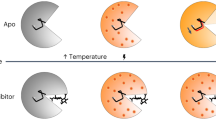
Abstract
WHEN the dynamic properties of many different proteins are plotted as a function of temperature, biphasic behaviour is observed, with a broad transition centred around 220 K. Atomic mean-square displacements from X-ray crystallography1 and Mbssbauer scattering2–3 show this behaviour, as do electron transfer rates4 and dynamic information from inelastic neutron scattering5. Molecular dynamics simulations over a range of temperatures also exhibit a transition at about 220 K: high-temperature atomic fluctuations are dominated by anharmonic collective motions of bonded and nonbonded groups of atoms, but below 220 K the predominant dynamic behaviour is harmonic vibration of individual atoms6. Here we show by high-resolution X-ray diffraction that crystalline ribonuclease A does not bind substrate or inhibitor at 212 K but will bind either rapidly at 228 K. Once bound at the higher temperature, inhibitor cannot be washed off after the enzyme is cooled to below the transition temperature. These results suggest that enzyme flexibility is required for catalytic function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Tilton, R. F. Jr., Dewan, J. C. & Petsko, G. A. Biochemistry 31, 2469–2481 (1992).
Parak, F. et al. FEBS Lett. 117, 368–372 (1980).
Keller, H. & Debrunner, P. Phys. Rev. Lett. 45, 68–71 (1980).
Nocek, J. M. et al. J. Am. chem. Soc. 113, 6822–6831 (1991).
Doster, W., Cusack, S. & Petry, W. Nature 337, 754–756 (1989).
Loncharich, R. J. & Brooks, B. R. J. molec. Biol. 215, 439–455 (1990).
Richards, F. M. & Wyckoff, H. W. The Enzymes 3rd edn Vol. IV (ed. Boyer, P. D.) 647–806 (1971).
Howlin, B., Harris, G. W., Moss, D. S. & Palmer, R. A. J. molec. Biol. 196, 159–164 (1987).
Petsko, G. A. Methods Enzym. 114, 141–146 (1985).
Douzou, P. Cryobiochemistry (Academic, London, 1977).
Fink, A. L., Kar, D. & Kotin, R. Biochemistry, 26, 8571–8579 (1987).
Rasmussen, B. F. & Petsko, G. A. J. appl. Crystallogr. (submitted).
Gilbert, W. A., Lord, R. C., Petsko, G. A. & Thamann, T. J. J. Raman Spectrosc. 12, 173–179 (1982).
Carlisle, C. H., Palmer, R. A., Mazumdar, S. K., Gorinsky, B. A. & Yeates, D. G. R. J. molec. Biol. 85, 1–18 (1974).
Doster, W., Bachleitner, A., Dunau, R., Hiebl, M. & Lüscher, E. Biophys. J. 50, 213–219 (1986).
Steinbach, P. J. et al. Biochemistry 30, 3988–4001 (1991).
Kartha, G., Ambady, G. & Viswamitra, M. A. Science 179, 495–496 (1973).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rasmussen, B., Stock, A., Ringe, D. et al. Crystalline ribonuclease A loses function below the dynamical transition at 220 K. Nature 357, 423–424 (1992). https://doi.org/10.1038/357423a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/357423a0
This article is cited by
-
A Minireview on Temperature Dependent Protein Conformational Sampling
The Protein Journal (2021)
-
Compared DNA and RNA quality of breast cancer biobanking samples after long-term storage protocols in − 80 °C and liquid nitrogen
Scientific Reports (2020)
-
A Quantitative Comparison of the Counting Significance of van Hove Integral Spectroscopy and Quasielastic Neutron Scattering
Scientific Reports (2020)
-
Evaluation of Predictors of Protein Relative Stability Obtained by Solid-State Hydrogen/Deuterium Exchange Monitored by FTIR
Pharmaceutical Research (2020)
-
Combining Neutron Scattering, Deuteration Technique, and Molecular Dynamics Simulations to Study Dynamics of Protein and Its Surface Water Molecules
Chinese Journal of Polymer Science (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.