Abstract
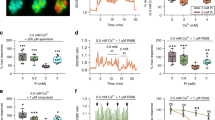
Blood pH is maintained in a narrow range around pH 7.4 mainly through regulation of respiration and renal acid extrusion1,2. The molecular mechanisms involved in pH homeostasis are not completely understood. Here we show that ovarian cancer G-protein-coupled receptor 1 (OGR1), previously described as a receptor for sphingosylphosphorylcholine3, acts as a proton-sensing receptor stimulating inositol phosphate formation. The receptor is inactive at pH 7.8, and fully activated at pH 6.8—site-directed mutagenesis shows that histidines at the extracellular surface are involved in pH sensing. We find that GPR4, a close relative of OGR1, also responds to pH changes, but elicits cyclic AMP formation. It is known that the skeleton participates in pH homeostasis as a buffering organ, and that osteoblasts respond to pH changes in the physiological range4, but the pH-sensing mechanism operating in these cells was hitherto not known. We detect expression of OGR1 in osteosarcoma cells and primary human osteoblast precursors, and show that these cells exhibit strong pH-dependent inositol phosphate formation. Immunohistochemistry on rat tissue sections confirms the presence of OGR1 in osteoblasts and osteocytes. We propose that OGR1 and GPR4 are proton-sensing receptors involved in pH homeostasis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Feldman, J. L., Mitchell, G. S. & Nattie, E. E. Breathing: rhythmicity, plasticity, chemosensitivity. Annu. Rev. Neurosci. 26, 239–266 (2003)
Swenson, E. R. Metabolic acidosis. Respir. Care 46, 342–353 (2001)
Xu, Y. et al. Sphingosylphosphorylcholine is a ligand for ovarian cancer G-protein-coupled receptor 1. Nature Cell Biol. 2, 261–267 (2000)
Krieger, N. S., Parker, W. R., Alexander, K. M. & Bushinsky, D. A. Prostaglandins regulate acid-induced cell-mediated bone resorption. Am. J. Physiol. Renal Physiol. 279, F1077–F1082 (2000)
Berridge, M. J., Downes, C. P. & Hanley, M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem. J. 206, 587–595 (1982)
Berridge, M. J. & Irvine, R. F. Inositol phosphates and cell signalling. Nature 341, 197–205 (1989)
Paris, S. & Pouyssegur, J. Pertussis toxin inhibits thrombin-induced activation of phosphoinositide hydrolysis and Na+/H+ exchange in hamster fibroblasts. EMBO J. 5, 55–60 (1986)
Simon, M. I., Strathmann, M. P. & Gautam, N. Diversity of G proteins in signal transduction. Science 252, 802–808 (1991)
Smith, G. D. et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418, 186–190 (2002)
Elling, C. E., Nielsen, S. M. & Schwartz, T. W. Conversion of antagonist-binding site to metal-ion site in the tachykinin NK-1 receptor. Nature 374, 74–77 (1995)
Sheikh, S. P., Zvyaga, T. A., Lichtarge, O., Sakmar, T. P. & Bourne, H. R. Rhodopsin activation blocked by metal-ion-binding sites linking transmembrane helices C and F. Nature 383, 347–350 (1996)
Zhu, K. et al. Sphingosylphosphorylcholine and lysophosphatidylcholine are ligands for the G protein-coupled receptor GPR4. J. Biol. Chem. 276, 41325–41335 (2001)
Brechter, A. B. & Lerner, U. H. Characterization of bradykinin receptors in a human osteoblastic cell line. Regul. Pept. 103, 39–51 (2002)
Bushinsky, D. A. Acid-base imbalance and the skeleton. Eur. J. Nutr. 40, 238–244 (2001)
Goldhaber, P. & Rabadjija, L. H+ stimulation of cell-mediated bone resorption in tissue culture. Am. J. Physiol. 253, E90–E98 (1987)
Meghji, S., Morrison, M. S., Henderson, B. & Arnett, T. R. pH dependence of bone resorption: Mouse calvarial osteoclasts are activated by acidosis. Am. J. Physiol. Endocrinol. Metab. 280, E112–E119 (2001)
Gijon, M. A. & Leslie, C. C. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J. Leucoc. Biol. 65, 330–336 (1999)
Waldmann, R. et al. H+-gated cation channels. Ann. NY Acad. Sci. 868, 67–76 (1999)
Caterina, M. J. et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 (2000)
An, S., Tsai, C. & Goetzl, E. J. Cloning, sequencing and tissue distribution of two related G protein-coupled receptor candidates expressed prominently in human lung tissue. FEBS Lett. 375, 121–124 (1995)
Ishizaka, H., Gudi, S. R., Frangos, J. A. & Kuo, L. Coronary arteriolar dilation to acidosis: role of ATP-sensitive potassium channels and pertussis toxin-sensitive G proteins. Circulation 99, 558–563 (1999)
Reeh, P. W. & Steen, K. H. Tissue acidosis in nociception and pain. Prog. Brain Res. 113, 143–151 (1996)
Heming, T. A. et al. Effects of extracellular pH on tumour necrosis factor-α production by resident alveolar macrophages. Clin. Sci. (Lond.) 101, 267–274 (2001)
Sottile, V., Halleux, C., Bassilana, F., Keller, H. & Seuwen, K. Stem cell characteristics of human trabecular bone-derived cells. Bone 30, 699–704 (2002)
Seuwen, K., Lagarde, A. & Pouyssegur, J. Deregulation of hamster fibroblast proliferation by mutated ras oncogenes is not mediated by constitutive activation of phosphoinositide-specific phospholipase C. EMBO J. 7, 161–168 (1988)
Salomon, Y. Adenylate cyclase assay. Adv. Cycl. Nucleot. Res. 10, 35–55 (1979)
Palczewski, K. et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 289, 739–745 (2000)
Bower, M. J., Cohen, F. E. & Dunbrack, R. L. Prediction of protein side-chain rotamers from a backbone-dependent rotamer library: A new homology modeling tool. J. Mol. Biol. 267, 1268–1282 (1997)
Cornell, W. D. et al. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J. Am. Chem. Soc. 117, 5179–5197 (1995)
Acknowledgements
We thank B. Wilmering-Wetter and H. Anklin for assistance in cell culture, IP formation and intracellular calcium assays, R. Brearley and M. Schäublin for support in molecular biology, and A Rebmann for IHC analysis. We also thank R. Bouhelal and I. Vranesic for advice concerning the fluorescence imaging plate reader, and J. Mosbacher for help with confocal microscopy.Authors' contributions The experiments shown in Figs 1, 3, 4, 5 were carried out by M.G.L., M.V., D.G. and K.S. J.A.G. performed the IHC on tissue sections. C.E.J. cloned GPR4 and performed expression profiling experiments. U.J. and H.H. participated in the cloning of OGR1, expression profiling, and experiments with lipid agonists. R.W. carried out receptor modelling.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ludwig, MG., Vanek, M., Guerini, D. et al. Proton-sensing G-protein-coupled receptors. Nature 425, 93–98 (2003). https://doi.org/10.1038/nature01905
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01905
This article is cited by
-
GPR68-ATF4 signaling is a novel prosurvival pathway in glioblastoma activated by acidic extracellular microenvironment
Experimental Hematology & Oncology (2024)
-
Identifying hub genes of sepsis-associated and hepatic encephalopathies based on bioinformatic analysis—focus on the two common encephalopathies of septic cirrhotic patients in ICU
BMC Medical Genomics (2024)
-
Bicarbonate signalling via G protein-coupled receptor regulates ischaemia-reperfusion injury
Nature Communications (2024)
-
pH-regulated single cell migration
Pflügers Archiv - European Journal of Physiology (2024)
-
Strengthening the basics: acids and bases influence vascular structure and function, tissue perfusion, blood pressure, and human cardiovascular disease
Pflügers Archiv - European Journal of Physiology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.