Abstract
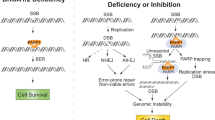
Synthetic lethality provides a potential mechanistic framework for the therapeutic targeting of genetic and functional deficiencies in cancers and is now being explored widely. The first clinical exemplification of synthetic lethality in cancer has been the exploitation of inhibitors of poly-(ADP-ribose) polymerase (PARP) for the treatment of cancers with defects in the BRCA1 or BRCA2 tumor suppressor proteins, which are involved in the repair of DNA damage. Although this approach has shown promise, multiple potential resistance mechanisms have been identified. In this Perspective, we discuss these mechanisms and their relevance to the development of selective therapies for BRCA-deficient cancers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Greaves, M. & Maley, C.C. Clonal evolution in cancer. Nature 481, 306–313 (2012).
Ashworth, A., Lord, C.J. & Reis-Filho, J.S. Genetic interactions in cancer progression and treatment. Cell 145, 30–38 (2011).
Stratton, M.R. & Rahman, N. The emerging landscape of breast cancer susceptibility. Nat. Genet. 40, 17–22 (2008).
TCGA. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
Dobzhansky, T. Genetics of natural populations. Xiii. Recombination and variability in populations of Drosophila pseudoobscura. Genetics 31, 269–290 (1946).
Hartwell, L.H., Szankasi, P., Roberts, C.J., Murray, A.W. & Friend, S.H. Integrating genetic approaches into the discovery of anticancer drugs. Science 278, 1064–1068 (1997).
Kaelin, W.G. Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat. Rev. Cancer 5, 689–698 (2005).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Bryant, H.E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005).
Fong, P.C. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009).
Fong, P.C. et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 28, 2512–2519 (2010).
Audeh, M.W. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376, 245–251 (2010).
Balmaña, J., Domchek, S.M., Tutt, A. & Garber, J.E. Stumbling blocks on the path to personalized medicine in breast cancer: the case of PARP inhibitors for BRCA1/2-associated cancers. Cancer Discov. 1, 29–34 (2011).
Lord, C.J. & Ashworth, A. The DNA damage response and cancer therapy. Nature 481, 287–294 (2012).
Gelmon, K.A. et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 12, 852–861 (2011).
McCabe, N. et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 66, 8109–8115 (2006).
Vilar, E. et al. MRE11 deficiency increases sensitivity to poly(ADP-ribose) polymerase inhibition in microsatellite unstable colorectal cancers. Cancer Res. 71, 2632–2642 (2011).
Mendes-Pereira, A.M. et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol. Med. 1, 315–322 (2009).
Brenner, J.C. et al. Mechanistic rationale for inhibition of poly(ADP-ribose) polymerase in ETS gene fusion–positive prostate cancer. Cancer Cell 19, 664–678 (2011).
Brenner, J.C. et al. PARP-1 inhibition as a targeted strategy to treat Ewing's sarcoma. Cancer Res. 72, 1608–1613 (2012).
Byers, L.A. et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2, 798–811 (2012).
Edwards, S.L. et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature 451, 1111–1115 (2008).
Sakai, W. et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 451, 1116–1120 (2008).
Ikeda, H. et al. Genetic reversion in an acute myelogenous leukemia cell line from a Fanconi anemia patient with biallelic mutations in BRCA2. Cancer Res. 63, 2688–2694 (2003).
Swisher, E.M. et al. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 68, 2581–2586 (2008).
Norquist, B. et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J. Clin. Oncol. 29, 3008–3015 (2011).
Barber, L.J. et al. Secondary mutations in BRCA2 associated with clinical resistance to a PARP inhibitor. J. Pathol. 229, 422–429 (2013).
Issaeva, N. et al. 6-thioguanine selectively kills BRCA2-defective tumors and overcomes PARP inhibitor resistance. Cancer Res. 70, 6268–6276 (2010).
Cao, L. et al. A selective requirement for 53BP1 in the biological response to genomic instability induced by Brca1 deficiency. Mol. Cell 35, 534–541 (2009).
Bunting, S.F. et al. BRCA1 functions independently of homologous recombination in DNA interstrand crosslink repair. Mol. Cell 46, 125–135 (2012).
Bouwman, P. et al. 53BP1 loss rescues BRCA1 deficiency and is associated with triple-negative and BRCA-mutated breast cancers. Nat. Struct. Mol. Biol. 17, 688–695 (2010).
Jaspers, J.E. et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 3, 68–81 (2013).
Zimmermann, M., Lottersberger, F., Buonomo, S.B., Sfeir, A. & de Lange, T. 53BP1 regulates DSB repair using Rif1 to control 5′ end resection. Science 339, 700–704 (2013).
Di Virgilio, M. et al. Rif1 prevents resection of DNA breaks and promotes immunoglobulin class switching. Science 339, 711–715 (2013).
Chapman, J.R. et al. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol. Cell 49, 858–871 (2013).
Escribano-Díaz, C. et al. A cell cycle–dependent regulatory circuit composed of 53BP1–RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Mol. Cell 49, 872–883 (2013).
Nakada, S., Yonamine, R.M. & Matsuo, K. RNF8 regulates assembly of RAD51 at DNA double-strand breaks in the absence of BRCA1 and 53BP1. Cancer Res. 72, 4974–4983 (2012).
Pennington, K.P. et al. 53BP1 expression in sporadic and inherited ovarian carcinoma: Relationship to genetic status and clinical outcomes. Gynecol. Oncol. 128, 493–499 (2013).
Choi, Y.H. & Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. published online, doi:10.2174/13816128113199990005 (13 May 2013).
Rottenberg, S. et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. USA 105, 17079–17084 (2008).
Oplustilova, L. et al. Evaluation of candidate biomarkers to predict cancer cell sensitivity or resistance to PARP-1 inhibitor treatment. Cell Cycle 11, 3837–3850 (2012).
Murai, J. et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 72, 5588–5599 (2012).
Satoh, M.S. & Lindahl, T. Role of poly(ADP-ribose) formation in DNA repair. Nature 356, 356–358 (1992).
McCabe, N. et al. BRCA2-deficient CAPAN-1 cells are extremely sensitive to the inhibition of poly(ADP-Ribose) polymerase: an issue of potency. Cancer Biol. Ther. 4, 934–936 (2005).
Pettitt, S.J. et al. A genetic screen using the piggyBac transposon in haploid cells identifies Parp1 as a mediator of olaparib toxicity. PLoS ONE 8, e61520 (2013).
Patel, A.G. et al. Enhanced killing of cancer cells by poly(ADP-ribose) polymerase inhibitors and topoisomerase I inhibitors reflects poisoning of both enzymes. J. Biol. Chem. 287, 4198–4210 (2012).
Liu, X. et al. Acquired resistance to combination treatment with temozolomide and ABT-888 is mediated by both base excision repair and homologous recombination DNA repair pathways. Mol. Cancer Res. 7, 1686–1692 (2009).
Klauke, M.L. et al. Higher cytoplasmic and nuclear poly(ADP-ribose) polymerase expression in familial than in sporadic breast cancer. Virchows Arch. 461, 425–431 (2012).
Gottipati, P. et al. Poly(ADP-ribose) polymerase is hyperactivated in homologous recombination-defective cells. Cancer Res. 70, 5389–5398 (2010).
Drost, R. et al. BRCA1 RING function is essential for tumor suppression but dispensable for therapy resistance. Cancer Cell 20, 797–809 (2011).
Graeser, M. et al. A marker of homologous recombination predicts pathologic complete response to neoadjuvant chemotherapy in primary breast cancer. Clin. Cancer Res. 16, 6159–6168 (2010).
Hidalgo, M. et al. A pilot clinical study of treatment guided by personalized tumorgrafts in patients with advanced cancer. Mol. Cancer Ther. 10, 1311–1316 (2011).
Luo, J., Solimini, N.L. & Elledge, S.J. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136, 823–837 (2009).
Tischler, J., Lehner, B. & Fraser, A.G. Evolutionary plasticity of genetic interaction networks. Nat. Genet. 40, 390–391 (2008).
Kumar, M.S. et al. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell 149, 642–655 (2012).
Brough, R. et al. Functional viability profiles of breast cancer. Cancer Discov. 1, 260–273 (2011).
Acknowledgements
We thank Cancer Research UK, Breakthrough Breast Cancer, the American Association for Cancer Research as part of the Stand Up to Cancer Breast Cancer Dream Team, The Komen Foundation, The Breast Cancer Research Foundation, The Wellcome Trust and the European Union as part of the FP7 DNA Damage Response (DDR) and EUROCAN programmes for funding our work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
C.J.L. and A.A. are named inventors on patents describing the use of PARP inhibitors and stand to gain from their development as part of the Institute of Cancer Research “Rewards to Inventors” scheme.
Rights and permissions
About this article
Cite this article
Lord, C., Ashworth, A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat Med 19, 1381–1388 (2013). https://doi.org/10.1038/nm.3369
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3369
This article is cited by
-
Genetic separation of Brca1 functions reveal mutation-dependent Polθ vulnerabilities
Nature Communications (2023)
-
Loss of the receptors ER, PR and HER2 promotes USP15-dependent stabilization of PARP1 in triple-negative breast cancer
Nature Cancer (2023)
-
Sequential targeting of PARP with carboplatin inhibits primary tumour growth and distant metastasis in triple-negative breast cancer
British Journal of Cancer (2023)
-
Suicide gene strategies applied in ovarian cancer studies
Cancer Gene Therapy (2023)
-
Inhibition of USP7 induces p53-independent tumor growth suppression in triple-negative breast cancers by destabilizing FOXM1
Cell Death & Differentiation (2023)