Abstract
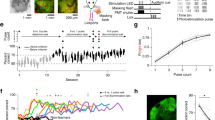
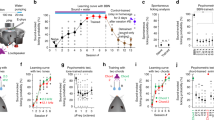
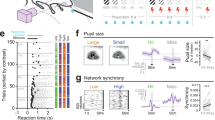
Electrical microstimulation can establish causal links between the activity of groups of neurons and perceptual and cognitive functions1,2,3,4,5,6. However, the number and identities of neurons microstimulated, as well as the number of action potentials evoked, are difficult to ascertain7,8. To address these issues we introduced the light-gated algal channel channelrhodopsin-2 (ChR2)9 specifically into a small fraction of layer 2/3 neurons of the mouse primary somatosensory cortex. ChR2 photostimulation in vivo reliably generated stimulus-locked action potentials10,11,12,13 at frequencies up to 50 Hz. Here we show that naive mice readily learned to detect brief trains of action potentials (five light pulses, 1 ms, 20 Hz). After training, mice could detect a photostimulus firing a single action potential in approximately 300 neurons. Even fewer neurons (approximately 60) were required for longer stimuli (five action potentials, 250 ms). Our results show that perceptual decisions and learning can be driven by extremely brief epochs of cortical activity in a sparse subset of supragranular cortical pyramidal neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Penfield, W. & Boldery, P. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60, 389–443 (1937)
Salzman, C. D., Britten, K. H. & Newsome, W. T. Cortical microstimulation influences perceptual judgements of motion direction. Nature 346, 174–177 (1990)
Romo, R., Hernandez, A., Zainos, A. & Salinas, E. Somatosensory discrimination based on cortical microstimulation. Nature 392, 387–390 (1998)
Libet, B. in Handbook of Sensory Physiology (ed. Iggo, A.) 743–790 (Springer, Berlin, 1973)
Leal-Campanario, R., Delgado-Garcia, J. M. & Gruart, A. Microstimulation of the somatosensory cortex can substitute for vibrissa stimulation during Pavlovian conditioning. Proc. Natl Acad. Sci. USA 103, 10052–10057 (2006)
Butovas, S. & Schwarz, C. Detection psychophysics of intracortical microstimulation in rat primary somatosensory cortex. Eur. J. Neurosci. 25, 2161–2169 (2007)
Tehovnik, E. J. Electrical stimulation of neural tissue to evoke behavioral responses. J. Neurosci. Methods 65, 1–17 (1996)
Ranck, J. B. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res. 98, 417–440 (1975)
Nagel, G. et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl Acad. Sci. USA 100, 13940–13945 (2003)
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neurosci. 8, 1263–1268 (2005)
Li, X. et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl Acad. Sci. USA 102, 17816–17821 (2005)
Ishizuka, T., Kakuda, M., Araki, R. & Yawo, H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci. Res. 54, 85–94 (2006)
Bi, A. et al. Ectopic expression of a microbial-type rhodopsin restores visual responses in mice with photoreceptor degeneration. Neuron 50, 23–33 (2006)
Hatanaka, Y., Hisanaga, S., Heizmann, C. W. & Murakami, F. Distinct migratory behavior of early- and late-born neurons derived from the cortical ventricular zone. J. Comp. Neurol. 479, 1–14 (2004)
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nature Neurosci. 10, 663–668 (2007)
Margrie, T. W. et al. Targeted whole-cell recordings in the mammalian brain in vivo . Neuron 39, 911–918 (2003)
Fee, M. S., Mitra, P. P. & Kleinfeld, D. Central versus peripheral determinants of patterned spike activity in rat vibrissa cortex during whisking. J. Neurophysiol. 78, 1144–1149 (1997)
Arenkiel, B. R. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007)
Gray, N. W., Weimer, R. M., Bureau, I. & Svoboda, K. Rapid redistribution of synaptic PSD-95 in the neocortex in vivo . PLoS Biol. 4, e370 (2006)
DeWeese, M. R., Wehr, M. & Zador, A. M. Binary spiking in auditory cortex. J. Neurosci. 23, 7940–7949 (2003)
Petersen, R. S., Panzeri, S. & Diamond, M. E. Population coding in somatosensory cortex. Curr. Opin. Neurobiol. 12, 441–447 (2002)
Ferezou, I., Bolea, S. & Petersen, C. C. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron 50, 617–629 (2006)
Zhang, Y. P. & Oertner, T. G. Optical induction of synaptic plasticity using a light-sensitive channel. Nature Methods 4, 139–141 (2006)
Nagel, G. et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284 (2005)
Wang, H. et al. High-speed mapping of synaptic connectivity using photostimulation in channelrhodopsin-2 transgenic mice. Proc. Natl Acad. Sci. USA 104, 8143–8148 (2007)
Schroll, C. et al. Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae. Curr. Biol. 16, 1741–1747 (2006)
Lima, S. Q. & Miesenbock, G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121, 141–152 (2005)
Salzman, C. D., Murasugi, C. M., Britten, K. H. & Newsome, W. T. Microstimulation in visual area MT: effects on direction discrimination performance. J. Neurosci. 12, 2331–2355 (1992)
Tehovnik, E. J., Tolias, A. S., Sultan, F., Slocum, W. M. & Logothetis, N. K. Direct and indirect activation of cortical neurons by electrical microstimulation. J. Neurophysiol. 96, 512–521 (2006)
Pologruto, T. A., Sabatini, B. L. & Svoboda, K. ScanImage: flexible software for operating laser-scanning microscopes. Biomed. Eng. Online 2, 13 (2003)
Acknowledgements
We thank B. Burbach, D. Flickinger, H. Kessels, D. O’Connor, T. Sato, R. Weimer and A. Zador for help with experiments, and D. O’Connor for comments on the manuscript. This work was supported by the Swiss National Science Foundation (to D.H.), the National Institutes of Health and the Howard Hughes Medical Institute.
Author Contributions D.H. and K.S. designed the experiments. D.H. performed the behavioral and in vivo physiological experiments. L.P., D.H. and K.S. performed the brain slice measurements. N.G. performed histology. S.R., T.H., Z.M. and K.S. provided advice and equipment. D.H. and K.S. wrote the paper. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information 1
The file contains Supplementary Methods, Supplementary Figures 1-7 with Legends and Legends to Supplementary Movies 1-2. (PDF 1707 kb)
Supplementary Video 1
The file contains Supplementary Movie 1. (MOV 4116 kb)
Supplementary Video 2
The file contains Supplementary Movie 2. (MOV 4613 kb)
Rights and permissions
About this article
Cite this article
Huber, D., Petreanu, L., Ghitani, N. et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature 451, 61–64 (2008). https://doi.org/10.1038/nature06445
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06445
This article is cited by
-
Learning in a sensory cortical microstimulation task is associated with elevated representational stability
Nature Communications (2023)
-
Thalamocortical loops as temporal demodulators across senses
Communications Biology (2023)
-
Spatio-temporal activation patterns of neuronal population evoked by optostimulation and the comparison to electrical microstimulation
Scientific Reports (2023)
-
Propagation of activity through the cortical hierarchy and perception are determined by neural variability
Nature Neuroscience (2023)
-
Optogenetics: implications for Alzheimer’s disease research and therapy
Molecular Brain (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.