Abstract
Purpose. The purpose of this study was to determine the usefulness of transferrin (TF)-pendant-type polyethyleneglycol (PEG)-liposomes (TF-PEG-liposomes), in which TF was covalently linked to the distal terminal of PEG chains on the external surface of PEG-liposomes as a carrier for in vivo cytoplasmic targeting to tumor cells.
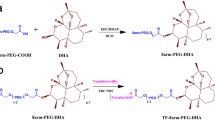
Methods. Small unilamellar TF-PEG-liposomes (100-140 nm in diameter) were prepared from DSPC, CH, DSPE-PEG, and DSPE-PEG-COOH (2:1:0.11:0.021, molar ratio), and were conjugated to TF via the carboxyl residue of DSPE-PEG-COOH. The intracellular targeting ability of TF-PEG-liposomes to tumor cells was examined in vitro and in Colon 26 tumor-bearing mice.
Results. TF-PEG-liposomes, bearing approximately 25 TF molecules per liposome, readily bound to mouse Colon 26 cells in vitro and were internalized by receptor-mediated endocytosis. TF-PEG-liposomes showed a prolonged residence time in the circulation and low RES uptake in Colon 26 tumor-bearing mice, resulting in enhanced extravasation of the liposomes into the solid tumor tissue. Electron microscopic studies in Colon 26 tumor-bearing mice revealed that the extravasated TF-PEG-liposomes were internalized into tumor cells by receptor-mediated endocytosis.
Conclusion. TF-PEG-liposomes had the capabilities of specific receptor binding and receptor-mediated endocytosis to target cells after extravasation into solid tumors in vivo. Such liposomes should be useful for in vivo cytoplasmic targeting of chemotherapeutic agents or plasmid DNAs to target cells.
Similar content being viewed by others
REFERENCES
T. M. Allen. Liposomal drug formulations. Drugs 56: 747-756 (1998).
K. Maruyama, O. Ishida, T. Takizawa, and K. Moribe. Possibility of active targeting to tumor tissues with liposomes. Adv. Drug Deliv. Rev. 40:89-102 (1999).
D. Aragnol and L. D. Leserman. Immune clearance of liposomes inhibited by an anti-Fc receptor antibody in vivo. Proc. Natl. Acad. Sci. USA 83:2699-2703 (1986).
J. T. P. Derksen, H. W. M. Marselt, and G. L. Scherphof. Uptake and processing of immunoglobulin-coated liposomes by subpopulation of rat liver macrophages. Biochim. Biophys. Acta 971:127-136 (1988).
K. Maruyama, E. Holmberg, S. J. Kennel, A. Klibanov, V. P. Torchilin, and L. Huang. Characterization of in vivo immunoliposome targeting to pulmonary endothelium. J. Pharm. Sci. 79:978-984 (1990).
K. Maruyama, T. Takizawa, T. Yuda, S. J. Kennel, L. Huang, and M. Iwatsuru. Targetability of novel immunoliposomes modified with amphipathic polyethyleneglycols conjugated at their distal terminals to monoclonal antibodies. Biochim. Biophys. Acta 1234:74-80 (1995).
K. Maruyama, N. Takahashi, T. Tagawa, K. Nagaike, and M. Iwatsuru. Immunoliposomes bearing polyethyleneglycol-coupled Fab' fragment show prolonged circulation time and high extravasation into target solid tumors in vivo. FEBS Lett. 413:177-180 (1997).
E. Wagner, D. Curiel, and M. Cotton. Delivery of drugs, proteins and genes into cells using transferrin as a ligand for receptor-mediated endocytosis. Adv. Drug Deliv. Rev. 14:113-135 (1994).
H. A. Huebers and C. A. Finch. The physiology of transferrin and transferrin receptors. Physiol. Rev. 67:520-582 (1987).
P. Aisen. The transferrin receptor and the release of iron from transferrin. Adv. Exp. Med. Biol. 365:31-40 (1994).
F. Szoka and D. Papahadjopoulos. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. USA 75:4194-4198 (1978).
A. Dautry-Varsat, A. Ciechanover, and H. F. Lodish. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc. Natl. Acad. Sci. USA 80:2258-2262 (1983).
S. K. Huang, K. Hong, K. D. Lee, D. Papahadjopoulos, and D. S. Friend. Light microscopic localization of silver-enhanced liposome-entrapped colloidal gold in mouse tissue. Biochim. Biophys. Acta 1069:117-121 (1991).
C. H. Fiske and Y. Subbarow. The colorimetric determination of phosphorus. J. Biol. Chem. 66:375-400 (1925).
H. G. Enoch and P. Strittmatter. Formation and properties of 1000-Å-diameter, single-bilayer phospholipid vesicles. Proc. Natl. Acad. Sci. USA 76:145-149 (1979).
R. D. Klausner, G. Ashwell, J. Renswoude, J. B. Harford, and K. R. Bridges. Binding of apotransferrin to K562 cells: Explanation of the transferrin cycle. Proc. Natl. Acad. Sci. USA 80:2263-2266 (1983).
O. Ishida, K. Maruyama, K. Sasaki, and M. Iwatsuru. Size-dependent extravasation ant interstitial localization of polyethyleneglycol liposomes in solid tumor-bearing mice. Int. J. Pharm. 190:49-56 (1999).
K. Sasaki. Dynamic change of the vascular wall from transmural to intracellular passage of red blood cells observed in splenic regeneration. Acta Anat. 139:315-319 (1990).
A. Klibanov, K. Maruyama, A. M. Beckerleg, V. P. Torchilin, and L. Huang. Activity of amphipatihic poly(ethylene glycol) 5000 to prolong the circulation time of liposomes depends on the liposome size and is unfavorable for immunoliposome binding to target. Biochim. Biophys. Acta 1062:142-148 (1991).
A. Klibanov and L. Huang. Long-circulating liposomes: Development and perspectives. Liposome Res. 2:321-334 (1992).
T. Yuda, Y. Pongpaibul, K. Maruyama, and M. Iwatsuru. Activity of amphipathic polyethyleneglycols to prolong the circulation time of liposomes. J. Pharm. Sci. Technol. Jpn. 59:32-42 (1999).
R. K. Jain and L. E. Gerlowski. Extravascular transport in normal and tumor tissue. Crit. Rev. Oncol. Hematol. 5:115-170 (1986).
Y. Matsumura and H. Maeda. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46:6387-6392 (1986).
S. Unezaki, K. Maruyama, J. Hosoda, I. Nagae, Y. Koyanagi, M. Nakata, O. Ishida, M. Iwatsuru, and S. Tsuchiya. Direct measurement of extravasation of polyethyleneglycol-coated liposomes into solid tumor tissue by in vivo fluorescence microscopy. Int. J. Pharm. 144:11-17 (1996).
D. Liu, A. Mori, and L. Huang. Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim. Biophys. Acta 1104:95-101 (1992).
P. K. Bali, O. Zak, and P. Aisen. A new role for the transferrin receptor in the release of iron from transferrin. Biochemistry 30:324-328 (1991).
D. C. Litzinger and L. Huang. Phosphatidylethanolamine liposomes: Drug delivery, gene transfer and immunodiagnostic applications. Biochim. Biophys. Acta 1113:201-227 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ishida, O., Maruyama, K., Tanahashi, H. et al. Liposomes Bearing Polyethyleneglycol-Coupled Transferrin with Intracellular Targeting Property to the Solid Tumors In Vivo. Pharm Res 18, 1042–1048 (2001). https://doi.org/10.1023/A:1010960900254
Issue Date:
DOI: https://doi.org/10.1023/A:1010960900254