Abstract
Left ventricular non-compaction (LVNC) is the third most prevalent cardiomyopathy in children and its pathogenesis has been associated with the developmental defect of the embryonic myocardium. We show that patient-specific induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) generated from LVNC patients carrying a mutation in the cardiac transcription factor TBX20 recapitulate a key aspect of the pathological phenotype at the single-cell level and this was associated with perturbed transforming growth factor beta (TGF-β) signalling. LVNC iPSC-CMs have decreased proliferative capacity due to abnormal activation of TGF-β signalling. TBX20 regulates the expression of TGF-β signalling modifiers including one known to be a genetic cause of LVNC, PRDM16, and genome editing of PRDM16 caused proliferation defects in iPSC-CMs. Inhibition of TGF-β signalling and genome correction of the TBX20 mutation were sufficient to reverse the disease phenotype. Our study demonstrates that iPSC-CMs are a useful tool for the exploration of pathological mechanisms underlying poorly understood cardiomyopathies including LVNC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Kohli, S. K. et al. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur. Heart J. 29, 89–95 (2008).
Nugent, A. W. et al. The epidemiology of childhood cardiomyopathy in Australia. N. Engl. J. Med. 348, 1639–1646 (2003).
Sedmera, D., Pexieder, T., Vuillemin, M., Thompson, R. P. & Anderson, R. H. Developmental patterning of the myocardium. Anat. Rec. 258, 319–337 (2000).
Chin, T. K., Perloff, J. K., Williams, R. G., Jue, K. & Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 82, 507–513 (1990).
Kosaka, Y. et al. 14-3-3epsilon plays a role in cardiac ventricular compaction by regulating the cardiomyocyte cell cycle. Mol. Cell. Biol. 32, 5089–5102 (2012).
Chen, Q. et al. Smad7 is required for the development and function of the heart. J. Biol. Chem. 284, 292–300 (2009).
DiMichele, L. A. et al. Transient expression of FRNK reveals stage-specific requirement for focal adhesion kinase activity in cardiac growth. Circ. Res. 104, 1201–1208 (2009).
Bartram, U. et al. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-β2-knockout mice. Circulation 103, 2745–2752 (2001).
Shou, W. et al. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature 391, 489–492 (1998).
Grego-Bessa, J. et al. Notch signaling is essential for ventricular chamber development. Dev. Cell 12, 415–429 (2007).
Luxan, G. et al. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat. Med. 19, 193–201 (2013).
Arndt, A. K. et al. Fine mapping of the 1p36 deletion syndrome identifies mutation of PRDM16 as a cause of cardiomyopathy. Am. J. Hum. Genet. 93, 67–77 (2013).
Chakraborty, S. & Yutzey, K. E. Tbx20 regulation of cardiac cell proliferation and lineage specialization during embryonic and fetal development in vivo. Dev. Biol. 363, 234–246 (2012).
Takeuchi, J. K. et al. Tbx20 dose-dependently regulates transcription factor networks required for mouse heart and motoneuron development. Development 132, 2463–2474 (2005).
Hammer, S. et al. Characterization of TBX20 in human hearts and its regulation by TFAP2. J. Cell. Biochem. 104, 1022–1033 (2008).
Posch, M. G. et al. A gain-of-function TBX20 mutation causes congenital atrial septal defects, patent foramen ovale and cardiac valve defects. J. Med. Genet. 47, 230–235 (2010).
Liu, C. et al. T-box transcription factor TBX20 mutations in Chinese patients with congenital heart disease. Eur. J. Med. Genet. 51, 580–587 (2008).
Qian, L. et al. Transcription factor neuromancer/TBX20 is required for cardiac function in Drosophila with implications for human heart disease. Proc. Natl Acad. Sci. USA 105, 19833–19838 (2008).
Kirk, E. P. et al. Mutations in cardiac T-box factor gene TBX20 are associated with diverse cardiac pathologies, including defects of septation and valvulogenesis and cardiomyopathy. Am. J. Hum. Genet. 81, 280–291 (2007).
Chen, G. et al. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods 8, 424–429 (2011).
Churko, J. M., Burridge, P. W. & Wu, J. C. Generation of human iPSCs from human peripheral blood mononuclear cells using non-integrative Sendai virus in chemically defined conditions. Methods Mol. Biol. 1036, 81–88 (2013).
Burridge, P. W. et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 11, 855–860 (2014).
Wu, H. et al. Epigenetic regulation of phosphodiesterases 2A and 3A underlies compromised β-adrenergic signaling in an iPSC model of dilated cardiomyopathy. Cell Stem Cell 17, 89–100 (2015).
D’Amato, G. et al. Sequential Notch activation regulates ventricular chamber development. Nat. Cell Biol. 18, 7–20 (2016).
Engelmann, G. L., Boehm, K. D., Birchenall-Roberts, M. C. & Ruscetti, F. W. Transforming growth factor-β 1 in heart development. Mech. Dev. 38, 85–97 (1992).
Kitamura, R. et al. Stage-specific role of endogenous Smad2 activation in cardiomyogenesis of embryonic stem cells. Circ. Res. 101, 78–87 (2007).
Hauck, L. et al. Critical role for FoxO3a-dependent regulation of p21CIP1/WAF1 in response to statin signaling in cardiac myocytes. Circ. Res. 100, 50–60 (2007).
Akli, S., Zhan, S., Abdellatif, M. & Schneider, M. D. E1A can provoke G1 exit that is refractory to p21 and independent of activating Cdk2. Circ. Res. 85, 319–328 (1999).
Brooks, G., Poolman, R. A. & Li, J. M. Arresting developments in the cardiac myocyte cell cycle: role of cyclin-dependent kinase inhibitors. Cardiovasc. Res. 39, 301–311 (1998).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Sakabe, N. J. et al. Dual transcriptional activator and repressor roles of TBX20 regulate adult cardiac structure and function. Hum. Mol. Genet. 21, 2194–2204 (2012).
Sun, N. et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci. Transl. Med. 4, 130ra147 (2012).
Davis, J. et al. A tension-based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell 165, 1147–1159 (2016).
Lan, F. et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 12, 101–113 (2013).
Hinson, J. T. et al. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 349, 982–986 (2015).
Itzhaki, I. et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471, 225–229 (2011).
Wang, Y. et al. Genome editing of isogenic human induced pluripotent stem cells recapitulates long QT phenotype for drug testing. J. Am. Coll. Cardiol. 64, 451–459 (2014).
Burridge, P. W. et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 22, 547–556 (2016).
Matsa, E., Ahrens, J. H. & Wu, J. C. Human induced pluripotent stem cells as a platform for personalized and precision cardiovascular medicine. Physiol. Rev. 96, 1093–1126 (2016).
Takahata, M. et al. SKI and MEL1 cooperate to inhibit transforming growth factor-β signal in gastric cancer cells. J. Biol. Chem. 284, 3334–3344 (2009).
Warner, D. R. et al. PRDM16/MEL1: a novel Smad binding protein expressed in murine embryonic orofacial tissue. Biochim. Biophys. Acta 1773, 814–820 (2007).
Bjork, B. C., Turbe-Doan, A., Prysak, M., Herron, B. J. & Beier, D. R. Prdm16 is required for normal palatogenesis in mice. Hum. Mol. Genet. 19, 774–789 (2010).
Oechslin, E. & Jenni, R. Left ventricular non-compaction revisited: a distinct phenotype with genetic heterogeneity? Eur. Heart J. 32, 1446–1456 (2011).
Sen-Chowdhry, S. & McKenna, W. J. Left ventricular noncompaction and cardiomyopathy: cause, contributor, or epiphenomenon? Curr. Opin. Cardiol. 23, 171–175 (2008).
Kodo, K. et al. GATA6 mutations cause human cardiac outflow tract defects by disrupting semaphorin-plexin signaling. Proc. Natl Acad. Sci. USA 106, 13933–13938 (2009).
Stevenson, K. R., Coolon, J. D. & Wittkopp, P. J. Sources of bias in measures of allele-specific expression derived from RNA-sequence data aligned to a single reference genome. BMC Genomics 14, 536 (2013).
Abel, E. D. et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J. Clin. Invest. 104, 1703–1714 (1999).
Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. & Luo, L. A global double-fluorescent Cre reporter mouse. Genesis 45, 593–605 (2007).
Chen, W. P., Liu, Y. H., Ho, Y. J. & Wu, S. M. Pharmacological inhibition of TGFβ receptor improves Nkx2.5 cardiomyoblast-mediated regeneration. Cardiovasc. Res. 105, 44–54 (2015).
Hall, B. E. et al. Conditional overexpression of TGF-β1 disrupts mouse salivary gland development and function. Lab. Invest. 90, 543–555 (2010).
Chang, B. et al. 14-3-3ε gene variants in a Japanese patient with left ventricular noncompaction and hypoplasia of the corpus callosum. Gene 515, 173–180 (2013).
Markell, L. M., Masiuk, K. E., Blazanin, N. & Glick, A. B. Pharmacologic inhibition of ALK5 causes selective induction of terminal differentiation in mouse keratinocytes expressing oncogenic HRAS. Mol. Cancer Res. 9, 746–756 (2011).
Ran, F. A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Ding, Q. et al. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell 12, 238–251 (2013).
Yusa, K. Seamless genome editing in human pluripotent stem cells using custom endonuclease-based gene targeting and the piggyBac transposon. Nat. Protoc. 8, 2061–2078 (2013).
Acknowledgements
We thank N. Sun, S. Hu and J. Lee for their help with functional assessments; H. Yamagishi (Keio University, Japan) for providing plasmid; A. B. Glick (Pennsylvania State University, USA) for providing dominant-negative TGFBRII overexpression adeno virus; B. Huber, B. Patlolla and G. Berry for their help with analysing the in vivo study. We are grateful for the support provided by the Neuroscience Microscopy Service (NMS), and FACS Core at the Institute for Stem Cell Biology and Regenerative Medicine, Stanford University. This work is supported by the Uehara Memorial Foundation Research Fellowship, American Heart Association Postdoctoral Fellowship 15POST25160016 (K.K.), American Heart Association Postdoctoral Fellowship 15POST22940013 and NIH K99HL130416 (S.-G.O.), The Ministry of Education, Culture, Sports, Science and Technology in Japan (F.I.), National Institutes of Health P01 GM099130 (M.P.S.), NIH K18 HL11708301, Children’s Cardiomyopathy Foundation (D.B.), AHA Established Investigator Award, NIH R01 HL113006, NIH R01 HL130020, NIH R01 126527, NIH R01 128170, NIH R01 HL123968 and NIH R24 HL117756 (J.C.W.).
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. K.K., S.-G.O., D.B. and J.C.W. designed research, performed the experiments and wrote the manuscript; F.I., D.B. and J.C.W. recruited the patients; K.K., J.M.C. and S.-G.O. generated and performed experiments on iPSCs/ESCs with the help of A.D.E. and G.J.; K.K., F.J., K.H., K.I. and J.M.C. performed sequencing and bioinformatics analysis; S.M.W. generated NK-TGCK transgenic mouse; K.K., V.T. and I.K. generated genome-corrected iPSC lines; K.K., P.S. and O.J.A. performed and interpreted the patch clamping and calcium imaging data; M.P.S. provided scientific advice; and J.C.W. provided funding and supervised the entire research project.
Corresponding authors
Ethics declarations
Competing interests
J.C.W. is co-founder of Stem Cell Theranostics. The remaining authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Characterization of patient-specific iPSCs.
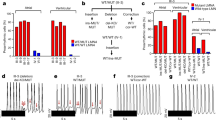
(a–b) Combined overexpression of cardiac transcription factors and wild-type, Y317∗, or T262M mutant TBX20 revealed lack of synergistic activity of mutant TBX20 protein with NKX2-5 and GATA4 (a), or TBX5 (b), on NPPA promoter in luciferase assay using HeLa cells. n = 6 independent experiments per each group. (c) TBX20 Y317∗ and T262M mutant protein could not repress the MSX2 promoter activity in luciferase assay using HeLa cells. n = 6 independent experiments per each group. (d) iPSCs derived from the family members could form teratomas when injected into a NOD/SCID mouse background, and could differentiate into all three germ layers (ectoderm, endoderm, and mesoderm) as shown by hematoxylin and eosin (HE) staining. (e) Validation of pluripotent gene expression profile using quantitative real-time PCR (qRT-PCR) in all cell lines n = 6 independent experiments per each group. (f) Immunofluorescence of representative iPSC colony derived from patient-specific fibroblasts or PBMCs staining with markers of pluripotency, including TRA-1-60 (green), OCT3/4 (red), SSEA-4 (green), and NANOG (red). Scale bars, 100 μm. (g) SNP-based analysis revealed a normal karyotype for all iPSC lines derived from the family members. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.005; ns, not significant in one-way ANOVA followed by Tukey post hoc test. The bar graphs show the mean and error bars represent s.e.m. Scale bars, 100 μm. Statistics source data can be found in Supplementary Table 12.
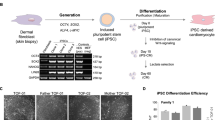
Supplementary Figure 2 Supplementary Figure 2. Characterization of patient-specific iPSC-CMs.
(a) Schematic of cardiac differentiation protocol. (b) Representative immunostaining for cardiac troponin T (TNNT2) and alpha-sarcomeric actin (αSA) demonstrating no significant differences in sarcomeric structures between iPSC-CMs from control and family members at 4 weeks after induction of cardiac differentiation. Scale bars, 20 μm. (c) Spontaneous action potentials in control, mild DCM, and LVNC iPSC-CMs at 4 weeks after induction of cardiac differentiation measured in current-clamp mode. (d–g) The results of Ca2+ imaging study using iPSC-CMs at 4 weeks. Time to peak (d), F/Frest (e), 50% decay (f), and 90% decay (g) were observed between control and patient-specific iPSC-CM lines with 1 Hz or 2 Hz electric stimulation. Data were recorded from n = 30 cells (control and mild DCM groups) or n = 60 cells (LVNC group). ∗P < 0.05; ns, not significant in one-way ANOVA. (h) Quantification of cell size for control and patient-specific iPSC-CMs (n = 60 cells per group) at 2, 4, 6, and 8 weeks after induction of cardiac differentiation. (i) Quantification of multinucleation in control and LVNC (III-4) iPSC-CMs at 2, 4, 6, and 8 weeks. n = 3 independent experiments per each group. (j) mRNA expression of sarcomere components in control and patient-specific iPSC-CMs were validated by qRT-PCR at 2 weeks after induction of cardiac differentiation. n = 6 independent experiments per each group. (k) mRNA expression profile of mesodermal transcription factors in differentiating iPSCs were validated by qRT-PCR at 2 days after induction of cardiac differentiation. n = 6 independent experiments per each group. CON, unrelated control. ∗P < 0.05; ns, not significant in one-way ANOVA followed by Tukey post hoc test. The bar graphs show the mean and error bars represent s.e.m. Statistics source data can be found in Supplementary Table 12.
Supplementary Figure 3 Gene expression profile and proliferative potential of differentiating iPSC-CMs.
(a) Relative mRNA expression profile of cardiac transcription factors including GATA4, TBX5, NKX2-5, MEF2C, TBX20, and ISL1 from day 0 to day 16 after induction of cardiac differentiation. The LVNC iPSCs showed significant decrease of mRNA expressions of cardiac transcription factors in day 6 and 9. n = 6 independent experiments per each group. ∗P < 0.05.∗∗∗P < 0.005; ns, not significant in one-way ANOVA followed by Tukey post hoc test. (b) The number (left) and population doubling level (right) of iPSC-CMs with or without serum or growth factors (IGF1, IGF2, FGF1 or NRG1) from day 14 to day 28 after induction of cardiac differentiation. n = 6 independent experiments per group. (c) The measurement of cumulative population doublings showed no significant difference in the growth speed between of control, mild DCM, and LVNC iPSC lines (n = 9, 3 and 7 independent experiments for control, mild DCM and LVNC per group respectively). The bar graphs show the mean and error bars represent s.e.m. Statistics source data can be found in Supplementary Table 12.
Supplementary Figure 4 Abnormal activation of TGFβ signaling in patient-specific LVNC iPSC-CMs.
(a) Upstream regulator analysis of growth factors comparing LVNC and control iPSC-CMs at 2 weeks. (b) Significant increase of TGFβ expression in patient-specific iPSC-CMs versus control iPSC-CMs in mRNA-sequencing. Fold-change of mRNA-sequencing data was obtained by FKPM (fragments per kilobase of exon per million) of LVNC (A-III-2, 3, 4; mean of four samples) or mild DCM (A-II-2; mean of two samples) iPSC-CMs per CON (unrelated controls; mean of two samples) iPSC-CMs. (c) mRNA expression of downstream target genes of TGFβ1 in control and patient-specific iPSC-CMs are validated by mRNA-sequencing at 2 weeks. LVNC iPSC-CMs showed increasing activity of TGFβ signaling compared with control iPSC-CMs (unrelated controls; mean of two samples, mild DCM; mean of two samples, LVNC; mean of four samples). (d–e) qRT-PCR analysis showed significant increase in TGFβ (d) and downstream target gene (e) mRNA expression in LVNC iPSC-CMs compared to control iPSC-CMs at 2 weeks. (f) Significant increase of CDKN1A expression in patient-specific iPSC-CMs versus control iPSC-CMs in mRNA-sequencing (unrelated controls; mean of two samples, mild DCM; mean of two samples, LVNC; mean of four samples). (g) Validation of mRNA expression of cyclin-dependent kinase inhibitors using qRT-PCR. †q < 0.05 in Benjamini-Hochberg correction. One-way ANOVA followed by Tukey post hoc test were performed for the validation of qRT-PCR. ∗P < 0.05.∗∗P < 0.01.∗∗∗P < 0.005; ns, not significant. The bar graphs show the mean and error bars represent s.e.m. Statistics source data can be found in Supplementary Table 12.
Supplementary Figure 5 Phenotype of cardiomyocyte-specific TGFβ1 overexpression mouse embryos.
(a) The schematic of the β1glo and αMHC-Cre transgene. (b) Activation of TGFB1 transgene in double transgenic (β1glo/αMHC-Cre) embryos at embryonic day (E) E10.5. Hearts of double transgenic embryos and wild-type littermates (-/-) were corrected and TGFβ1 and Gapdh mRNA expression were validated by RT-PCR. (c) Immunostaining of nuclear (blue), phosphor-histone H3 (phospho-HH3) (red), and αSA (green) in coronal sections of control and β1glo/αMHC-Cre double transgenic embryo hearts at E10.5. (d) Percentage of phospho-histone H3 positive (pHH3+) cardiomyocytes in compact layer of control (n = 7 hearts) and β1glo/αMHC-Cre double transgenic embryo hearts (n = 4 hearts) at E10.5. (e) The schematic of the β1glo and NK-TGCK transgene. NK enhancer/promoter-driven eGFP-Cre fusion proteins are expressed without doxycycline (DOX) and tTA proteins inhibit eGFP-Cre expression with existence of DOX. (f) Schematic of DOX-treatment protocol. (g) Cre activities of αMHC-Cre and NK-TGCK transgene with or without DOX treatment. αMHC-Cre and NK-TGCK transgenic mice were crossed with mT/mG reporter mice which express cell membrane-localized green fluorescence in Cre recombinase expressing cells. Immunostaining for nuclear (blue), αSA (red), and GFP (green) showed ∼90% Cre activity in αMHC-Cre transgenic mouse, ∼40% in DOX-untreated NK-TGCK transgenic mouse, and ∼25% in DOX-treated NK-TGCK transgenic mouse at E12.5. (h) mRNA expression of TGFB1 transgene in αMHC-Cre/β1glo (n = 6 hearts) and NK-TGCK/β1glo double transgenic mouse embryos with (n = 9 hearts) or without DOX treatment (n = 9 hearts) at E10.5. (i) mRNA expression of TGFB1 downstream target genes including Cdkn1a and Serpine1 in NK-TGCK/β1glo double transgenic mouse embryos with (n = 5 hearts) or without DOX treatment (n = 8 hearts) compared with wild-type littermates (CON; n = 5 hearts per group with or without DOX) at E10.5. (j) Immunostaining of nuclear (blue), phospho-histone H3 (phosphor-HH3) (red), and αSA (green) in coronal sections of wild-type (control) and β1glo/NE-TGCK double transgenic embryo hearts with (DOX+) or without (DOX−) doxycycline treatment at E12.5. (k) Percentage of phospho-histone H3 positive (pHH3+) cardiomyocytes in compact layer of control (n = 7 hearts) and β1glo/NE-TGCK double transgenic embryo hearts with (DTG+; n = 6 hearts) or without (DTG-; n = 7 hearts) doxycycline treatment at E12.5. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.005; ns, not significant in unpaired two-tailed t-test or one-way ANOVA followed by Tukey post hoc test. The bar graphs show the mean and error bars represent s.e.m. Scale bars, 100 μm. Statistics source data can be found in Supplementary Table 12. Unprocessed original scans of gels are shown in Supplementary Fig. 8.
Supplementary Figure 6 Modification of TGFβ signaling rescues proliferation in LVNC iPSC-CMs and TBX20 knockdown ESC-CMs.
(a) Representative immunostaining for nuclear (blue), TNNT2 (red), and EdU (green) in control and LVNC (III-3) iPSC-CMs at 2 weeks after induction of cardiac differentiation with or without treatment of TGFβ receptor-1 inhibitor (SD208 or RepSox) for 2 continuous days. (b) Representative immunostaining for nuclear (blue), TNNT2 (green), and EdU (red) in scramble and TBX20 knockdown ESC-CMs (TBX20KD ESC-CMs) at 2 weeks after induction of cardiac differentiation with or without treatment of TGFβ receptor-1 inhibitor (SD208 or RepSox) for 2 continuous days. (c) Representative immunostaining for nuclear (blue), TNNT2 (green), and EdU (red) in LVNC iPSC-CMs at 2 weeks after induction of cardiac differentiation with adenoviral mediated overexpression of GFP (GFP-Ad) or dominant negative form of TGFBRII (TGFBRIIDN-Ad). Scale bars, 100 μm.
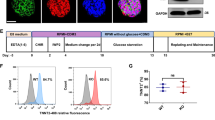
Supplementary Figure 7 Generation of TBX20 mutation corrected patient-specific iPSC lines using TALEN.
(a) TALEN recognition sites on TBX20 exon 7. TALEN pair was designed to bind to the specific sequence of mutant allele including stop-gain mutation site (c.951C > A, red character) using the rapid TALEN assembly system. (b) Sequence of left and right arms of the targeting vector. To make a targeting vector, 500 bp fragments of left and right arms were designed to share homologies with TBX20 exon 7 carrying wild-type sequence (c.951C, blue character) and flanking introns. TTAA site for piggyBac excision was created by introducing silent mutations near the intended modification site (red character in left arm). To avoid re-cleavage of substituted genomic sequence, three silent mutations (red character) were created into the TALEN recognition sites on right arm (yellow box). Both arms were cloned into a vector carrying piggyBac transposon with PGK promoter puroΔtk selection cassette (PGK-puroΔtk) to make the targeting vector. (c) Overview of targeted correction of TBX20 Y317∗ mutation using TALEN and piggyBac transposon system. TALEN-mediated double strand DNA break promotes homologous recombination of the targeting vector into cleaved site, and clones with integrated PGK-puroΔtk cassette were selected by puromycin. Subsequently, piggyBac-PGK-puroΔtk cassette was excised by transient expression of piggyBac transposase. Mutation-corrected lines were obtained after negative selection using ganciclovir treatment. Genotyping primer positions are indicated as Seq Fw, Seq Rv, 5′ Fw, 5′ Rv, 3′ Fw and 3′ Rv. d, PCR analysis showing transposon removal. Pre, pre-transfection of TALEN pairs and targeting vector; PB, after puromycin selection; Ex, after gancyclovir selection; 5′ Fw-Rv, PCR using 5′ Fw and 5′ Rv primers; 3′ Fw-Rv, PCR using 3′ Fw and 5′ Rv primers; Seq Fw-Rv, PCR using Seq Fw and Seq Rv primers. Unprocessed original scans of gels are shown in Supplementary Fig. 8. (e) Precise repair of the stop-gain mutation site. Mutation-corrected lines showed the wild-type sequence (c.951C, yellow box) instead of stop-gain mutation (c.951C > M, red box) and possessed the designed synonymous mutation as indicated in the orange box. (f) Mechanistic schema of the pathology of LVNC caused by TBX20 loss of function mutation. During the myocardium development, Tbx20 interacts with other cardiac transcription factors, including NKX2-5, GATA4, and TBX5, and initiates cardiac chamber-specific gene expression and myocardium growth. On the other hand, TBX20 inhibits activation of TGFβ signaling partially via PRDM16 and promotes embryonic cardiomyocyte proliferation. TBX20 mutation disturbs synergistic regulation of cardiac chamber specification and myocardium growth. Furthermore, failed suppression of TGFβ leads to improper upregulation of CDKN1A and promotes early exit of cardiomyocyte cell cycle. Decreased ventricular cardiomyocyte specification and proliferation leads to failed formation of compact layer of developing myocardium and results in LVNC.
Supplementary Figure 8 Unprocessed original scans.
Uncropped images of scanned western blots and gels shown in the Figures are provided.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1080 kb)
Supplementary Table 1
Supplementary Information (XLSX 10 kb)
Supplementary Table 2
Supplementary Information (XLSX 10 kb)
Supplementary Table 3
Supplementary Information (XLSX 10 kb)
Supplementary Table 4
Supplementary Information (XLSX 11 kb)
Supplementary Table 5
Supplementary Information (XLSX 20 kb)
Supplementary Table 6
Supplementary Information (XLSX 9 kb)
Supplementary Table 7
Supplementary Information (XLSX 9 kb)
Supplementary Table 8
Supplementary Information (XLSX 64 kb)
Supplementary Table 9
Supplementary Information (XLSX 18 kb)
Supplementary Table 10
Supplementary Information (XLSX 11 kb)
Supplementary Table 11
Supplementary Information (XLSX 11 kb)
Supplementary Table 12
Supplementary Information (XLSX 115 kb)
Rights and permissions
About this article
Cite this article
Kodo, K., Ong, SG., Jahanbani, F. et al. iPSC-derived cardiomyocytes reveal abnormal TGF-β signalling in left ventricular non-compaction cardiomyopathy. Nat Cell Biol 18, 1031–1042 (2016). https://doi.org/10.1038/ncb3411
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3411
This article is cited by
-
Cardiac manifestations of human ACTA2 variants recapitulated in a zebrafish model
Journal of Human Genetics (2024)
-
Endothelial deletion of PTBP1 disrupts ventricular chamber development
Nature Communications (2023)
-
Human centenarian–associated SIRT6 mutants modulate hepatocyte metabolism and collagen deposition in multilineage hepatic 3D spheroids
GeroScience (2023)
-
Modeling Nonischemic Genetic Cardiomyopathies Using Induced Pluripotent Stem Cells
Current Cardiology Reports (2022)
-
Decellularized extracellular matrix mediates tissue construction and regeneration
Frontiers of Medicine (2022)