Abstract
The mammalian immune system has an extraordinary potential for making receptors that sense and neutralize any chemical entity entering the body. Inevitably, some of these receptors recognize components of our own body, and so cellular mechanisms have evolved to control the activity of these ‘forbidden’ receptors and achieve immunological self tolerance. Many of the genes and proteins involved are conserved between humans and other mammals. This provides the bridge between clinical studies and mechanisms defined in experimental animals to understand how sets of gene products coordinate self-tolerance mechanisms and how defects in these controls lead to autoimmune disease.
Similar content being viewed by others
Main
Our immune system is the body's sixth sense. It can react to any chemical structure imaginable to fight off every possible microorganism. The receptors coordinating this feat are antibodies expressed on the surface of B cells as B-cell receptors (BCRs) and T-cell receptors (TCRs) displayed on T cells. Huge receptor diversity is encoded in the mammalian genome by two processes of somatic genome modification that occur selectively in lymphocytes. First, V(D)J recombination assembles unique BCR and TCR genes from three separate gene segments, the variable (V), diversity (D) and joining (J) genes, during B- and T-cell differentiation. This takes place in the ‘central lymphoid tissues’, which are principally the bone marrow for B cells and the thymus for T cells. Second, somatic hypermutation substitutes single nucleotides of BCR genes during a late phase of the immune response in peripheral lymphoid tissues (such as the spleen, lymph nodes and tonsils). A significant fraction of the receptors generated by both these processes bind to one or more self components in the body — a by-product of a deliberately random receptor-generating process. Between 20 and 50% of TCRs and BCRs generated by V(D)J recombination bind with a potentially dangerous affinity to a self antigen1,2,3,4. Since only 3–8% of the population develops an autoimmune disease5, it is remarkable that this enormous burden of self-reactive receptors is so well regulated in most of us.
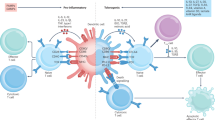
Each lymphocyte usually produces only a single receptor out of the billions possible. Experiments have established that if this receptor is self reactive, then four cellular strategies are employed to deal with them (Fig. 1). First, the cell displaying the ‘forbidden’, or self-reactive, receptor can be triggered to die, as originally envisaged in Burnet's concept of clonal deletion. Second, a cell bearing a forbidden receptor can ‘edit’ the offending receptor by further V(D)J recombination or somatic hypermutation to display a different receptor that is not self reactive6. Third, intrinsic biochemical and gene-expression changes can reduce the ability of the cell to be triggered by self-reactive receptors. This is generally termed clonal anergy or tuning7,8,9. Finally, even if the cells have evaded the three mechanisms above, collectively called ‘immunological ignorance’, extrinsic controls can limit the danger of self-reactive receptors. These extrinsic controls limit the supply of essential growth factors, costimuli, pro-inflammatory mediators and other factors, and also include active suppression by regulatory T (TR) cells, through a mechanism that is poorly understood. The latter topic is reviewed separately in this issue by Kronenberg and Rudensky (page 598).
a, The cell is deleted through induction of cell death. b, The receptor is edited to one that is less self-reactive. c, Biochemical or gene-expression changes intrinsically dampen the self-reactive receptor's ability to activate the cell. d, The ability of self-reactive cells or antibody to cause autoimmunity is limited by using extrinsic suppression and by limiting essential growth factors, costimuli and inflammatory mediators.
These four mechanisms act as checkpoints on the pathway leading to the production of secreted antibodies and effector T cells. The cellular map in Fig. 2 derives primarily from experiments tracing the fate of forbidden receptors and the cells bearing them in mice that had exceptionally high frequencies of receptor for particular antigens. In humans, cells with self-reactive receptors are too heterogeneous and infrequent to visualize these cellular mechanisms at work. The gulf between experimental animals and the clinic is now being bridged by the discovery of individual genes and proteins that are essential both for specific cellular tolerance mechanisms in mice and for preventing autoimmunity in humans. Here, we review the state of the field, following the map shown in Fig. 2. We focus on how our understanding of cellular and gene maps of self tolerance and immunity will guide rational solutions to autoimmune disease.
The range of different BCRs or TCRs, with varying degrees of avidity for self antigens, are denoted by different colours as marked in the key. Specific cellular mechanisms are shown by red numbers. BCR tolerance mechanisms in central lymphoid organs: (1) Arrest of immature B-cell maturation; (2) BCR light chain editing by V(D)J recombination; (3) Death and deletion of immature B cells. TCR tolerance mechanisms in central lymphoid organs: (4) TCR α-chain editing by V(D)J recombination; (5) Death and deletion of semi-mature T cells. Intrinsic regulation of self-reactive receptors by anergy and biochemical tuning: (6) BCR tuning/anergy; (7) TCR tuning/anergy. Extrinsic regulation of self-reactive receptors by competitive mechanisms: (8) Follicular exclusion of B cells; (9) B-cell competition for BAFF; (10) T-cell competition for IL-7. Extrinsic regulation of self-reactive receptors by limiting immunogenic co-stimuli: (11) Controls on availability of extrafollicular T-cell help; (12) Control of TLR ligands and signalling; (13) B-cell death induced by FASL from T cells; (14) BCR inhibition of plasma-cell differentiation; (15) Control of B7 ligands and other costimulatory molecules; (16) T-cell death induced by FASL; (17) T-cell suppression by TR cells. Regulation of self-reactive receptors in follicles: (18) Control of ICOS and follicular T helper cell differentiation; (19) BCR-induced death of germinal-centre B cells; (20) Germinal-centre B-cell death from competition for follicular T helper cells. Tolerance of self-reactive receptors at the final effector phase: (21) Control of autoantibody accumulation and inflammation in tissues.
BCR tolerance mechanisms in central lymphoid organs
Analysis of transgenic mice with a restricted repertoire of BCRs revealed a series of cellular events that are triggered immediately after an immature B cell displays a self-reactive receptor in the bone marrow. If the strength of receptor crosslinking and intracellular signalling exceeds a certain threshold, the immature B cell rapidly internalizes the offending BCR and temporarily halts its maturation programme6,10,11. This has three consequences. First, homing receptors, such as CD62 ligand (CD62L), are not expressed. Such receptors are needed for B cells to enter the lymph nodes10. Second, receptors for B-cell-activating factor (BAFF), a circulating cytokine required to sustain peripheral B-cell survival, are poorly induced12. Third, RAG1 (recombination-activating gene 1) and RAG2, which encode the core enzymes for V(D)J recombination, continue to be expressed. This allows the BCRs to be edited by rearranging a replacement BCR light chain6,13.
If a B cell with a forbidden receptor fails to edit to a less self-reactive receptor, cell death occurs within 1–2 days, either in the bone marrow or shortly after arriving in the spleen10. The process of clonal deletion may result partly from growth-factor withdrawal, owing to low expression of BAFF receptors on immature B cells. Deletion also involves BCR-induced cell death through increasing the levels of BIM (BCL-2-interacting mediator of cell death). This pro-apoptotic factor inhibits essential B-cell survival proteins from the BCL-2 family14 (Fig. 3c). Interestingly, BIM-deficient mice spontaneously produce anti-DNA autoantibodies after a latent period of many months14.
Three examples are shown of what is likely to be a continuum. a, A BCR with no self-reactivity, where BCR and BAFFR survival pathways dominate. b, A BCR with intermediate self-reactivity, where BCR signalling activates survival and death pathways, so that death dominates unless increased BAFF is supplied. c, A BCR with avid self-reactivity, where BCR internalization and maturation arrest cripples the BCR and BAFFR survival pathways, while BIM induction promotes death.
It is not known whether defects in BCR editing or deletion contribute to human autoimmunity. Antibodies that recognize human nuclear antigens and DNA — such as those found in patients with systemic lupus erythematosus (SLE) — are more frequently borne by the subset of immature B cells with little or no surface BCR in human bone marrow4. This suggests that the BCRs have been internalized and provides evidence for conservation in humans of the processes described above. As opposed to B cells producing antibodies to systemic antigens, which can be regulated in this way, B cells bearing autoantibodies to organ-specific antigens15 such as those causing Graves' disease are not encountered in the bone marrow. The failure to edit or delete this important class of self-reactive BCRs may put extra pressure on other mechanisms described below.
TCR tolerance mechanisms in central lymphoid organs
Receptor editing and cellular deletion also accompany V(D)J recombination in developing T cells in the thymus6, although deletion appears to be the predominant process. Unlike BCRs, which are designed to recognize native antigen, TCRs are selected to recognize a composite ligand comprising peptide fragments of antigen bound to MHC molecules. Composites of self peptides and MHC are displayed on the surface of cortical thymic epithelial cells, and TCRs that weakly bind these ligands trigger maturation signals that inhibit RAG gene expression (thereby closing off the option of editing)16, increase TCR cell-surface expression and induce the expression of homing receptors for chemokines found in the thymic medulla and the peripheral lymphoid tissues. The thymic cortical epithelium is unique in supporting this essential process of positive selection, through as yet unknown factors3. A minority of self-reactive TCRs trigger an editing process; in this case, TCRs are downregulated, RAG expression continues and the offending TCR α-chain is replaced or diluted with a second α-chain that is less self reactive6.
As positively selected thymocytes move from the cortex towards the medulla, they continue to test their TCRs for self reactivity, but now this occurs on medullary thymic epithelial cells and dendritic cells of haemopoietic origin16 (Fig. 4). These medullary cells express T-cell costimulatory molecules, such as CD80 (also known as B7.1) and CD86 (B7.2), the ligands for CD28. At this point, TCRs that bind strongly to self-peptide–MHC combinations trigger the death (negative selection) of thymocytes. The crucial role of medullary cells in ensuring self tolerance is clearly illustrated in studies of mice where medullary MHC molecules are missing or B7 molecules are missing or blocked by antibodies. In such animals, T cells with self-reactive TCRs reach the periphery and cause systemic inflammatory conditions resembling graft-versus-host disease3,17. The well established association between particular MHC molecules and susceptibility to specific autoimmune diseases may stem from inefficient presentation of particular self peptides during this phase of TCR deletion18,19. Development of peripheral nerve-specific autoimmunity in CD86-deficient non-obese diabetic (NOD) mice20 may arise from a similar problem with thymic deletion of nerve-specific self peptides.
Two examples are shown of what is likely to be a continuum: a, a TCR with weak self reactivity, where TCR and IL-7R pro-survival pathways dominate; b, a TCR with strong self reactivity where TCR-induced BIM and FAS death pathways predominate. Similar pathways also act in mature peripheral T cells, where competition for limiting IL-7 and self-peptide/MHC, and induction of B7 ligands for CD28, add extra levels of extrinsic control.
A strong connection between thymic TCR deletion and human autoimmune disease has been forged by understanding the function of the autoimmune regulator (AIRE) gene. Crippling mutations in the human gene are responsible for autoimmune polyendocrine syndrome 1 (refs 21, 22), an infrequent but devastating disorder that targets many discrete organs. Corresponding Aire mutations in mice cause similar, albeit milder, organ-specific autoimmunity23,34 and a marked failure to delete organ-specific TCRs in the thymus because tissue-specific genes are not switched on in rare medullary thymic epithelial cells24,25,26. Promiscuous expression of many different tissue-specific proteins, such as insulin, was noted in rare medullary thymic cells over a decade ago. This expression was proposed as a way to couple central thymic tolerance mechanisms to peripherally expressed self proteins27. The crucial role of this mechanism is established by the severe autoimmune syndrome in AIRE-deficient humans, and it may explain why inherited promoter variants that decrease thymic expression of the insulin gene are associated selectively with autoimmune diabetes in humans28,29.
A less-defined abnormality of medullary thymic epithelium appears to be responsible for myasthenia gravis, where hyperproliferative or neoplastic thymic epithelial cells that display subunits of the acetylcholine receptor activate T cells and precipitate the formation of circulating anti-acetylcholine receptor autoantibodies30. Surgical removal of the thymus in some cases cures the autoimmunity.
The signalling events involved in negative selection of T cells are still poorly understood. The tyrosine kinase ZAP70 (ζ-chain-associated protein kinase of 70 kDa) is required for thymocyte deletion because partial deficiency of ZAP70 in mice allows cells with forbidden TCRs to escape death and cause a systemic inflammatory disorder resembling rheumatoid arthritis31. The onset of negative selection also requires GRB2 (growth-factor-receptor-bound protein 2) (ref. 32) and MINK (misshapen-Nck-interacting kinase (NIK) related kinase) (ref. 33) plus intense but transient activation of ERK (extracellular signal-regulated kinase) and prolonged p38 and JNK (Jun kinase) activation16. At the distal end of the pathway, deletion partly requires induction of BIM expression at the messenger RNA and protein level14. BIM antagonizes BCL-2 and related proteins to release pro-apoptotic BAX and BAK, which are also required for deletion34. Deletion also involves induction of members of the Nur77 family of orphan nuclear receptors, and TCR-induced thymocyte death is blocked by a dominant-negative Nur77 mutant35. Induction of BIM and Nur77 expression and cell death in thymocytes with forbidden TCRs is selectively defective in the NOD mouse strain, owing to the cumulative T-cell-intrinsic effects of four of the chromosomal loci that contribute to diabetes susceptibility in this strain36. The resistance of NOD thymocytes to deletion, observed in three separate experimental systems37,38,39, thus appears to be an important component of the susceptibility of this strain to a range of autoimmune diseases.
The combination of strong stimulatory signals through TCR and CD28 is, paradoxically, a potent trigger of nuclear factor-κB (NF-κB) activation, the pro-survival pathway that induces expression of BCL-2 proteins in mature peripheral T cells40. NF-κB activation may counteract BIM-induced cell death (Fig. 4), and indeed gene-expression profiling demonstrates specific induction of NF-κB genes in semi-mature thymocytes with self-reactive TCRs36. Strong TCR engagement also induces expression of an extracellular protein, FAS ligand (FASL, also known as CD95L), and triggers T-cell death independently from the BIM/BCL-2 mechanism through FASL–FAS interaction and the caspase-8 proteolytic cascade. The requirement for this pathway in thymocyte deletion, though, is still debated41,42. The severe systemic autoimmune syndromes that develop in mice lacking BIM14, and in both mice and humans with defects in FASL and FAS43, underscore the crucial roles of BIM and FAS at several T- and B-cell tolerance checkpoints.
Marked disruption of thymic deletion by defects in a single gene, exemplified by homozygous AIRE deficiency, is an informative but apparently rare cause of human autoimmune disease. More commonly, autoimmune disease reflects subtle decreases at multiple points in the thymic deletion pathways (summarized in Fig. 4), as seen in the NOD mouse36. It is striking that loss of one copy of a key gene, such as Aire26 and Bim14, in this pathway is sufficient to create a small but measurable decrease in the efficiency of deletion. Equally small changes in insulin gene expression in the thymus correlate with human susceptibility to type 1 diabetes28,29. These findings indicate that the process of thymic deletion is on a knife-edge rather than buffered by a large safety margin for deleting forbidden receptors. Therefore, there is likely to be large individual variation in the efficiency of deleting particular TCRs among people.
Intrinsic regulation by anergy and biochemical tuning
In addition to deletion and editing, self-reactive receptors are regulated in primary and secondary lymphoid tissues by intrinsic biochemical changes in the cells displaying them. Several intrinsic cellular mechanisms of anergy are well documented in B cells with self-reactive BCRs (refs 7, 11, 44; Fig. 1c). The first is decreased display of self-reactive BCRs on the cell surface, varying from as little as 50% to more than 99% reduction, owing to accelerated endocytosis (M. Blery, unpublished observations) and blocked transport of new BCRs out of the endoplasmic reticulum45. Partly independent from receptor downregulation, self-reactive BCRs activate tyrosine kinase signalling poorly, limiting cell survival because of weak NF-κB1 activation. The self-reactive BCRs nevertheless continue to signal for BIM induction to promote death46 and to ERK pathways that block Toll-like receptor 9 (TLR9)-induced differentiation into plasma cells47. Each of these changes is reversible if the BCR stops binding to the self antigen, as would occur if the BCR is edited to lose self reactivity through somatic hypermutation in germinal centres.
Two other mechanisms of molecular feedback dampen self-reactive BCR signalling. Purely biochemical tuning involves proteins that increase the threshold for B-cell activation and are expressed constitutively regardless of BCR specificity. An example is the recruitment of the tyrosine phosphatase SHP1 (SH2-domain-containing protein tyrosine phosphatase 1) to the activated BCR through the cell-surface proteins CD22 and PD1. Another example is the recruitment of the lipid phosphatase SHIP (SH2-domain-containing inositol-5-phosphatase) to the activated BCR through Fc receptor-γ (refs 7, 48). Defects in either of these pathways dysregulate responses to foreign and self antigens, and create susceptibility to spontaneous autoantibody production. The changes in gene expression that occur selectively in cells bearing self-reactive BCRs are a second adaptation. An example is expression of the CD5 cell-surface protein induced selectively by self-reactive BCRs, which provides an additional inhibitory receptor to recruit SHP1 and inhibit BCR signalling and activation49.
Biochemical and genetic feedback loops are equally important for tuning T cells because all the T cells that leave the thymus will have been selected to have a moderate degree of TCR self reactivity8,9,16. Whereas antigen-receptor downregulation is generally less marked in self-reactive T cells than B cells, expression of the inhibitory receptor CD5 is induced to 10–50-fold higher levels in self-reactive T cells than in B1 cells or anergic B cells. CD5 levels are dynamically adjusted on individual thymic and peripheral T cells in proportion to the strength of TCR self reactivity, downregulating the response of TCRs to self peptides to avoid T-cell activation or deletion50,51. Expression of another inhibitory receptor, cytotoxic T-lymphocyte antigen 4 (CTLA4), is induced at a high threshold of TCR self reactivity and inhibits T-cell activation by competing with CD28 for ligation with B7 molecules and by transmitting inhibitory signals48,52,53. Lack of CTLA4 causes massive accumulation of self-reactive T cells in peripheral lymphoid and nonlymphoid tissues, both by disrupting intrinsic regulation of TCR-induced proliferation53,54 and by impairing the suppressive function of TR cells. Subtle functional variants of the CTLA4 gene are associated with susceptibility to thyroid autoimmunity and type 1 diabetes in humans and mice55.
Increased expression of the ubiquitin ligases CBL-B, GRAIL and ITCH can also accompany chronic TCR signalling in vitro56,57,58. These proteins interfere with TCR, CD28 and cytokine receptor signalling by tagging the TCR–CD28 or cytokine receptor signalling molecules with ubiquitin. This tag can trigger endocytosis and alter intracellular trafficking of TCRs, promote proteolytic degradation of receptors or signalling subunits, or allosterically interfere with signalling56,57,59,60. It is not known whether these ubiquitin ligases are induced to higher levels in strongly self-reactive T cells in vivo. But their importance for preventing autoimmunity in rodents is clear: cbl-b deficiency coupled with a particular MHC haplotype causes type 1 diabetes in the KDP (Komeda diabetes prone) rat strain61. Mice lacking Itch, or cbl-b and its close relative c-cbl, develop large numbers of activated T cells and produce high titres of autoantibodies59,60. Although the animal studies indicate crucial roles for anergy and tuning, little is known about the genes involved in human autoimmune disease other than the association with CTLA4 variants discussed above.
Extrinsic regulation of self-reactive receptors
A significant fraction of self-reactive BCRs and TCRs fail to be edited or trigger deletion in primary lymphoid tissues, either because the self antigens are bound with only low avidity or because they are not sufficiently abundant in the primary lymphoid organs. For receptors with intermediate avidity for self antigens, the risk they pose for autoimmunity may not overshadow their potential use in fighting infection. B cells with receptors that fall into this zone undergo a conditional type of clonal deletion that is extrinsically regulated through competition with B cells bearing less self-reactive BCRs (ref. 62). The recently revealed molecular basis46,63 for this illuminates general principles of broad significance (Fig. 3b).
The survival of peripheral B cells depends on BAFF, which is produced in limiting quantities primarily by radioresistant lymphoid stromal cells12. BAFF activates its receptor, BAFFR, on B cells and triggers an increase in the activity of the transcription factor NF-κB2, which maintains peripheral B-cell survival through induction of BCL-2 expression12,64. BAFF also induces expression of the serine–threonine kinase, PIM2 (refs 46, 65), which has potent pro-survival effects by phosphorylating and inhibiting the activity of the pro-apoptotic protein BAD66. Constant engagement of self-reactive BCRs, below the threshold required to trigger maturation-arrest in the bone marrow, is still sufficient to increase BIM expression and consequently elevate the survival requirement for BAFF (ref. 46; Fig. 3b). With large numbers of circulating B cells, the self-reactive cells fail to receive enough BAFF and are competitively deleted. This mechanism provides a ‘survival of the fittest’ process that can select against subtle differences in affinity for self antigen63. Conversely, self-reactive BCRs are more likely to survive when competition for BAFF is reduced, for example, in states of B-cell lymphopenia, or when BAFF synthesis is increased during infection or in pathological conditions12,46,63. In a clinical setting, partial antagonism of BAFF may be a particularly powerful way to enhance autoimmune B-cell sensitivity to natural tolerance mechanisms. It may also be a valuable adjunct to B-cell-depleting therapeutics, such as anti-CD20 (Rituxan), which might otherwise increase BAFF availability.
In common with B cells, the survival of mature T cells depends upon continuous signalling in the peripheral lymphoid tissues. Keeping T cells alive requires TCR signalling through contact with ubiquitous MHC ligands as well as exposure to interleukin-7 (IL-7) (refs 67–69). Normally, IL-7 levels are low and maintain T cells in interphase, and strongly self-reactive TCRs trigger only a transient proliferation that is overwhelmed by BIM-induced cell death14. In the case of T lymphocytopenia, however, IL-7 levels rise and amplify TCR signalling, causing naive T cells to proliferate and fill up ‘space’. Such homeostatic proliferation may activate T cells reactive to tissue-specific antigens and promote migration of these cells into extralymphoid sites, thereby risking the development of autoimmune disease at these sites. This scenario may explain why T lymphocytopenia predisposes people to autoimmune disease. Thus, some children with limited T- and B-cell production owing to a partial loss of RAG1 or RAG2 V(D)J recombinase activity develop Omenn syndrome, a massive expansion of peripheral T cells that infiltrate the skin and intestine, which resembles graft-versus-host disease70. In Wiskott–Aldrich syndrome, lymphopenia and defective T-cell function are accompanied by an array of autoimmune and inflammatory conditions71. Relaxation of competitive regulation may also explain the development of thyroid autoimmunity in one-third of patients with multiple sclerosis treated with the lymphocyte-depleting antibody, CAMPATH-1H (ref. 72). T-cell lymphopenia is an essential contributing factor to autoimmune diabetes susceptibility in the BB rat strain73,74 and is implicated in causing autoimmunity in the NOD mouse75.
Extrinsic regulation by limiting immunogenic costimuli
Antibody responses specific for many antigens depend upon B cells receiving two signals in short succession: signal one from the antigen binding to the BCR, and signal two from T helper cells. The T-cell surface protein CD40 ligand (CD40L) and secreted cytokines IL-2, IL-4, IL-5 and IL-21 comprise the second signals for B-cell proliferation and differentiation into antibody-secreting plasma cells76,77. Owing to thymic tolerance, the capacity of T cells to provide help for self-reactive B cells is limited. Nevertheless, signal two from T helper cells responding to foreign antigen can be misdirected to self-reactive BCRs that cross-react with a component of a microorganism or recognize a self component that associates with microbial components. It can also be misdirected by delivery of helper signals to bystander B cells. However, even in the rare instances when infection can be shown to trigger autoantibody diseases, notably Guillain–Barré syndrome, where cross-reactivity between antigens from Campylobacter jejuni and components of peripheral nerves elicits neuropathic autoantibodies78, only one in 1,000 infected individuals develop the autoantibodies. The resistance of 99.9% of people must be accounted for by efficient B-cell-intrinsic tolerance mechanisms.
A second pathway for antibody production involves the signalling of B cells through contact with stimulatory ligands released by microorganisms, notably bacterial flagellins, cell-wall lipopolysaccharides and unmethylated CpG dinucleotides. These are recognized by a set of TLRs on B cells79, and can partially substitute for T-cell help to allow antibody control of many microorganisms even in T-cell-deficient individuals. Relatively little is known about how TLR signalling is dampened to ensure that the threshold for stimulation is high enough to stop activation of self-reactive B cells. Experiments in mice have shown that dysregulated activity of the TLR9 pathway (which senses CpG DNA), owing to pathological accumulation of circulating IgG–self-DNA complexes, is a potent driver of the production of autoantibodies against IgG and DNA (ref. 80). TLR9 signalling may explain the production of anti-DNA autoantibodies in a subset of people given procainamide (a drug used to correct an irregular heartbeat), through interference with DNA methyltransferases that mask CpG motifs in our own DNA (ref. 81). It may also explain why SLE can sometimes be treated with chloroquine, an inhibitor of TLR9 signalling. Inadequate clearance of apoptotic cells with exposed CpG DNA and other nuclear antigens may account for the striking association between SLE and genetic deficiencies in classical complement pathway components82.
Activation of mature T cells requires a combination of TCR ligation plus a costimulatory signal. T-cell tolerance is favoured when antigen is recognized without immunogenic costimuli. Although there are multiple T-cell costimuli, interaction of CD28 on T cells with the B7 proteins CD80 and CD86 on antigen-presenting cells is especially important52. Expression of B7 proteins and other costimulatory ligands is induced on the surface of B cells and dendritic cells in response to TLR signalling and potentiates the survival and clonal expansion of T cells, in part by activating the NF-κB1 pathway and inducing the expression of BCL-2-related pro-survival proteins40. When B7–CD28 signalling is blocked, TCR-induced proliferation is cut short and T-cell death ensues, as a result of either BIM expression or FAS–FASL signalling52,53,68. Blocking B7–CD28 costimulation is thus a promising avenue to switch immune responses into tolerance, for example by using a competing soluble receptor for B7 proteins such as a CTLA4–immunoglobulin fusion protein (see the review in this issue by Feldmann and Steinman, page 612). This treatment may also interfere with tolerance by decreasing thymic deletion, TR function (see the review by Kronenberg and Rudensky, page 598) and intrinsic T-cell regulation by CTLA4.
Regulation of self-reactive receptors in follicles
Each of the checkpoints described above deals with self-reactive receptors generated by V(D)J recombination in the primary lymphoid organs; however, self-reactive BCRs are also generated in a second wave of receptor-gene diversification through somatic hypermutation in germinal-centre follicles of peripheral lymphoid tissues30,83,84 (Fig. 2). Somatic hypermutation poses a particularly severe threat of autoimmunity for three reasons. First, this process can markedly increase the affinity of antibodies for self antigens, so that the same concentration of secreted antibody may cause 100-fold more damage. Second, the follicular pathway of B-cell differentiation generates long-lived plasma cells and memory cells, which can continue to produce antibody indefinitely85. Third, autologous DNA, an important self-antigen target in SLE, is presented by the numerous apoptotic cells86 in germinal centres, and organ-specific self components are trapped and displayed as immune complexes on follicular dendritic cells in autoimmune disease30,87, where they represent a powerful potential stimulus for autoantibody production.
In animal models of SLE, most, if not all, anti-double-stranded DNA antibodies are somatically mutated, and the pattern of somatic mutations indicates that autoreactive B cells are positively selected for higher affinity83. Germinal centres and hypermutated, autoantigen-specific BCRs form in ectopic sites immediately adjacent to sources of autoantigen in a range of human autoimmune diseases30,87, as well as arising in lymph nodes that drain organs affected by autoimmunity in experimental animals88.
Remarkably little is known about the mechanisms that normally deal with self-reactive BCRs arising in germinal centres or how these are disrupted in human autoimmunity. Self-reactive BCRs induce subtle differences in B-cell responsiveness to the opposing chemokine gradients between follicles and extrafollicular zones11,62,89, excluding them from follicles and thus minimizing their participation in germinal-centre responses. Rapid deletion of B cells within germinal centres can be induced in less than 4 hours — comparable to or faster than the rapid death of self-reactive thymocytes — when the BCR binds to an antigen that is not recognized by the T cells in the germinal centre90,91. In common with deletion of thymocyte and extrafollicular B cells, deletion of germinal-centre B cells may involve the induction of BIM expression or trigger the FAS death receptor. Like extrafollicular B cells (Fig. 2), self-reactive B cells in the germinal centres may be extrinsically regulated by competition for BAFF, or by competition for CD40L, IL-21 and ICOS (inducible T-cell costimulator) signals from follicular T cells (see below). Cells with cross-reactive BCRs that recognize both self and foreign antigen84 would, in principle, be able to survive, but self-antigen-induced BIM expression would put them at a disadvantage when competing with cells bearing purely foreign reactive BCRs whose BCR engagement is less chronic and more tightly linked to T-cell help.
The small subset of CD4+ T cells found in germinal centres follows a unique programme of differentiation compared with their extrafollicular counterparts. Like extrafollicular helper T cells, follicular T cells express CD40L, which is required to sustain survival and proliferation of germinal-centre B cells92. Follicular T cells, however, also display high levels of ICOS, which is specifically required to help germinal-centre antibody responses in mice and humans52,93. Follicular T cells also require costimulation through OX40L (ref. 94) and an intracellular signalling adaptor for the SLAM family of co-stimulatory receptors, SH2D1a (also known as SAP) (ref. 95). The fact that antigen stimulation in the absence of microbial TLR agonists, as would normally occur for self antigens, does not induce T-cell entry into follicles96 suggests that strict regulation of follicular T-helper cell differentiation may block self-reactive T cells from delivering help to germinal-centre B cells. Recently, we discovered a novel ubiquitin ligase, Roquin, that is essential for repressing ICOS expression in T cells. When Roquin is defective in mice, self-antigen-directed follicular T-cell help becomes uncontrolled, generating large numbers of germinal centres and extraordinary levels of autoantibody production97. These advances begin to open up analysis of the tolerance checkpoints controlling autoantibody selection during the crucial germinal-centre phase of antibody evolution.
Self-reactive receptor tolerance at the final effector phase
Even when high-affinity antibodies do form and circulate in sufficient quantity to cause disease upon transplacental transfer to a foetus, there is often little or no disease in individuals with these antibodies98. Moreover, when pathology does occur, it is often limited to focal points, illustrated by the circumscribed skin lesions in pemphigus despite ubiquitous skin distribution of the target autoantigen. Analysis of a mouse model of rheumatoid arthritis shows that even when sufficient autoantibody is present in the circulation, its capacity to localize in joints to produce joint pathology depends on inflammatory cascades involving Fc receptors, mast cells, neutrophils and complement99,100. There is clearly considerable scope for tolerating self-reactive receptors even at this level, and much more research is needed into this phase of regulation.
Concluding remarks
Although many self-tolerance mechanisms exist, defects in a single checkpoint, such as AIRE, can lead to autoimmune disease. The clinical manifestation is nevertheless seen only after a latent period of many years and then only against a few proteins or organs. There are obvious parallels here with inherited defects in tumour-suppressor genes, favouring the view that successive and parallel tolerance checkpoints provide back-up mechanisms to control all but a few exceptional forbidden receptors. There seems to be hundreds of genes such as AIRE, BIM, ZAP70, CBLB, FAS and ROQUIN involved in these checkpoints, and most of the candidate genes are yet to be discovered on the basis of the rate at which new autoimmunity genes are currently being identified. Since heterozygous mutation in any one of these genes may predispose a person to autoimmunity, the sheer number of genes involved may collectively account for the ∼5% of people with autoimmune disease despite a low population frequency for heterozygous mutations in any one of these genes. Population-wide scans based on common DNA polymorphisms will not be effective tools to identify predisposing defects of this type: instead, exon resequencing of individuals with autoimmune disease will be required.
Regardless of whether predisposition to autoimmunity is caused by rare or common genetic variants, interventions aimed at preventing or treating autoimmunity will need to be tailored to correct weak cellular checkpoints, shore up back-up mechanisms and avoid doing more harm by interfering with these mechanisms and thus exacerbating the breakthrough of forbidden receptors. The development of thyroid autoimmunity through lymphopenia as a result of antibody therapy in multiple sclerosis patients72 and the development of systemic autoimmunity when B7 molecules are blocked in experimental animals17 highlight the risks. Well-targeted interventions require a more complete map of the cellular mechanisms and genes underpinning self tolerance, and more ways to test for individual variation. The conserved genes and proteins now laid out by mammalian genome sequencing, and the relative ease of moving back and forward between human and rodent gene analysis, provide the vehicle for solving both of these challenges.
References
Ignatowicz, L., Kappler, J. & Marrack, P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell 84, 521–529 (1996).
Zerrahn, J., Held, W. & Raulet, D. H. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell 88, 627–636 (1997).
Laufer, T. M., DeKoning, J., Markowitz, J. S., Lo, D. & Glimcher, L. H. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature 383, 81–85 (1996).
Wardemann, H. et al. Predominant autoantibody production by early human B cell precursors. Science 301, 1374–1377 (2003).
Jacobson, D. L., Gange, S. J., Rose, N. R. & Graham, N. M. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin. Immunol. Immunopathol. 84, 223–243 (1997).
Nemazee, D. & Hogquist, K. A. Antigen receptor selection by editing or downregulation of V(D)J recombination. Curr. Opin. Immunol. 15, 182–189 (2003).
Healy, J. I. & Goodnow, C. C. Positive versus negative signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 16, 645–670 (1998).
Schwartz, R. H. T cell anergy. Annu. Rev. Immunol. 21, 305–334 (2003).
Grossman, Z. & Paul, W. E. Self-tolerance: context-dependent tuning of T cell antigen recognition. Semin. Immunol. 12, 197–203 (2000).
Hartley, S. B. et al. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell 72, 325–335 (1993).
Fields, M. L. & Erikson, J. The regulation of lupus-associated autoantibodies: immunoglobulin transgenic models. Curr. Opin. Immunol. 15, 709–717 (2003).
Mackay, F., Schneider, P., Rennert, P. & Browning, J. BAFF and APRIL: a tutorial on B cell survival. Annu. Rev. Immunol. 21, 231–264 (2003).
Jankovic, M., Casellas, R., Yannoutsos, N., Wardemann, H. & Nussenzweig, M. C. RAGs and regulation of autoantibodies. Annu. Rev. Immunol. 22, 485–501 (2004).
Strasser, A. & Bouillet, P. The control of apoptosis in lymphocyte selection. Immunol. Rev. 193, 82–92 (2003).
Akkaraju, S., Canaan, K. & Goodnow, C. C. Self-reactive B cells are not eliminated or inactivated by autoantigen expressed on thyroid epithelial cells. J. Exp. Med. 186, 2005–2012 (1997).
Palmer, E. Negative selection — clearing out the bad apples from the T-cell repertoire. Nature Rev. Immunol. 3, 383–391 (2003).
Gao, J. X. et al. Perinatal blockade of B7-1 and B7-2 inhibits clonal deletion of highly pathogenic autoreactive T cells. J. Exp. Med. 195, 959–971 (2002).
Kanagawa, O., Martin, S. M., Vaupel, B. A., Carrasco-Marin, E. & Unanue, E. R. Autoreactivity of T cells from nonobese diabetic mice: an I-Ag7-dependent reaction. Proc. Natl Acad. Sci. USA 95, 1721–1724 (1998).
Wicker, L. S., Todd, J. A. & Peterson, L. B. Genetic control of autoimmune diabetes in the NOD mouse. Annu. Rev. Immunol. 13, 179–200 (1995).
Salomon, B. et al. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. J. Exp. Med. 194, 677–684 (2001).
Nagamine, K. et al. Positional cloning of the APECED gene. Nature Genet. 17, 393–398 (1997).
Aaltonen, J. et al. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nature Genet. 17, 399–403 (1997).
Ramsey, C. et al. Aire-deficient mice develop multiple features of APECED phenotype and show altered immune response. Hum. Mol. Genet. 11, 397–409 (2002).
Anderson, M. S. et al. Projection of an immunological self-shadow within the thymus by the Aire protein. Science 298, 1395–1403 (2002).
Liston, A., Lesage, S., Wilson, J., Peltonen, L. & Goodnow, C. C. Aire regulates negative selection of organ-specific T cells. Nature Immunol. 4, 350–354 (2003).
Liston, A. et al. Gene dosage-limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J. Exp. Med. 200, 1015–1026 (2004).
Hanahan, D. Peripheral-antigen-expressing cells in thymic medulla: factors in self-tolerance and autoimmunity. Curr. Opin. Immunol. 10, 656–662 (1998).
Pugliese, A. et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nature Genet. 15, 293–297 (1997).
Vafiadis, P. et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nature Genet. 15, 289–292 (1997).
Shiono, H. et al. Scenarios for autoimmunization of T and B cells in myasthenia gravis. Ann. NY Acad. Sci. 998, 237–256 (2003).
Sakaguchi, N. et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426, 454–460 (2003).
Gong, Q. et al. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nature Immunol. 2, 29–36 (2001).
McCarty, N. et al. Signaling by the kinase MINK is essential in the negative selection of autoreactive thymocytes. Nature Immunol. 6, 65–72 (2005).
Rathmell, J. C., Lindsten, T., Zong, W. X., Cinalli, R. M. & Thompson, C. B. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nature Immunol. 3, 932–939 (2002).
Zhou, T. et al. Inhibition of Nur77/Nurr1 leads to inefficient clonal deletion of self-reactive T cells. J. Exp. Med. 183, 1879–1892 (1996).
Liston, A. et al. Generalized resistance to thymic deletion in the NOD mouse; a polygenic trait characterized by defective induction of Bim. Immunity 21, 817–830 (2004).
Kishimoto, H. & Sprent, J. A defect in central tolerance in NOD mice. Nature Immunol. 2, 1025–1031 (2001).
Lesage, S. et al. Failure to censor forbidden clones of CD4 T cells in autoimmune diabetes. J. Exp. Med. 196, 1175–1188 (2002).
Choisy-Rossi, C. M., Holl, T. M., Pierce, M. A., Chapman, H. D. & Serreze, D. V. Enhanced pathogenicity of diabetogenic T cells escaping a non-MHC gene-controlled near death experience. J. Immunol. 173, 3791–3800 (2004).
Kane, L. P., Lin, J. & Weiss, A. It's all Rel-ative: NF-κB and CD28 costimulation of T-cell activation. Trends Immunol. 23, 413–420 (2002).
Sprent, J. & Kishimoto, H. The thymus and negative selection. Immunol. Rev. 185, 126–135 (2002).
Villunger, A. et al. Negative selection of semimature CD4(+)8(-)HSA+ thymocytes requires the BH3-only protein Bim but is independent of death receptor signaling. Proc. Natl Acad. Sci. USA 101, 7052–7057 (2004).
Nagata, S. Human autoimmune lymphoproliferative syndrome, a defect in the apoptosis-inducing Fas receptor: a lesson from the mouse model. J. Hum. Genet. 43, 2–8 (1998).
Benschop, R. J. et al. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity 14, 33–43 (2001).
Bell, S. E. & Goodnow, C. C. A selective defect in IgM antigen receptor synthesis and transport causes loss of cell surface IgM expression on tolerant B lymphocytes. EMBO J. 13, 816–826 (1994).
Lesley, R. et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity 20, 441–453 (2004).
Rui, L., Vinuesa, C. G., Blasioli, J. & Goodnow, C. C. Resistance to CpG DNA-induced autoimmunity through tolerogenic B cell antigen receptor ERK signaling. Nature Immunol. 4, 594–600 (2003).
Ravetch, J. V. & Lanier, L. L. Immune inhibitory receptors. Science 290, 84–89 (2000).
Hippen, K. L., Tze, L. E. & Behrens, T. W. CD5 maintains tolerance in anergic B cells. J. Exp. Med. 191, 883–890 (2000).
Wong, P., Barton, G. M., Forbush, K. A. & Rudensky, A. Y. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J. Exp. Med. 193, 1179–1187 (2001).
Smith, K. et al. Sensory adaptation in naive peripheral CD4 T cells. J. Exp. Med. 194, 1253–1261 (2001).
Sharpe, A. H. & Freeman, G. J. The B7-CD28 superfamily. Nature Rev. Immunol. 2, 116–126 (2002).
Walker, L. S. & Abbas, A. K. The enemy within: keeping self-reactive T cells at bay in the periphery. Nature Rev. Immunol. 2, 11–19 (2002).
Inobe, M. & Schwartz, R. H. CTLA-4 engagement acts as a brake on CD4+ T cell proliferation and cytokine production but is not required for tuning T cell reactivity in adaptive tolerance. J. Immunol. 173, 7239–7248 (2004).
Ueda, H. et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423, 506–511 (2003).
Heissmeyer, V. et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nature Immunol. 5, 255–265 (2004).
Anandasabapathy, N. et al. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity 18, 535–547 (2003).
Jeon, M. S. et al. Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity 21, 167–177 (2004).
Naramura, M. et al. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nature Immunol. 3, 1192–1199 (2002).
Liu, Y. C. Ubiquitin ligases and the immune response. Annu. Rev. Immunol. 22, 81–127 (2004).
Yokoi, N. et al. Cblb is a major susceptibility gene for rat type 1 diabetes mellitus. Nature Genet. 31, 391–394 (2002).
Cyster, J. G., Hartley, S. B. & Goodnow, C. C. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature 371, 389–395 (1994).
Thien, M. et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity 20, 785–798 (2004).
Claudio, E., Brown, K., Park, S., Wang, H. & Siebenlist, U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nature Immunol. 3, 958–965 (2002).
Xu, L. G., Wu, M., Hu, J., Zhai, Z. & Shu, H. B. Identification of downstream genes up-regulated by the tumor necrosis factor family member TALL-1. J. Leukoc. Biol. 72, 410–416 (2002).
Fox, C. J. et al. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 17, 1841–1854 (2003).
Sprent, J. & Surh, C. D. T cell memory. Annu. Rev. Immunol. 20, 551–579 (2002).
Marrack, P. & Kappler, J. Control of T cell viability. Annu. Rev. Immunol. 22, 765–787 (2004).
Barthlott, T., Kassiotis, G. & Stockinger, B. T cell regulation as a side effect of homeostasis and competition. J. Exp. Med. 197, 451–460 (2003).
Le Deist, F., Poinsignon, C., Moshous, D., Fischer, A. & de Villartay, J. P. Artemis sheds new light on V(D)J recombination. Immunol. Rev. 200, 142–155 (2004).
Dupuis-Girod, S. et al. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics 111, e622–e627 (2003).
Coles, A. J. et al. Pulsed monoclonal antibody treatment and autoimmune thyroid disease in multiple sclerosis. Lancet 354, 1691–1695 (1999).
MacMurray, A. J. et al. Lymphopenia in the BB rat model of type 1 diabetes is due to a mutation in a novel immune-associated nucleotide (Ian)-related gene. Genome Res. 12, 1029–1039 (2002).
Hornum, L., Romer, J. & Markholst, H. The diabetes-prone BB rat carries a frameshift mutation in Ian4, a positional candidate of Iddm1. Diabetes 51, 1972–1979 (2002).
King, C., Ilic, A., Koelsch, K. & Sarvetnick, N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 117, 265–277 (2004).
Foy, T. M., Aruffo, A., Bajorath, J., Buhlmann, J. E. & Noelle, R. J. Immune regulation by CD40 and its ligand gp39. Annu. Rev. Immunol. 14, 591–617 (1996).
Kovanen, P. E. & Leonard, W. J. Cytokines and immunodeficiency diseases: critical roles of the gamma(c)-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol. Rev. 202, 67–83 (2004).
Ang, C. W., Jacobs, B. C. & Laman, J. D. The Guillain-Barré syndrome: a true case of molecular mimicry. Trends Immunol. 25, 61–66 (2004).
Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 430, 257–263 (2004).
Leadbetter, E. A. et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416, 603–607 (2002).
Richardson, B. DNA methylation and autoimmune disease. Clin. Immunol. 109, 72–79 (2003).
Taylor, P. R. et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo . J. Exp. Med. 192, 359–366 (2000).
Radic, M. Z. & Weigert, M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu. Rev. Immunol. 12, 487–520 (1994).
Ray, S. K., Putterman, C. & Diamond, B. Pathogenic autoantibodies are routinely generated during the response to foreign antigen: a paradigm for autoimmune disease. Proc. Natl Acad. Sci. USA 93, 2019–2024 (1996).
Slifka, M. K., Antia, R., Whitmire, J. K. & Ahmed, R. Humoral immunity due to long-lived plasma cells. Immunity 8, 363–372 (1998).
Rosen, A. & Casciola-Rosen, L. Clearing the way to mechanisms of autoimmunity. Nature Med. 7, 664–665 (2001).
Weyand, C. M., Kurtin, P. J. & Goronzy, J. J. Ectopic lymphoid organogenesis: a fast track for autoimmunity. Am. J. Pathol. 159, 787–793 (2001).
Mandik-Nayak, L., Wipke, B. T., Shih, F. F., Unanue, E. R. & Allen, P. M. Despite ubiquitous autoantigen expression, arthritogenic autoantibody response initiates in the local lymph node. Proc. Natl Acad. Sci. USA 99, 14368–14373 (2002).
Reif, K. et al. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature 416, 94–99 (2002).
Shokat, K. M. & Goodnow, C. C. Antigen-induced B-cell death and elimination during germinal-centre immune responses. Nature 375, 334–338 (1995).
Pulendran, B., Kannourakis, G., Nouri, S., Smith, K. G. & Nossal, G. J. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature 375, 331–334 (1995).
Han, S. et al. Cellular interaction in germinal centers: roles of CD40 ligand and B7-2 in established germinal centers. J. Immunol. 155, 556–567 (1995).
Kroczek, R. A., Mages, H. W. & Hutloff, A. Emerging paradigms of T-cell co-stimulation. Curr. Opin. Immunol. 16, 321–327 (2004).
Walker, L. S., Gulbranson-Judge, A., Flynn, S., Brocker, T. & Lane, P. J. Co-stimulation and selection for T-cell help for germinal centres: the role of CD28 and OX40. Immunol. Today 21, 333–337 (2000).
Crotty, S., Kersh, E. N., Cannons, J., Schwartzberg, P. L. & Ahmed, R. SAP is required for generating long-term humoral immunity. Nature 421, 282–287 (2003).
Kearney, E. R., Pape, K. A., Loh, D. Y. & Jenkins, M. K. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo . Immunity 1, 327–339 (1994).
Vinuesa, C. G. et al. A novel RING-type ubiquitin ligase family member essential to repress follicular helper T cells and autoimmunity. Nature doi:10.1038/nature03555 (this issue).
Scofield, R. H. Autoantibodies as predictors of disease. Lancet 363, 1544–1546 (2004).
Wipke, B. T., Wang, Z., Nagengast, W., Reichert, D. E. & Allen, P. M. Staging the initiation of autoantibody-induced arthritis: a critical role for immune complexes. J. Immunol. 172, 7694–7702 (2004).
Monach, P. A., Benoist, C. & Mathis, D. The role of antibodies in mouse models of rheumatoid arthritis, and relevance to human disease. Adv. Immunol. 82, 217–248 (2004).
Acknowledgements
This is a broad field, and with very limited space we needed to cite selected reviews and articles. We sincerely apologise for not directly citing all of the important work on which the points discussed are based. We thank our colleagues at The ANU, Oxford University, UCSF, Centenary Institute, Garvan Institute and The Scripps Research Institute for helpful discussions, and thank the Wellcome Trust, NHMRC, JDRF and NIH for grant support. Competing interests statement The authors declare that they have no competing financial interests.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Goodnow, C., Sprent, J., de St Groth, B. et al. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature 435, 590–597 (2005). https://doi.org/10.1038/nature03724
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature03724
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.