brain barrier opening after reperfusion in rat brain. Stroke 29:2189–2195
Schreibelt G, Kooij G, Reijerkerk A, Van Doorn R, Gringhuis SI, van der Pol SM, Weksler BB, Romero IA, Couraud PO, Piontek J, Blasig IE, Dijkstra CD, Ronken E, de Vries HE (2007) Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J 21:3666–3676
Sheth P, Basuroy S, Li CY, Naren AP, Rao RK (2003) Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J Biol Chem 278:49239–49245
Shimakura A, Kamanaka Y, Ikeda Y, Kondo K, Suzuki Y, Umemura K (2000) Neutrophil elastase inhibition reduces cerebral ischemic damage in the middle cerebral artery occlusion. Brain Res 858:55–60
Takagi K, Ginsberg MD, Globus MY, Dietrich WD, Martinez E, Kraydieh S, Busto R (1993) Changes in amino acid neurotransmitters and cerebral blood flow in the ischemic penumbral region following middle cerebral artery occlusion in the rat: correlation with histopathology. J Cereb Blood Flow Metab 13:575–585
Wolburg H, Lippoldt A (2002) Tight junctions of the blood–brain barrier: development, composition and regulation. Vascul Pharmacol 38:323–337
Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA (2007) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27:697–709
Youakim A, Ahdieh M (1999) Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol 276:G1279–G1288
Zhao H (2009) Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab 29:873–885
Acknowledgments
This work is supported by grants from the Natural Science Foundation of China (No. 30670723, 30700249, 30700861, 30800451, 30872656, and 30973079), the special fund for Scientific Research of Doctor-degree Subjects in Colleges and Universities (No. 20092104110015), and Shenyang Science and Technology Plan Projects (No. F10-205-1-22 and F10-205-1-37).
Author information
Authors and Affiliations
Corresponding author
Additional information
Haixia Jiao and Zhenhua Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jiao, H., Wang, Z., Liu, Y. et al. Specific Role of Tight Junction Proteins Claudin-5, Occludin, and ZO-1 of the Blood–Brain Barrier in a Focal Cerebral Ischemic Insult. J Mol Neurosci 44, 130–139 (2011). https://doi.org/10.1007/s12031-011-9496-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9496-4