Abstract
The hippocampus is critical for encoding declarative memory, our repository of knowledge of who, what, where and when1. Mnemonic information is processed in the hippocampus through several parallel routes involving distinct subregions. In the classic trisynaptic pathway, information proceeds from entorhinal cortex (EC) to dentate gyrus to CA3 and then to CA1, the main hippocampal output2. Genetic lesions of EC (ref. 3) and hippocampal dentate gyrus (ref. 4), CA3 (ref. 5) and CA1 (ref. 6) regions have revealed their distinct functions in learning and memory. In contrast, little is known about the role of CA2, a relatively small area interposed between CA3 and CA1 that forms the nexus of a powerful disynaptic circuit linking EC input with CA1 output7. Here we report a novel transgenic mouse line that enabled us to selectively examine the synaptic connections and behavioural role of the CA2 region in adult mice. Genetically targeted inactivation of CA2 pyramidal neurons caused a pronounced loss of social memory—the ability of an animal to remember a conspecific—with no change in sociability or several other hippocampus-dependent behaviours, including spatial and contextual memory. These behavioural and anatomical results thus reveal CA2 as a critical hub of sociocognitive memory processing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Squire, L. R. & Wixted, J. T. The cognitive neuroscience of human memory since H.M. Annu. Rev. Neurosci. 34, 259–288 (2011)
van Strien, N. M., Cappaert, N. L. & Witter, M. P. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nature Rev. Neurosci. 10, 272–282 (2009)
Suh, J., Rivest, A. J., Nakashiba, T., Tominaga, T. & Tonegawa, S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science 334, 1415–1420 (2011)
Nakashiba, T. et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149, 188–201 (2012)
Nakashiba, T., Young, J. Z., McHugh, T. J., Buhl, D. L. & Tonegawa, S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 319, 1260–1264 (2008)
Tsien, J. Z., Huerta, P. T. & Tonegawa, S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338 (1996)
Chevaleyre, V. & Siegelbaum, S. A. Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron 66, 560–572 (2010)
Lorente de Nó, R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J. Psychol. Neurol. 46, 113–177 (1934)
Franklin, K. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates (Academic, 2007)
Lein, E. S., Callaway, E. M., Albright, T. D. & Gage, F. H. Redefining the boundaries of the hippocampal CA2 subfield in the mouse using gene expression and 3-dimensional reconstruction. J. Comp. Neurol. 485, 1–10 (2005)
Fanselow, M. S. & Dong, H. W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19 (2010)
Lee, S. E. et al. RGS14 is a natural suppressor of both synaptic plasticity in CA2 neurons and hippocampal-based learning and memory. Proc. Natl Acad. Sci. USA 107, 16994–16998 (2010)
Cui, Z., Gerfen, C. R. & Young, W. S. III. Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J. Comp. Neurol. 521, 1844–1866 (2013)
Kohara, K. et al. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nature Neurosci. 10.1038/nn.3614. (2013)
Wall, N. R., De La Parra, M., Callaway, E. M. & Kreitzer, A. C. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron 79, 347–360 (2013)
Rowland, D. C. et al. Transgenically targeted rabies virus demonstrates a major monosynaptic projection from hippocampal area CA2 to medial entorhinal layer II neurons. J. Neurosci. 33, 14889–14898 (2013)
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neurosci. 8, 1263–1268 (2005)
Hargreaves, E. L., Rao, G., Lee, I. & Knierim, J. J. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science 308, 1792–1794 (2005)
Hensler, J. G. Serotonergic modulation of the limbic system. Neurosci. Biobehav. Rev. 30, 203–214 (2006)
Pan, W. X & McNaughton, N. The supramammillary area: its organization, functions and relationship to the hippocampus. Prog. Neurobiol. 74, 127–166 (2004)
Young, W. S., Li, J., Wersinger, S. R. & Palkovits, M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience 143, 1031–1039 (2006)
Wersinger, S. R., Ginns, E. I., O’Carroll, A. M., Lolait, S. J. & Young, W. S. III. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry 7, 975–984 (2002)
DeVito, L. M. et al. Vasopressin 1b receptor knock-out impairs memory for temporal order. J. Neurosci. 29, 2676–2683 (2009)
Stevenson, E. L. & Caldwell, H. K. The vasopressin 1b receptor and the neural regulation of social behavior. Horm. Behav. 61, 277–282 (2012)
Kogan, J. H., Frankland, P. W. & Silva, A. J. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus 10, 47–56 (2000)
Ferguson, J. N. et al. Social amnesia in mice lacking the oxytocin gene. Nature Genet. 25, 284–288 (2000)
Brennan, P. A. & Zufall, F. Pheromonal communication in vertebrates. Nature 444, 308–315 (2006)
Corkin, S. What’s new with the amnesic patient H.M.? Nature Rev. Neurosci. 2, 153–160 (2002)
Benes, F. M., Kwok, E. W., Vincent, S. L. & Todtenkopf, M. S. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol. Psychiatry 44, 88–97 (1998)
Meyer-Lindenberg, A., Domes, G., Kirsch, P. & Heinrichs, M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Rev. Neurosci. 12, 524–538 (2011)
Heintz, N. GENSAT Brain Atlas of gene expression in EGFP Transgenic Mice http://www.gensat.org (2003)
Lein, E. S. ISH Data: Allen Brain Atlas: Mouse Brain http://mouse.brain-map.org/ (2007)
de Jong, P. J. BAC Clones Distribution Center – BACPAC Resources Center https://bacpac.chori.org/ (2000)
Warming, S., Costantino, N., Court, D. L., Jenkins, N. A. & Copeland, N. G. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33, e36 (2005)
Gong, S. et al. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 27, 9817–9823 (2007)
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neurosci. 13, 133–140 (2010)
Wickersham, I. R., Sullivan, H. A. & Seung, H. S. Production of glycoprotein-deleted rabies viruses for monosynaptic tracing and high-level gene expression in neurons. Nature Protocols 5, 595–606 (2010)
Watabe-Uchida, M., Zhu, L., Ogawa, S. K., Vamanrao, A. & Uchida, N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 (2012)
Yamamoto, M. et al. Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J. Neurosci. 23, 6759–6767 (2003)
Jiao, Y. et al. A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J. Neurosci. Methods 93, 149–162 (1999)
Boulanger, L. M. et al. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J. Neurosci. 15, 1532–1544 (1995)
Takeda, K. et al. WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum. Mol. Genet. 10, 477–484 (2001)
Rasband, W. S. Image J. http://imagej.nih.gov/ij/ (1997)
Vorhees, C. V. & Williams, M. T. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols 1, 848–858 (2006)
Bielsky, I. F., Hu, S., Szegda, K. L., Westphal, H. & Young, L. J. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 29, 483–493 (2004)
Yang, M. & Crawley, J. N. Simple behavioral assessment of mouse olfaction. Curr. Protocols Neurosci. 84, 8.24.1–8.24.12 (2009)
Acknowledgements
We thank T. R. Reardon for providing the rabies virus; J. Kupferman and F. Lema for experimental assistance; and C. Denny, Z. Donaldson, R. Hen, J. Gordon, J. Basu and M. Russo for discussions and comments on the manuscript. This work was supported by a Ruth L. Kirschstein F30 National Research Service Award from the National Institute of Mental Health (F.L.H.) and the Howard Hughes Medical Institute (S.A.S.).
Author information
Authors and Affiliations
Contributions
F.L.H. planned and performed the experiments, analysed the data and wrote the manuscript. S.A.S. oversaw the overall execution of the project, contributed to the experimental design and the interpretation of the data, provided financial support and helped write the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Generation of Amigo2-Cre mouse line.
λ Red-mediated homologous recombination with galK positive and negative selection was used to make seamless changes to the bacterial artificial chromosome (BAC). PCR cassettes are shown in orange, and Amigo2 locus is shown in blue. The PCR cassette contained two homology arms (H1, 58 nucleotides; H2, 62 nucleotides) that flanked the galactose kinase (galK) cassette. The homology arms flanked the Amigo2 start codon. Recombination followed by positive selection was used to obtain the galK integrate. Recombination of the modified BAC with a PCR cassette containing the Cre open reading frame (ORF) and poly(A) (PA) flanked by the same homology arms yielded the final BAC used to generate the transgenic line.
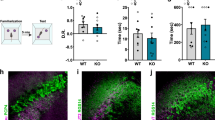
Extended Data Figure 2 Amigo2-Cre mice express Cre in a genetically defined population of CA2 PNs.
Coronal sections of hippocampus from Amigo2-Cre mice injected in dorsal hippocampus with a Cre-dependent AAV to express YFP (shown in green) in CA2. a, Coronal section of ventral hippocampus (about 2.8 mm caudal to bregma; see Fig. 54 of ref. 9 for a reference image) showing CA2 axons (green) from dorsal CA2. Note absence of YFP from ventral CA2 neurons (RGS14 stain in red). b, 97.22 ± 0.46% of YFP+ cells (n = 4 mice, 2,948 cells) express the CA2 marker PCP4 (red). c, 98.45 ± 0.33% of YFP+ cells (n = 4 mice, 2,870 cells) express the CA2 marker STEP (red). d, Almost no YFP+ cells (0.17 ± 0.13%; n = 4 mice, 2,870 cells) express the CA1 marker WFS1 (red). e–g, Magnification of boxed area in b, showing YFP signal (e), PCP4 staining (f) and a merge of the two (g). h–j, Magnification of boxed area in c, showing YFP signal (h), STEP staining (i) and a merge of the two (j). k–m, Magnification of boxed area in d, showing YFP signal (k), WFS1 staining (l) and a merge of the two (m). Nissl stain shown in blue. Scale bars, 400 μm (a–d); 100 μm (e–m).
Extended Data Figure 3 Amigo2-Cre mice express Cre in RGS14+ CA2 PNs but not in GABA+ inhibitory neurons.
Cre+ neurons expressing YFP (shown in green) co-label with RGS14 staining (shown in red), but do not co-label with GABA staining (shown in red in separate images). a, Reproduction of section −1.06 mm shown in Fig. 1b. b, e, Magnification of boxed area in a. c, RGS14 staining of section shown in b. d, Merge of b and c, showing YFP and RGS14 co-labelling. f, GABA staining of section shown in e. g, Merge of e and f, showing no overlap of GABA and YFP. h, Reproduction of section −1.46 mm shown in Fig. 1b. i, l, Magnification of boxed area in h. j, RGS14 staining of section shown in i. k, Merge of i and j, demonstrating YFP and RGS14 co-labelling. m, GABA staining of section shown in l. n, Merge of l and m, showing no overlap of GABA and YFP. o, Reproduction of section −2.18 mm shown in Fig. 1b. p, s, Magnification of boxed area in o. q, RGS14 staining of section shown in p. r, Merge of p and q, demonstrating YFP and RGS14 co-labelling. t, GABA staining of section shown in s. u, Merge of s and t, showing no overlap of GABA and YFP. Scale bars, 200 μm. Nissl stain shown in blue.
Extended Data Figure 4 Specificity of the pseudotyped rabies virus.
a, b, No labelled cells were observed (n = 3 mice) after injection of the (EnvA)SAD-ΔG-mCherry virus when TVA was not expressed in CA2. b, Magnification of boxed area in a. Rabies labelling would have appeared in magenta; Nissl stain shown in green. Scale bars, 200 μm.
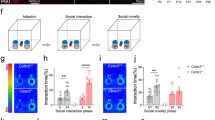
Extended Data Figure 5 Inactivation of CA2 does not alter locomotor activity or anxiety-like behaviour.
a, There was no significant difference (P = 0.31, two-tailed unpaired Student’s t-test) between CA2-YFP and CA2-TeNT groups in the distance travelled in the open field (OF) test (YFP, 53.14 ± 4.62 m, n = 8; TeNT, 47.04 ± 3.70 m, n = 10). b, There was also no significant difference (P = 0.55, two-tailed unpaired Student’s t-test) between the groups in the number of rearing events recorded during the OF session (YFP, 378.0 ± 17.36, n = 8; TeNT, 354.7 ± 30.99, n = 10). c, d, Inactivation of CA2 did not alter anxiety-like behaviour measured in the elevated plus maze (EPM). The number of open arm entries was not significantly different (P > 0.99, two-tailed unpaired Student’s t-test) between the groups (YFP, 14.00 ± 1.46, n = 8; TeNT, 14.00 ± 1.54, n = 10). Additionally, the time spent in the open arms (YFP, 163.7 ± 10.43 s, n = 8; TeNT, 155.1 ± 16.38 s, n = 10) did not differ significantly (P = 0.68, two-tailed unpaired Student’s t-test) between the groups. Results are means ± s.e.m.
Extended Data Figure 6 Spatial learning and memory assayed with the Morris water maze task is unaltered by CA2 inactivation.
a, Diagram of the experimental design. On days 1 and 2 mice were trained to find a platform with a visible flag. On days 3–7 mice were trained to find a hidden platform located in the southwest quadrant of the water maze. Spatial memory was assayed on day 8 with the platform removed. Reversal training was conducted on days 9–13 with the platform now hidden in the northwest quadrant. Spatial memory of the novel location was tested on day 14. b, Path length to the platform was not altered significantly by CA2 inactivation (two-way repeated-measures ANOVA: treatment × time F(11,770) = 0.67, P = 0.77; time F(11,770) = 21.87, P < 0.0001; treatment F(1,70) = 2.85, P = 0.10). c, Latency to find the platform did not differ significantly between the two groups (two-way repeated-measures ANOVA: treatment × time F(11,770) = 0.78, P = 0.66; time F(11,770) = 25.23, P < 0.0001; treatment F(1,70) = 2.84, P = 0.10). YFP, n = 8; TeNT, n = 10. d, Spatial memory during the probe trial was unaffected by CA2 inactivation. The percentage of time spent in the target quadrant (YFP, 33.00 ± 2.66%; TeNT, 38.6 ± 4.79%) was not significantly different between the two groups (P = 0.36, two-tailed unpaired Student’s t-test). e, Spatial memory after reversal training was unaffected by CA2 inactivation. There was no significant difference between the groups in the percentage of time spent in the target quadrant during the probe trial after reversal training (YFP, 36.38 ± 5.75%; TeNT, 36.40 ± 2.92%; P > 0.99, two-tailed unpaired Student’s t-test). Results are means ± s.e.m.
Extended Data Figure 7 Contextual fear-conditioning memory and auditory fear-conditioning memory are unaffected by inactivation of CA2.
a, Diagram of the experimental design. Delay fear conditioning was employed to test hippocampus-dependent contextual fear memory and amygdala-dependent auditory fear memory. b, There was no significant difference in percentage freezing between the groups (two-way repeated-measures ANOVA: treatment × day F(4,68) = 0.31, P = 0.87; treatment F(1,17) = 0.13, P = 0.73; day F(4,68) = 100.8, P < 0.0001; YFP, n = 11; TeNT, n = 8). Before training on day 1, neither group showed a fear response to context A (YFP, 2.45 ± 1.06%; TeNT, 0.75 ± 0.49%) or to the tone (YFP, 3.09 ± 1.31%; TeNT, 1.63 ± 0.84%). On day 2 after training, robust fear responses to context A were measured in both groups (YFP, 24.09 ± 2.88%; TeNT, 26.00 ± 4.10%). Both groups showed low levels of freezing on day 3 in novel context B (YFP, 6.55 ± 1.52%; TeNT, 4.00 ± 0.87%), demonstrating context specificity of the fear memory and a lack of fear generalization. Both groups showed robust freezing to the tone on day 3 (YFP, 35.82 ± 4.93%; TeNT, 34.63 ± 3.96%), demonstrating intact auditory fear memory. c, Freezing data plotted in 30-s bins. Shaded areas represent tone presentation. Red line represents shock delivery. Left: two-way repeated-measures ANOVA revealed no significant difference between groups in freezing on day 1 (treatment × time F(6,102) = 1.135, P = 0.3474; treatment F(1,17) = 1.116, P = 0.3056; time F(6,102) = 6.348, P < 0.0001). Middle: two-way repeated-measures ANOVA revealed no significant difference between groups in freezing on day 2 (treatment × time F(9,153) = 0.9741, P = 0.4637; treatment F(1,17) = 0.1326, P = 0.7203; time F(9,153) = 6.335, P < 0.0001). Right: two-way repeated-measures ANOVA revealed no significant difference between groups in freezing on day 3 (treatment × time F(7,119) = 0.2490, P = 0.9716; treatment F(1,17) = 0.6517, P = 0.4307; time F(7,119) = 50.87, P < 0.0001). Results are means ± s.e.m.
Extended Data Figure 8 Object recognition memory and preference for novelty is preserved in CA2-TeNT animals.
a, Diagram of the experimental design for the novel-object-recognition task. b, The groups did not differ significantly in exploration of object 1 (YFP, 16.75 ± 1.57 s; TeNT, 19.60 ± 2.24 s) or object 2 (YFP, 16.50 ± 1.97 s; TeNT, 15.90 ± 1.66 s) averaged over the course of the first four trials (two-way ANOVA: treatment × object F(1,32) = 0.80, P = 0.38; object F(1,32) = 1.05, P = 0.31; treatment F(1,32) = 0.34, P = 0.56; YFP, n = 8; TeNT, n = 10). c, Both groups explored the novel object (YFP, 21.23 ± 2.37 s; TeNT, 24.37 ± 2.81 s) more than the familiar object (YFP, 7.41 ± 0.92 s; TeNT, 8.57 ± 1.48 s). Statistical analysis revealed a significant effect of object, but not CA2 inactivation or interaction of the two (two-way ANOVA: treatment × object F(1,28) = 0.22, P = 0.64; object F(1,28) = 48.46, P < 0.0001; treatment F(1,28) = 1.02, P = 0.32). Multiple comparison testing revealed a significant difference between exploration of the novel object compared with exploration of the old object for both the YFP group (P = 0.0002) and the TeNT group (P < 0.0001). d, Diagram of the experimental design for another variation of the novel-object-recognition task. e, The groups did not differ significantly in time spent exploring object 1 (YFP, 21.50 ± 2.31 s; TeNT, 22.18 ± 3.57 s) or object 2 (YFP, 22.02 ± 2.23 s; TeNT, 22.36 ± 2.81 s) during trial 1 of day 4 (two-way ANOVA: treatment × object F(1,44) = 0.004, P = 0.95; object F(1,44) = 0.02, P = 0.90; treatment F(1,44) = 0.03, P = 0.85; YFP, n = 12; TeNT, n = 12). f, Both groups explored the novel object (YFP, 21.49 ± 1.91 s; TeNT, 22.73 ± 1.82 s) more than the familiar object (YFP, 13.74 ± 1.83 s; TeNT, 16.53 ± 1.64 s). Statistical analysis revealed a significant effect of object, but not CA2 inactivation or interaction of the two (two-way ANOVA: treatment × object F(1,44) = 0.18, P = 0.67; object F(1,44) = 15.02, P = 0.0004; treatment F(1,44) = 1.25, P = 0.27). Multiple comparison testing revealed a significant difference between exploration of the novel object compared with exploration of the old object for both the YFP group (P = 0.008) and the TeNT group (P = 0.02). Results are means ± s.e.m.
Extended Data Figure 9 Olfaction is unaffected by CA2 inactivation.
a, There was no significant difference between the groups in latency to find a buried food pellet (YFP, 63.93 ± 8.22 s, n = 15; TeNT, 67.06 ± 9.42 s, n = 16; P = 0.81, two-tailed unpaired Student’s t-test). b, There was no significant difference between the groups (YFP, n = 15; TeNT, n = 14) in performance on the olfactory habituation/dishabituation task (two-way repeated-measures ANOVA: treatment × trial F(11,297) = 0.933, P = 0.51; treatment F(1,27) = 0.08, P = 0.78; trial F(11,297) = 60.21, P < 0.0001). Results are means ± s.e.m.
Rights and permissions
About this article
Cite this article
Hitti, F., Siegelbaum, S. The hippocampal CA2 region is essential for social memory. Nature 508, 88–92 (2014). https://doi.org/10.1038/nature13028
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13028
This article is cited by
-
Molecular profiling of the hippocampus of children with autism spectrum disorder
Molecular Psychiatry (2024)
-
Silencing CA1 pyramidal cells output reveals the role of feedback inhibition in hippocampal oscillations
Nature Communications (2024)
-
A prefrontal-thalamic circuit encodes social information for social recognition
Nature Communications (2024)
-
A zinc finger transcription factor enables social behaviors while controlling transposable elements and immune response in prefrontal cortex
Translational Psychiatry (2024)
-
A new AAV tool for highly preferentially targeting hippocampal CA2
Molecular Brain (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.