Abstract
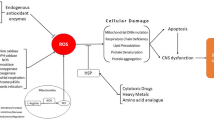
Senescence of the brain seems to be related to increased levels of free oxygen radical (FOR). FOR may damage macromolecular compounds such as: proteins, lipids, and DNA. In the aging brain, increased FOR levels damage DNA, mitochondrial DNA (mtDNA), and nuclear DNA (nDNA). In DNA they damage single and double strands, leading to mutations in mtDNA and nDNA. Damage to mtDNA seems to result in decay of mitochondria, decreased production of ATP, and in the activation of the apoptotic process. In the aging brain, apoptosis does not seem to be activated in wild-type p53-expressing cells because the elevated levels of the p53 protein are no longer accompanied by decreased levels of the Bcl-2 protein and increased levels of the Bax protein. It seems that, in the aging brain, changes in the metabolism of neurons may lead to their decreased numbers in the cerebral and cerebellar cortex, hippocampus, basal nucleus of Meynert, locus ceruleus, and substantia nigra, as well as to decreased numbers of synapses and disturbed stimulation of synaptic plasticity in the senescent brain. Simultaneously, a decrease in neurogenesis in the aging brain may lead to a decline in the maintenance of tissue integrity, function, and regenerative response. Environmental enrichment and physical activity may improve hippocampal neurogenesis and induce neuronal plasticity. The morphological lesions in the senescent brain are undoubtedly followed by a disturbed balance between various types of neurons in the CNS. Nevertheless, the high plasticity of the CNS in humans most probably does not allow for the development of abnormalities in higher functions.




Similar content being viewed by others
References
Harman D (1981) The aging process. Proc Natl Acad Sci USA 78:7124–7128
Sohal RS, Weindruch R (1996) Oxidative stress, caloric restriction, and aging. Science 273:59–63
Goldstein S, Shmookler RJ (1984) Genomic plasticity in aging human cells. Annu Rev Gerontol Geriatr 4:33–57
Culter RG (1984) Urate and ascorbate: their possible roles as antioxidants in determining longevity of mammalian species. Arch Gerontol Geriatr 3:321–348
Brunk UT, Jones CB, Sohal RS (1992) A novel hypothesis of lipofuscinogenesis and cellular aging based on interactions between oxidative stress and autophagocytosis. Mutat Res 275:395–403
Ikeda H, Tauchi H, Sato T (1985) Fine structural analysis of lipofuscin in various tissues of rats of different ages. Mech Ageing Dev 33:77–93
Hashemzadeh-Bonehi L, Phillips RG, Cairns NJ, Mosaheb S, Thorpe JR (2006) Pin1 protein associates with neuronal lipofuscin: potential consequences in age-related neurodegeneration. Exp Neurol 199:328–338
Stoub T, Barnes CA, Shah RC, Stebbins GT, Ferrari C, Detoledo-Morrell L (2012) Age-related changes in the mesial temporal lobe: the parahippocampal white matter region. Neurobiol Aging 33:1168–1176
Lister JP, Barnes CA (2009) Neurobiological changes in the hippocampus during normative aging. Arch Neurol 66:829–833
Rogers J, Zornetzer SF, Bloom FE, Mervis RE (1984) Senescent microstructural changes in rat cerebellum. Brain Res 292:23–32
Glick R, Bondareff W (1979) Loss of synapses in the cerebellar cortex of the senescent rat. J Gerontol 34:818–822
Huang CM, Brown N, Huang RH (1999) Age-related changes in the cerebellum: parallel fibers. Brain Res 840:148–152
Kabaso D, Coskren PJ, Henry BI, Hof PR, Wearne SL (2009) The electrotonic structure of pyramidal neurons contributing to prefrontal cortical circuits in macaque monkeys is significantly altered in aging. Cereb Cortex 19:2248–2268
Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR (2007) Changes in the structural complexity of the aged brain. Aging Cell 6:275–284
Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464:529–535
Muller WE, Stoll L, Schubert T, Gelbmann CM (1991) Central cholinergic functioning and aging. Acta Psychiatr Scand 366:34–39
Anglade P, Vyas S, Hirsch EC, Agid Y (1997) Apoptosis in dopaminergic neurons of the human substantia nigra during normal aging. Histol Histopathol 12:603–610
Mielke R, Kessler J, Szelies B, Herholz K, Wienhard K, Heiss WD (1998) Normal and pathological aging-findings of positron-emission-tomography. J Neural Transm 105:821–837
Akiyama H, Meyer JS, Mortel KF, Terayama Y, Thornby JI, Konno S (1997) Normal human aging: factors contributing to cerebral atrophy. J Neurol Sci 152:39–49
Rossini PM, Rossi S, Babiloni C, Polich J (2007) Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Prog Neurobiol 83:375–400
Shankar SK (2010) Biology of aging brain. Indian J Pathol Microbiol 53:595–604
Toescu EC, Verkhratsky A (2004) Ca2+ and mitochondria as substrates for deficits in synaptic plasticity in normal brain ageing. J Cell Mol Med 8:181–190
Brewer GJ, Lim A, Capps NG, Torricelli JR (2005) Age-related calcium changes, oxyradical damage, caspase activation and nuclear condensation in hippocampal neurons in response to glutamate and beta-amyloid. Exp Gerontol 40:426–437
Yamada K, Noda Y, Komori Y, Sugihara H, Hasegawa T, Nabeshima T (1996) Reduction in the number of NADPH-diaphorase-positive cells in the cerebral cortex and striatum in aged rats. Neurosci Res 24:393–402
Finch CE (2003) Neurons, glia, and plasticity in normal brain aging. Neurobiol Aging 24:123–127
Woodruff-Pak DS (2001) Eyeblink classical conditioning differentiates normal aging from Alzheimer’s disease. Integr Physiol Behav Sci 36:87–108
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300
Fridovich I (1983) Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol 23:239–257
Minotti G (1993) Sources and role of iron in lipid peroxidation. Chem Res Toxicol 6:134–146
Stadtman ER (1992) Protein oxidation and aging. Science 257:1220–1224
Goldman EH, Chen L, Fu H (2004) Activation of apoptosis signal-regulating kinase 1 by reactive oxygen species through dephosphorylation at serine 967 and 14-3-3 dissociation. J Biol Chem 279:10442–10449
Ames BN, Shigenaga MK, Hagen TM (1993) Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA 90:7915–7922
Manczak M, Jung Y, Park BS, Partovi D, Reddy PH (2005) Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrom c in aging. J Neurochem 92:494–504
Poon HF, Shepherd HM, Reed TT et al (2006) Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiol Aging 27:1020–1034
Harman D (1972) The biologic clock: the mitochondria? J Am Geriatr Soc 20:145–147
Brawek B, Loffler M, Wagner K et al (2010) Reactive oxygen species (ROS) in the human neocortex: role of aging and cognition. Brain Res Bull 81:484–490
Ventura B, Genova ML, Bovina C, Formiggini G, Lenaz G (2002) Control of oxidative phosphorylation by Complex I in rat liver mitochondria: implications for aging. Biochim Biophys Acta 1553:249–260
Gilmer LK, Ansari MA, Roberts KN, Scheff SW (2010) Age-related changes in mitochondrial respiration and oxidative damage in the cerebral cortex of the Fischer 344 rat. Mech Ageing Dev 131:133–143
Calabrese V, Giuffrida Stella AM, Calvani M, Butterfield DA (2006) Acetylcarnitine and cellular stress response: roles in nutritional redox homeostasis and regulation of longevity genes. J Nutr Biochem 17:73–88
Iverson SL, Orrenius S (2004) The cardiolipin-cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch Biochem Biophys 423:37–46
Guo X, Popadin KY, Markuzon N et al (2010) Repeats, longevity and the sources of mtDNA deletions: evidence from ‘deletional spectra’. Trends Genet 26:340–343
Wang X, Michaelis ML, Michaelis EK (2010) Functional genomics of brain aging and Alzheimer’s disease: focus on selective neuronal vulnerability. Curr Genomics 11:618–633
Ozawa T, Tanaka M, Ikebe S, Ohno K, Kondo T, Mizuno Y (1990) Quantitative determination of deleted mitochondrial DNA relative to normal DNA in parkinsonian striatum by a kinetic PCR analysis. Biochem Biophys Res Commun 172:483–489
Shigenaga MK, Hagen MT, Ames BN (1994) Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA 91:10771–10778
Sastre J, Pallardo FV, Vina J (2000) Mitochondrial oxidative stress plays a key role in aging and apoptosis. IUBMB Life 49:427–435
Lindahl T (1993) Instability and decay of the primary structure of DNA. Nature 362:709–715
Sedelnikova OA, Horikawa I, Zimonjic DB, Popescu NC, Bonner WM, Barrett JC (2004) Senescing human cells and ageing mice accumulate DNA lesions with unrepairable double-strand breaks. Nat Cell Biol 6:168–170
Zhang L, Kokkonen G, Roth GS (1995) Identification of neuronal programmed cell death in situ in the striatum of normal adult rat brain and its relationship to neuronal death during aging. Brain Res 677:177–179
Dorszewska J, Adamczewska-Goncerzewicz Z, Szczech J (2004) Apoptotic proteins in the course of aging of central nervous system in the rat. Respir Physiol Neurobiol 139:145–155
Floyd RA, Hensley K (2002) Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging 23:795–807
Mecocci P, MacGarvey U, Kaufman AE et al (1993) Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol 34:609–616
Miquel J (1992) An update on the mitochondrial-DNA mutation hypothesis of cell aging. Mutat Res 275:209–216
Ames BN, Gold LS (1991) Endogenous mutagens and the causes of aging and cancer. Mutat Res 250:3–16
Dorszewska J, Adamczewska-Goncerzewicz Z (2004) Oxidative damage to DNA, p53 gene expression and p53 protein level in the process of aging in rat brain. Respir Physiol Neurobiol 139:227–236
Kennedy C, Sakurada O, Shinohara M, Jehle J, Sokoloff L (1978) Local cerebral glucose utilization in the normal conscious macaque monkey. Ann Neurol 4:293–301
Tian F, Tong TJ, Zhang ZY, McNutt MA, Liu XW (2009) Age-dependent down-regulation of mitochondrial 8-oxoguanine DNA glycosylase in SAM-P/8 mouse brain and its effect on brain aging. Rejuvenation Res 12:209–215
Bruner SD, Norman DP, Verdine GL (2000) Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature 403:859–866
Yanagawa H, Ogawa Y, Ueno N (1992) Redox ribonucleosides. Isolation and characterization of 5-hydroxyuridine, 8-hydroxyguanosine and 8-hydroxyadenosine from Torula yeast RNA. J Biol Chem 19:13320–13326
Hollstein M, Shomer B, Greenblatt M et al (1996) Somatic point mutations in the p53 gene of human tumors and cell lines: updated compilation. Nucleic Acids Res 24:141–146
Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA (1992) 8-Hydroxyguanine, an abundant from of oxidative DNA damage, causes G–T and A–C substitutions. J Biol Chem 267:166–172
Fernandez-Silva P, Petruzzella V, Fracasso F, Gadaleta MN, Cantatore P (1991) Reduced synthesis of mtRNA in isolated mitochondria of senescent rat brain. Biochem Biophys Res Commun 176:645–653
Peacocke M, Campisi J (1991) Cellular senescence: a reflection of normal growth control, differentiation, or aging? J Cell Biochem 45:147–155
Chung YH, Shin C, Kim JM, Lee B, Park KH, Cha CI (2000) Immunocytochemical study on the distribution of p53 in the hippocampus and cerebellum of the aged rat. Brain Res 885:137–141
Marchal G, Rioux P, Petit-Taboue MC et al (1992) Regional cerebral oxygen consumption, blood flow and blood volume in healthy human aging. Arch Neurol 42:1013–1020
Kawiak J, Hoser G, Skorski T (1998) Apoptosis and some of its medical implications. Folia Histochem Cytobiol 3:99–110
Shimohama S, Tanino H, Fujimoto S (2001) Differential expression of rat brain caspase family proteins during development and aging. Biochem Biophys Res Commun 289:1063–1066
Kapasi AA, Singhal PC (1999) Aging splenocyte and thymocyte apoptosis is associated with enhanced expression of p53, bax and caspase-3. Mol Cell Biol Res Commun 1:78–81
Fraker PJ, Lill-Elghanian DA (2004) The many roles of apoptosis in immunity as modified by aging and nutritional status. J Nutr Health Aging 8:56–63
Viviani B, Boraso M (2011) Cytokines and neuronal channels: a molecular basis for age-related decline of neuronal function? Exp Gerontol 46:199–206
Wang E (1995) Senescent human fibroblasts resist programmed cell death, and failure to suppress bcl-2 is involved. Cancer Res 55:2284–2292
Aggarwal S, Gupta S (1998) Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2 and Bax. J Immunol 160:1627–1637
Migheli A, Cavalla P, Piva R, Giordana MT, Schiffer D (1994) bcl-2 protein expression in aged brain and neurodegenerative diseases. NeuroReport 5:1906–1908
Kaufmann JA, Bickford PC, Taglialatela G (2001) Oxidative-stress-dependent up-regulation of Bcl-2 expression in the central nervous system of aged Fisher-344 rats. J Neurochem 76:1099–1108
Sastry PS, Rao KS (2000) Apoptosis and the nervous system. J Neurochem 74:1–20
Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124:319–335
Gage FH (2000) Mammalian neural stem cells. Science 287:1433–1438
Kintner C (2002) Neurogenesis in embryos and adult neural stem cells. J Neurosci 22:639–643
Chen Q, Nakajima A, Choi SH, Xiong X, Sisodia SS, Tang YP (2008) Adult neurogenesis is functionally associated with AD-like neurodegeneration. Neurobiol Dis 29:316–326
Serrano F, Klann E (2004) Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev 3:431–443
Bernal GM, Peterson DA (2004) Neural stem cells as therapeutic agents for age-related brain repair. Aging Cell 3:345–351
Lazarov O, Mattson MP, Peterson DA, Pimplikar SW, van Praag H (2010) When neurogenesis encounters aging and disease. Trends Neurosci 33:569–579
Mirochnic S, Wolf S, Staufenbiel M, Kempermann G (2009) Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus 19:1008–1018
Lee J, Seroogy KB, Mattson MP (2002) Dietary restriction enhance neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem 80:539–547
Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA (2003) Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol 460:563–572
Qiu L, Zhu C, Wang X et al (2007) Less neurogenesis and inflammation in the immature than in the juvenile brain after cerebral hypoxia–ischemia. J Cereb Blood Flow Metab 27:785–794
Klempin F, Kempermann G (2007) Adult hippocampal neurogenesis and aging. Eur Arch Psychiatry Clin Neurosci 257:271–280
Yirmiya R, Goshen I (2011) Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25:181–213
Bosco D, Fava A, Plastino M, Montalcini T, Pujia A (2011) Possible implications in insulin resistance and glucose metabolism in Alzheimer’s disease pathogenesis. J Cell Mol Med 15:1807–1821
Piriz J, Muller A, Trejo JL, Torres-Aleman I (2011) IGF-I and the aging mammalian brain. Exp Gerontol 46:96–99
Trejo JL, Piriz J, Llorens-Martin MV et al (2007) Central actions of liver-derived insulin-like growth factor I underlying its pro-cognitive effects. Mol Psychiatry 12:1118–1128
Muller AP, Fernandez AM, Haas C, Zimmer E, Portela LV, Torres-Aleman I (2012) Reduced brain insulin-like growth factor I function during aging. Mol Cell Neurosci 49:9–12
Shetty AK, Hattiangady B, Shetty GA (2005) Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia 51:173–186
Erickson KI, Voss MW, Prakash RS et al (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 108:3017–3022
Foster PP, Rosenblatt KP, Kuljis RO (2011) Exercise-induced cognitive plasticity, implications for mild cognitive impairment and Alzheimer’s disease. Front Neurol 2:28
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39
Bliss TV, Lomo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331–356
McNaughton BL, Douglas RM, Goddard GV (1978) Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res 157:277–293
Hatanpaa K, Isaacs KR, Shirao T, Brady DR, Rapoport SI (1999) Loss of proteins regulating synaptic plasticity in normal aging of the human brain and in Alzheimer disease. J Neuropathol Exp Neurol 58:637–643
He Q, Dent EW, Meiri KF (1997) Modulation of actin filament behavior by GAP-43 (neuromodulin) is dependent on the phosphorylation status of serine 41, the protein kinase C site. J Neurosci 17:3515–3524
Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR (2000) Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci 20:6587–6593
Aletta JM (1996) Phosphorylation of type III beta-tubulin PC12 cell neurites during NGF-induced process outgrowth. J Neurobiol 31:461–475
Acknowledgments
The author wishes to acknowledge the help of Dr. Margarita Lianeri.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dorszewska, J. Cell biology of normal brain aging: synaptic plasticity–cell death. Aging Clin Exp Res 25, 25–34 (2013). https://doi.org/10.1007/s40520-013-0004-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-013-0004-2