Abstract
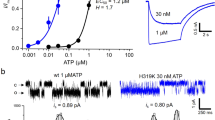
5-Hydroxytryptamine type 3 (5-HT3) receptors are cation-selective transmitter-gated ion channels of the Cys-loop superfamily1,2,3,4,5,6,7,8,9. The single-channel conductance of human recombinant 5-HT3 receptors assembled as homomers of 5-HT3A subunits, or heteromers of 5-HT3A and 5-HT3B subunits, are markedly different, being 0.4 pS (refs 6, 9) and 16 pS (ref. 7), respectively. Paradoxically, the channel-lining M2 domain of the 5-HT3A subunit would be predicted to promote cation conduction, whereas that of the 5-HT3B subunit would not7. Here we describe a determinant of single-channel conductance that can explain these observations. By constructing chimaeric 5-HT3A and 5-HT3B subunits we identified a region (the ‘HA-stretch’)10 within the large cytoplasmic loop of the receptor that markedly influences channel conductance. Replacement of three arginine residues unique to the HA-stretch of the 5-HT3A subunit by their 5-HT3B subunit counterparts increased single-channel conductance 28-fold. Significantly, ultrastructural studies of the Torpedo nicotinic acetylcholine receptor11 indicate that the key residues might frame narrow openings that contribute to the permeation pathway. Our findings solve the conundrum of the anomalously low conductance of homomeric 5-HT3A receptors and indicate an important function for the HA-stretch in Cys-loop transmitter-gated ion channels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Derkach, V., Surprenant, A. & North, R. A. 5-HT3 receptors are membrane ion channels. Nature 339, 706–709 (1989)
Yakel, J. L. & Jackson, M. B. 5-HT3 receptors mediate rapid responses in cultured hippocampus and a clonal cell line. Neuron 1, 615–621 (1988)
Yakel, J. L., Shao, X. M. & Jackson, M. B. The selectivity of the channel coupled to the 5-HT3 receptor. Brain Res. 533, 46–52 (1990)
Yang, J. Ion permeation through 5-hydroxytryptamine-gated channels in neuroblastoma N18 cells. J. Gen. Physiol. 96, 1177–1198 (1990)
Maricq, A. V., Peterson, A. S., Brake, A. J., Myers, R. M. & Julius, D. Primary structure and functional expression of the 5-HT3 receptor, a serotonin-gated ion channel. Science 254, 432–437 (1991)
Brown, A. M., Hope, A. G., Lambert, J. J. & Peters, J. A. Ion permeation and conduction in a human recombinant 5-HT3 receptor subunit (h5-HT3A). J. Physiol. (Lond.) 507, 653–665 (1998)
Davies, P. A. et al. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 397, 359–363 (1999)
Dubin, A. E. et al. The pharmacological and functional characteristics of the serotonin 5-HT3A receptor are specifically modified by a 5-HT3B receptor subunit. J. Biol. Chem. 274, 30799–30810 (1999)
Hussy, N., Lukas, W. & Jones, K. A. Functional properties of a cloned 5-hydroxytryptamine ionotropic receptor subunit: Comparison with native mouse receptors. J. Physiol. (Lond.) 481, 311–323 (1994)
Finer-Moore, J. & Stroud, R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc. Natl Acad. Sci. USA 81, 155–159 (1984)
Miyazawa, A., Fujiyoshi, Y., Stowell, M. & Unwin, N. Nicotinic acetylcholine receptor at 4.6 Å resolution: Transverse tunnels in the channel wall. J. Mol. Biol. 288, 765–786 (1999)
Boyd, G. W. et al. Assembly and cell surface expression of homomeric and heteromeric 5-HT3 receptors: The role of oligomerization and chaperone proteins. Mol. Cell. Neurosci. 21, 38–50 (2002)
Gill, C. H., Peters, J. A. & Lambert, J. J. An electrophysiological investigation of the properties of a murine recombinant 5-HT3 receptor stably expressed in HEK 293 cells. Br. J. Pharmacol. 114, 1211–1221 (1995)
Hanna, M. C., Davies, P. A., Hales, T. G. & Kirkness, E. F. Evidence for expression of heteromeric serotonin 5-HT3 receptors in rodents. J. Neurochem. 75, 240–247 (2002)
Bouzat, C., Bren, N. & Sine, S. M. Structural basis of the different gating kinetics of fetal and adult acetylcholine receptors. Neuron 13, 1395–1402 (1994)
Akk, G. & Steinbach, J. H. Structural elements near the C-terminus are responsible for changes in nicotinic receptor gating kinetics following patch excision. J. Physiol. (Lond.) 527, 405–417 (2000)
Yu, X. M. & Hall, Z. W. A sequence in the main cytoplasmic loop of the alpha subunit is required for assembly of mouse muscle nicotinic acetylcholine receptor. Neuron 13, 247–255 (1994)
Imoto, K. et al. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature 335, 645–648 (1988)
Reeves, D. C., Goren, E. N., Akabas, M. H. & Lummis, S. C. R. Structural and electrostatic properties of the 5-HT3 receptor pore revealed by substituted cysteine accessibility mutagenesis. J. Biol. Chem. 276, 42035–42042 (2001)
Panicker, S., Cruz, H., Arrabit, C. & Slesinger, P. A. Evidence for a centrally located gate in the pore of a serotonin-gated ion channel. J. Neurosci. 22, 1629–1639 (2002)
Gunthorpe, M. J. & Lummis, S. C. Conversion of the ion selectivity of the 5-HT3A receptor from cationic to anionic reveals a conserved feature of the ligand-gated ion channel superfamily. J. Biol. Chem. 276, 10977–10983 (2001)
Unwin, N. The Croonian Lecture 2000. Nicotinic acetylcholine receptor and the structural basis of fast synaptic transmission. Phil. Trans. R. Soc. Lond. B 355, 1813–1829 (2000)
Sokolova, O., Kolmakova-Partensky, L. & Grigorieff, N. Three-dimensional structure of a voltage-gated potassium channel at 2.5 nm resolution. Structure 9, 215–220 (2001)
Kobertz, W. R., Williams, C. & Miller, C. Hanging gondola structure of the T1 domain in a voltage-gated K+ channel. Biochemistry 39, 10347–10352 (2000)
Bass, R. B., Strop, P., Barclay, M. & Rees, D. C. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science 298, 1582–1587 (2002)
Sato, C. et al. The voltage-sensitive sodium channel is a bell-shaped molecule with several cavities. Nature 409, 1047–1051 (2001)
Higgins, M. K., Weitz, D., Warne, T., Schertler, G. F. & Kaupp, U. B. Molecular architecture of a retinal cGMP-gated channel: The arrangement of the cytoplasmic domains. EMBO J. 21, 2087–2094 (2002)
Sambrook, J., Fritsch, E. F. & Maniatis, T. Molecular Cloning—A Laboratory Manual, 2nd edn (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1989)
Davies, P. A., Wang, W., Hales, T. G. & Kirkness, E. F. A novel class of ligand-gated ion channel is activated by Zn2+. J. Biol. Chem. 278, 712–717 (2002)
Acknowledgements
We thank M.L.J. Ashford for constructive comments on an earlier version of this manuscript. This work was supported by grants from the Wellcome Trust and Tenovus Tayside to J.A.P. and J.J.L. and from the Anonymous Trust to J.A.P.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests
Rights and permissions
About this article
Cite this article
Kelley, S., Dunlop, J., Kirkness, E. et al. A cytoplasmic region determines single-channel conductance in 5-HT3 receptors. Nature 424, 321–324 (2003). https://doi.org/10.1038/nature01788
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01788
This article is cited by
-
Derepression may masquerade as activation in ligand-gated ion channels
Nature Communications (2023)
-
Modulation of glycine receptor single-channel conductance by intracellular phosphorylation
Scientific Reports (2020)
-
Evidence for an effect of receptor density on ligand occupancy and agonist EC50
Scientific Reports (2019)
-
Cryo-EM reveals two distinct serotonin-bound conformations of full-length 5-HT3A receptor
Nature (2018)
-
Structural principles of distinct assemblies of the human α4β2 nicotinic receptor
Nature (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.