Abstract
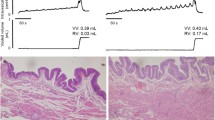
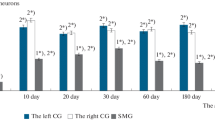
The distribution of afferent axons in the bladder of rats was studied by means of immunohistochemistry for calcitonin gene-related peptide (CGRP), in frozen sections and in wholemount preparations of mucosa and muscle coat. Synaptophysin-immunofluorescence was used for the general detection of all intramural axons. The afferent axons were distributed over four distinct targets: at the base of the epithelium, inside the epithelium, on blood vessels (both arteries and veins) and along muscle bundles. In the mucosa, all the afferent axons, except the perivascular ones, lay either inside the epithelium or in a subepithelial plexus very close to the basal surface of the epithelium. The plexus was thickest in the neck of the bladder and in the initial portion of the urethra, and it became progressively less dense in the adjacent regions; it did not extend beyond the equatorial region, and therefore the mucosa of the cranial region of the bladder had no afferent axons. Most of the axons in the subepithelial plexus were terminal axons and included conspicuous varicosities arranged in very long chains; branching points were numerous, usually at right angles and located at the level of a varicosity; some axons split and then rejoined, forming closed axonal loops. The afferent innervation of the musculature was more diffuse, and appeared uniform throughout the bladder. After unilateral surgical denervation (by excision of the pelvic ganglion 5–7 days earlier) areas of complete denervation were observed, but there were large areas where the innervation was only reduced. The results showed that there is a bilateral innervation of many regions of the mucosa and the musculature, including individual muscle bundles. A substantial number of fibres crossed the midline into the contralateral side of the bladder. CGRP-immunofluorescence in mucosal afferent axons is enhanced in the surviving axons 5 days after contralateral denervation, a change which is interpreted as an early sign of regeneration.
Similar content being viewed by others
References
Alian, M. & Gabella, G. (1996) Decrease and disappearance of intramural neurons in the rat bladder during post-natal development. Neuroscience Letters 218, 103–6.
Applebaum, A. E., Vance, W. H. & Coggeshall, R. E. (1980) Segmental localization of sensory cells that innervate the bladder. Journal of Comparative Neurology 192, 203–9.
Carpenter, F. G. & Rubin, R. M. (1967) The motor innervation of the rat urinary bladder. Journal of Physiology 192, 609–17.
Cervero, F. & Morrison, J. F. B. (Editors) (1986) Visceral Sensation. Amsterdam: Elsevier.
Changeux, J. P., Duclert, A. & Sekine, S. (1992) Calcitonin gene-related peptides and neuromuscular interactions. Annals of the New York Academy of Science 657, 361–78.
de Camilli, P., Vitadello, M., Canevini, M. P., Zanoni, R., Jahn, R. & Gorio, A. (1988) The synaptic vesicle proteins synapsin I and synaptophysin (protein p38) are concentrated both in efferent and afferent nerve endings of skeletal muscle. Journal of Neuroscience 8, 1625–31.
de Groat, W. C. (1987) Neuropeptides in pelvic afferent pathways. Experientia 43, 801–13.
de Groat, W. C., Nadelhaft, I., Milne, R. J., Booth, A. M., Morgan, C. & Thor, K. (1981) Organization of the sacral parasympathetic reflex top the urinary bladder and large intestine. Journal of the Autonomic Nervous System 3, 339–50.
Dixon, J. S. & Gilpin, C. J. (1987) Presumptive sensory axons of the human urinary bladder: a fine structural study. Journal of Anatomy 151, 199–207.
Donovan, M. K., Winternitz, S. R. & Wyss, J. M. (1983) An analysis of the sensory innervation of the urinary system in the rat. Brain Research Bulletin 11, 321–4.
EkstrÖm, J., Malmberg, L. & Öberg, S. (1986) Unilateral denervation of the rat urinary bladder and reinnervation: a predominance for ipsilateral changes. Acta Physiologica Scandinavica 127, 223–31.
Fall, M., LindstrÖm, S. & MeziÈres (1990) A bladder-to-bladder cooling reflex in the cat. Journal of Physiology 427, 281–300.
Gabella, G. (1995) The structural relations between nerve fibres and muscle cells in the urinary bladder of the rat. Journal of Neurocytology 24, 159–87.
Ghatei, M. A., Gu, J., Mulderry, P. K., Blank, M. A., Allen, J. M., Morrison, J. F. B., Polak, J. M. & Bloom, S. R. (1985) Calcitonin gene-related peptide (CGRP) in the female rat urogenital tract. Peptides 6, 809–15.
Green, T. & Dockray, G. J. (1988) Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse and guinea-pig. Neuroscience 25, 181–93.
Inoue, T. & Gabella, G. (1991) A vascular network closely linked to the epithelium of the urinary bladder of the rat. Cell and Tissue Research 263, 137–43.
Inyama, C. O., Hacker, G. W., Gu, J., Dahl, D., Bloom, S. R. & Polak, J. M. (1985) Cytochemical relationships in the paracervical ganglion (Frankenhäuser) of rat studied by immunocytochemistry. Neurocience Letters 55, 311–16.
Ishida-Yamamoto, A. & Tohyama, M. (1989) Calcitonin gene-related peptide in the nervous tissue. Progress in Neurobiology 33, 335–86.
Ishida-Yamamoto, A., Senba, E. & Tohyama, M. (1989) Distribution and fine structure of calcitonin gene-related peptide-like immunoreactive nerve fibres in rat skin. Brain Research 491, 93–101.
Iuchi, H., Satoh, Y. & Ono, K. (1994) Postnatal development of neuropeptide Y-and calcitonin gene-related peptide-immunoreactive nerves in the rat urinary bladder. Anatomy and Embryology 189, 361–73.
JÄnig, W. & Morrison, J. F. B. (1986) Functional properties of spinal visceral afferent supplying abdominal and pelvic organs, with special emphasis on visceral nociception. In Visceral Sensation (edited by F. Cervero & J. F. B. Morrison) pp. 87–114. Amsterdam: Elsevier.
Koltzenburg, M. & McMahon, S. B. (1986) Plasma extravasation in the rat urinary bladder following mechanical, electrical and chemical stimuli: evidence for a new population of chemosensitive primary sensory afferents. Neuroscience Letters 72, 352–6.
Louis, S. M., Jamieson, A., Russell, N. J. W. & Dockray, G. J. (1989) The role of substance P and calcitonin gene-related peptide in neurogenic plasma extravasation and vasodilatation in the rat. Neuroscience 32, 581–6.
Maggi, C. A. (1993) The dual, sensory and 'efferent' function of the capsaicin-sensitive primary neurons in the urinary bladder and urethra. In Nervous Control of the Urogenital System (edited by C. A. Maggi) pp. 383–422. Switzerland: Harwood Academic Publishers, Chur.
Maggi, C. A., Santicioli, P., Giuliani, S., Furio, M. & Meli, A. (1986) The capsaicin-sensitive innervation of the rat urinary bladder: further studies on mechanisms regulating micturition threshold. Journal of Urology 136, 696–700.
Mattiasson, A., Akblade, E., Sundler, F. & Uvelius, B. (1985) Origin and distribution of neuropeptide Y-, vasoactive intestinal polypeptideand substance P-containing nerve fibres in the urinary bladder of the rat. Cell and Tissue Research 239, 141–6.
Navone, F., Jahn, R., di Gioia, G., Stukenbrok, H., Greengard, P. & de Camilli, P. (1986) Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. Journal of Cell Biology 103, 2511–27.
Nimmo. A. J., Morrison, J. F. B. & Whitaker, E. M. (1988) A comparison of the distribution of substance P and calcitonin gene-related peptide receptors in the rat bladder. Quarterly Journal of Experimental Physiology 73, 789–92.
Papka, R. E. & MCNeill, D. L. (1993) Light-and electron-microscopic study of synaptic connections in the paracervical ganglion of the female rat: special reference to calcitonin gene-related peptide-, galanin-and tachykinin (substance P and neurokinin A)-immunoreactive nerve fibers and terminals. Cell and Tissue Research 271, 417–28.
Papka, R. E., Traurig, H. H. & Klenn, P. (1987) Paracervical ganglia of the female rat: histochemistry and immunohistochemistry of neurons, SIF cells, and nerve terminals. American Journal of Anatomy 179, 243–57.
Powley, T. L., Holst, M. C., Boyd, D. B. & Kelly, J. B. (1994) Three-dimensional of autonomic projections to the gastrointestinal tract. Microscopy Research Techniques 29, 297–304.
Rodrigo, J., Pedrosa, J. A., Alvarez, F. J., Bentura, M. L., Uttenthal, O., Martinezmurillo, R. & Polak, J. M. (1994) Presence of calcitonin gene-related peptide in intraepithelial nerve fibres and motor end plates of the esophagus: a light and electron microscopic study. Journal of the Autonomic Nervous System 49, 21–31.
Sala, C., Andreose, J. S. Fumagalli, G. & LØmo, T. (1995) Calcitonin gene-related peptide: possible role in formation and maintenance of neuromuscular junctions. Journal of Neuroscience 15, 520–8.
Santicioli, P., Maggi, C. A., Geppetti, P., Bianco, E. D., Theodorsson, E. & Meli, A. (1988) Release of calcitonin gene-related peptidelike immunoreactivity (CGRP-LI) from organs of the genitourinary tract of rat. Neuroscience Letters 92, 197–201.
Senba, E. & Tohyama, M. (1988) Calcitonin generelated peptide containing autonomic efferent pathways to the pelvic ganglia of the rat. Brain Research 449, 386–90.
Sharkey, K. A., Williams, R. G., Schultzberg, M. & Dockray, G. J. (1983) Sensory substance P-innervation of the urinary bladder: possible site of action of capsaicin in causing urine retention in rats. Neuroscience 10, 861–8.
Su, H. C., Wharton, J., Polak, J. M., Mulderry, P. K., Ghatei, M. A., Gibson, S. J., Terenghi, G., Morrison, J. F. B., Ballesta, J. & Bloom, S. R. (1986) Calcitonin gene-related peptide immunoreactivity in afferent neurons supplying the urinary tract: combined retrograde tracing and immunohistochemistry. Neuroscience 18, 727–47.
SzolcsÀnyi, J. (1984) Capsaicin-sensitive chemoceptive neural system with dual sensory-efferent function. In Antidromic Vasodilatation and Neurogenic Inflammation (edited by L. A. Chahl, J. SzolcsÀnyi & F. Lembeck) pp. 26–39. Budapest: Akademiai Kiado.
Tamaki, M., Iwanaga, T., Takeda, M., Adachi, I., Sato, S. & Fujita, T. (1992) Calcitonin gene-related peptide (CGRP)-immunoreactive nerve terminals in the wholemount preparations of the dog urethra. Archives of Histology and Cytology 55, 1–11.
Torrens, M. & Morrison, J. F. B. (1987) The Physiology of the Lower Urinary Tract. Berlin: Springer.
Uemura, E., Fletcher, T. F., Dirks, V. A. & Bradley, W. E. (1973) Distribution of sacral afferent axons in cat urinary bladder. American Journal of Anatomy 136, 305–14.
Uemura, E., Fletcher, T. F. & Bradley, W. E. (1974) Distribution of lumbar afferent axons in muscle coat of cat urinary bladder. American Journal of Anatomy 139, 389–98.
Uemura, E., Fletcher, T. F. & Bradley, W. E. (1975) Distribution of lumbar and sacral afferent axons in submucosa of cat urinary bladder. Anatomical Record 183, 579–88.
Vaughan, C. W. & Satchell, P. M. (1995) Urine storage mechanisms. Progress in Neurobiology 46, 215–37.
Vera, P. L. & Nadelhaft, I. (1990) Conduction velocity distribution of afferent fibers innervating the rat urinary bladder. Brain Research 520, 83–9.
Wakabayashi, Y., Tomoyoshi, T., Fujimiya, M., Arai, R. & Maeda, T. (1993) Substance P-containing axon terminals in the mucosa of the urinary bladder: pre-embedding immunohistochemistry using cryostat sections for electron microscopy. Histochemistry 100, 401–7.
Widdicombe, J. G. (1981) Nervous receptors in the respiratory tract and lungs. In Regulation of Breathing: Part II (edited by T. F. Hornbein) pp. 429–472. New York: Marcel Dekker.
Yokokawa, K., Sakanaka, M., Shiosaka, S., Toyama, M., Shiotani, Y. & Sonoda, T. (1985) Three-dimensional distribution of substance P-like immunoreactivity in the urinary bladder of rat. Journal of Neural Transmission 63, 209–22.
Yokokawa, K., Toyama, M., Shiosaka, S., Shiotani, Y., Sonoda, T., Emson, P. C., Hillyard, C. V., Girgis, S. & Macintyre, S. (1986) Distribution of calcitonin gene-related peptide containing fibers in the urinary bladder of the rat and their origin. Cell and Tissue Research 244, 271–8.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gabella, G., Davis, C. Distribution of afferent axons in the bladder of rats. J Neurocytol 27, 141–155 (1998). https://doi.org/10.1023/A:1006903507321
Issue Date:
DOI: https://doi.org/10.1023/A:1006903507321