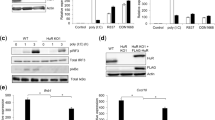
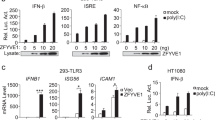
Abstract
Toll-like receptor 4 (TLR4) induces two distinct signaling pathways controlled by the TIRAP-MyD88 and TRAM-TRIF pairs of adaptor proteins, which elicit the production of proinflammatory cytokines and type I interferons, respectively. How TLR4 coordinates the activation of these two pathways is unknown. Here we show that TLR4 activated these two signaling pathways sequentially in a process organized around endocytosis of the TLR4 complex. We propose that TLR4 first induces TIRAP-MyD88 signaling at the plasma membrane and is then endocytosed and activates TRAM-TRIF signaling from early endosomes. Our data emphasize a unifying theme in innate immune recognition whereby all type I interferon–inducing receptors signal from an intracellular location.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Janeway, C.A. Jr & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Hirotani, T. et al. Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-β. Biochem. Biophys. Res. Commun. 328, 383–392 (2005).
Yamamoto, M. et al. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301, 640–643 (2003).
Yamamoto, M. et al. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat. Immunol. 4, 1144–1150 (2003).
Yamamoto, M. et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 420, 324–329 (2002).
Horng, T., Barton, G.M., Flavell, R.A. & Medzhitov, R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420, 329–333 (2002).
Barton, G.M., Kagan, J.C. & Medzhitov, R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 7, 49–56 (2006).
Latz, E. et al. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5, 190–198 (2004).
Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103 (2001).
Toshchakov, V. et al. TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages. Nat. Immunol. 3, 392–398 (2002).
Alexopoulou, L., Holt, A.C., Medzhitov, R. & Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
Lund, J.M. et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101, 5598–5603 (2004).
Diebold, S.S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
Kawai, T. & Akira, S. Antiviral signaling through pattern recognition receptors. J. Biochem. 141, 137–145 (2007).
Honda, K. et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434, 772–777 (2005).
Kawai, T. et al. Interferon-α induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5, 1061–1068 (2004).
Doyle, S. et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17, 251–263 (2002).
Oshiumi, H., Matsumoto, M., Funami, K., Akazawa, T. & Seya, T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-β induction. Nat. Immunol. 4, 161–167 (2003).
Barton, G.M. & Medzhitov, R. Toll-like receptor signaling pathways. Science 300, 1524–1525 (2003).
Takaoka, A. et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 448, 501–505 (2007).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
O'Neill, L.A. & Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7, 353–364 (2007).
Kagan, J.C. & Medzhitov, R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 125, 943–955 (2006).
Ulrichts, P., Peelman, F., Beyaert, R. & Tavernier, J. MAPPIT analysis of TLR adaptor complexes. FEBS Lett. 581, 629–636 (2007).
Oshiumi, H. et al. TIR-containing adapter molecule (TICAM)-2, a bridging adapter recruiting to toll-like receptor 4 TICAM-1 that induces interferon-β. J. Biol. Chem. 278, 49751–49762 (2003).
Rowe, D.C. et al. The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc. Natl. Acad. Sci. USA 103, 6299–6304 (2006).
Husebye, H. et al. Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 25, 683–692 (2006).
Praefcke, G.J. & McMahon, H.T. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 5, 133–147 (2004).
Akashi, S. et al. Lipopolysaccharide interaction with cell surface Toll-like receptor 4-MD-2: higher affinity than that with MD-2 or CD14. J. Exp. Med. 198, 1035–1042 (2003).
Macia, E. et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10, 839–850 (2006).
Boll, W., Ehrlich, M., Collier, R.J. & Kirchhausen, T. Effects of dynamin inactivation on pathways of anthrax toxin uptake. Eur. J. Cell Biol. 83, 281–288 (2004).
Damke, H., Baba, T., van der Bliek, A.M. & Schmid, S.L. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J. Cell Biol. 131, 69–80 (1995).
Seeger, M. & Payne, G.S. A role for clathrin in the sorting of vacuolar proteins in the Golgi complex of yeast. EMBO J. 11, 2811–2818 (1992).
Racoosin, E.L. & Swanson, J.A. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J. Cell Biol. 121, 1011–1020 (1993).
Radhakrishna, H. & Donaldson, J.G. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J. Cell Biol. 139, 49–61 (1997).
McDonald, P.H. et al. β-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science 290, 1574–1577 (2000).
Sandilands, E., Brunton, V.G. & Frame, M.C. The membrane targeting and spatial activation of Src, Yes and Fyn is influenced by palmitoylation and distinct RhoB/RhoD endosome requirements. J. Cell Sci. 120, 2555–2564 (2007).
Martin, T.F. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu. Rev. Cell Dev. Biol. 14, 231–264 (1998).
Oganesyan, G. et al. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439, 208–211 (2006).
Hacker, H. et al. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439, 204–207 (2006).
Rothe, M., Sarma, V., Dixit, V.M. & Goeddel, D.V. TRAF2-mediated activation of NF-κB by TNF receptor 2 and CD40. Science 269, 1424–1427 (1995).
Botelho, R.J. et al. Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J. Cell Biol. 151, 1353–1368 (2000).
Nunez Miguel, R. et al. A dimer of the Toll-like receptor 4 cytoplasmic domain provides a specific scaffold for the recruitment of signalling adaptor proteins. PLoS ONE 2, e788 (2007).
Vieira, A.V., Lamaze, C. & Schmid, S.L. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science 274, 2086–2089 (1996).
Schneider-Brachert, W. et al. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity 21, 415–428 (2004).
Xu, Y., Cheng, G. & Baltimore, D. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity 5, 407–415 (1996).
Hostager, B.S., Catlett, I.M. & Bishop, G.A. Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J. Biol. Chem. 275, 15392–15398 (2000).
Kagan, J.C. & Roy, C.R. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nat. Cell Biol. 4, 945–954 (2002).
Horng, T., Barton, G.M. & Medzhitov, R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2, 835–841 (2001).
Acknowledgements
We thank K. Miyake (Institute for Medical Sciences, University of Toyko) for Sa15-21; S. Akira (Osaka University) for TRAM-KO mice; C. Roy (Yale University) for Rab5 plasmids; T. Kirchhausen (Immune Disease Institute and Harvard Medical School) for dynasore; L. Marek, D. Hargreaves and C. Sokol for discussions; and T. Medjitov for help with bioinformatics analysis. Supported by the National Institutes of Health (1K99AI072955-01 to J.C.K., and R37 AI046688, P01 AI44220 and AI 061360 to R.M.) and the Howard Hughes Medical Institute (R.M.).
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figure 1 (PDF 994 kb)
Rights and permissions
About this article
Cite this article
Kagan, J., Su, T., Horng, T. et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat Immunol 9, 361–368 (2008). https://doi.org/10.1038/ni1569
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1569
This article is cited by
-
IKKε and TBK1 prevent RIPK1 dependent and independent inflammation
Nature Communications (2024)
-
A second-generation M1-polarized CAR macrophage with antitumor efficacy
Nature Immunology (2024)
-
The crucial regulatory role of type I interferon in inflammatory diseases
Cell & Bioscience (2023)
-
Scavenger receptor B1 facilitates the endocytosis of Escherichia coli via TLR4 signaling in mammary gland infection
Cell Communication and Signaling (2023)
-
Distinct changes in endosomal composition promote NLRP3 inflammasome activation
Nature Immunology (2023)