Abstract
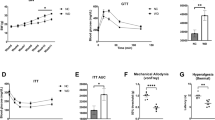
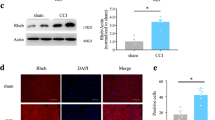
Lysophosphatidic acid (LPA) is a bioactive lipid with activity in the nervous system mediated by G-protein-coupled receptors. Here, we examined the role of LPA signaling in the development of neuropathic pain by pharmacological and genetic approaches, including the use of mice lacking the LPA1 receptor. Wild-type animals with nerve injury develop behavioral allodynia and hyperalgesia paralleled by demyelination in the dorsal root and increased expression of both the protein kinase C γ-isoform within the spinal cord dorsal horn and the α2δ1 calcium channel subunit in dorsal root ganglia. Intrathecal injection of LPA induced behavioral, morphological and biochemical changes similar to those observed after nerve ligation. In contrast, mice lacking a single LPA receptor (LPA1, also known as EDG2) that activates the Rho–Rho kinase pathway do not develop signs of neuropathic pain after peripheral nerve injury. Inhibitors of Rho and Rho kinase also prevented these signs of neuropathic pain. These results imply that receptor-mediated LPA signaling is crucial in the initiation of neuropathic pain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
20 June 2004
Replaced Figure 2c, corrected text
Notes
NOTE: In the version of this article initially published online, on p. 715, at the beginning of the second column, the antisense oligonucleotide was identified incorrectly. The first complete sentence of the column should begin, “Moreover, preinjury injection with LPA1 AS-ODN...” In addition, the bottom curve in Figure 2c was labeled incorrectly. The label should read “Wild-type”. These errors have been corrected for the HTML and print versions of the article.
References
Bridges, D., Thompson, S.W. & Rice, A.S. Mechanisms of neuropathic pain. Br. J. Anaesth. 87, 12–26 (2001).
Woolf, C.J. & Mannion, R.J. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 353, 1959–1964 (1999).
Cui, J.G., Holmin, S., Mathiesen, T., Meyerson, B.A. & Linderoth, B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain 88, 239–248 (2000).
Sommer, C. & Schafers, M. Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 784, 154–162 (1998).
Pezet, S., Malcangio, M. & McMahon, S.B. BDNF: a neuromodulator in nociceptive pathways? Brain Res. Brain Res. Rev. 40, 240–249 (2002).
Bennett, G.J. Does a neuroimmune interaction contribute to the genesis of painful peripheral neuropathies? Proc. Natl. Acad. Sci. USA 96, 7737–7738 (1999).
Ma, W. & Eisenach, J.C. Morphological and pharmacological evidence for the role of peripheral prostaglandins in the pathogenesis of neuropathic pain. Eur. J. Neurosci. 15, 1037–1047 (2002).
Ramer, M.S., Thompson, S.W. & McMahon, S.B. Causes and consequences of sympathetic basket formation in dorsal root ganglia. Pain 6 (Suppl.), S111–S120 (1999).
Schumacher, K.A., Classen, H.G. & Spath, M. Platelet aggregation evoked in vitro and in vivo by phosphatidic acids and lysoderivatives: identity with substances in aged serum (DAS). Thromb. Haemost. 42, 631–640 (1979).
Eichholtz, T., Jalink, K., Fahrenfort, I. & Moolenaar, W.H. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem. J. 291, 677–680 (1993).
Fukushima, N., Ishii, I., Contos, J.J., Weiner, J.A. & Chun, J. Lysophospholipid receptors. Annu. Rev. Pharmacol. Toxicol. 41, 507–534 (2001).
Ishii, I., Fukukshima, N., Ye, X. & Chun, J. Lysophospholipid receptors: signaling and biology. Annu. Rev. Biochem. (in press).
Kusaka, S., Kapousta-Bruneau, N., Green, D.G. & Puro, D.G. Serum-induced changes in the physiology of mammalian retinal glial cells: role of lysophosphatidic acid. J. Physiol. 506, 445–458 (1998).
Ye, X., Fukushima, N., Kingsbury, M.A. & Chun, J. Lysophosphatidic acid in neural signaling. Neuroreport 13, 2169–2175 (2002).
Fukushima, N., Ye, X. & Chun, J. Neurobiology of lysophosphatidic acid signaling. Neuroscientist 8, 540–550 (2002).
Kingsbury, M.A., Rehen, S.K., Contos, J.J., Higgins, C.M. & Chun, J. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat. Neurosci. 6, 1292–1299 (2003).
Ishii, I., Contos, J.J., Fukushima, N. & Chun, J. Functional comparisons of the lysophosphatidic acid receptors, LP(A1)/VZG-1/EDG-2, LP(A2)/EDG-4, and LP(A3)/EDG-7 in neuronal cell lines using a retrovirus expression system. Mol. Pharmacol. 58, 895–902 (2000).
Renback, K., Inoue, M. & Ueda, H. Lysophosphatidic acid-induced, pertussis toxin-sensitive nociception through a substance P release from peripheral nerve endings in mice. Neurosci. Lett. 270, 59–61 (1999).
Renback, K., Inoue, M., Yoshida, A., Nyberg, F. & Ueda, H. Vzg-1/lysophosphatidic acid-receptor involved in peripheral pain transmission. Brain Res. Mol. Brain Res. 75, 350–354 (2000).
Hecht, J.H., Weiner, J.A., Post, S.R. & Chun, J. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell. Biol. 135, 1071–1083 (1996).
Fukushima, N., Kimura, Y. & Chun, J. A single receptor encoded by vzg-1/lpA1/edg-2 couples to G proteins and mediates multiple cellular responses to lysophosphatidic acid. Proc. Natl. Acad. Sci. USA 95, 6151–6156 (1998).
Ye, X., Inoue, M. & Ueda, H. Botulinum toxin C3 inhibits hyperalgesia in mice with partial sciatic nerve injury. Jpn. J. Pharmacol. 83, 161–163 (2000).
Uehata, M. et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990–994 (1997).
Weiner, J.A. & Chun, J. Schwann cell survival mediated by the signaling phospholipid lysophosphatidic acid. Proc. Natl. Acad. Sci. USA 96, 5233–5238 (1999).
Ueda, H. In vivo molecular signal transduction of peripheral mechanisms of pain. Jpn. J. Pharmacol. 79, 263–268 (1999).
Rashid, M.H. et al. Novel expression of vanilloid receptor 1 on capsaicin-insensitive fibers accounts for the analgesic effect of capsaicin cream in neuropathic pain. J. Pharmacol. Exp. Ther. 304, 940–948 (2003).
Malmberg, A.B. & Basbaum, A.I. Partial sciatic nerve injury in the mouse as a model of neuropathic pain: behavioral and neuroanatomical correlates. Pain 76, 215–222 (1998).
Malmberg, A.B., Chen, C., Tonegawa, S. & Basbaum, A.I. Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science 278, 279–283 (1997).
Luo, Z.D. et al. Upregulation of dorsal root ganglion α2δ calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J. Neurosci. 21, 1868–1875 (2001).
Luo, Z.D. et al. Injury type-specific calcium channel α2 δ-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J. Pharmacol. Exp. Ther. 303, 1199–1205 (2002).
Weiner, J.A., Fukushima, N., Contos, J.J., Scherer, S.S. & Chun, J. Regulation of Schwann cell morphology and adhesion by receptor-mediated lysophosphatidic acid signaling. J. Neurosci. 21, 7069–7078 (2001).
Brancolini, C. et al. Rho-dependent regulation of cell spreading by the tetraspan membrane protein Gas3/PMP22. Mol. Biol. Cell 10, 2441–2459 (1999).
Pentland, B. & Donald, S.M. Pain in the Guillain-Barre syndrome: a clinical review. Pain 59, 159–164 (1994).
Carter, G.T. et al. Neuropathic pain in Charcot-Marie-Tooth disease. Arch. Phys. Med. Rehabil. 79, 1560–1564 (1998).
Gillespie, C.S. et al. Peripheral demyelination and neuropathic pain behavior in periaxin-deficient mice. Neuron 26, 523–531 (2000).
Hokfelt, T. et al. Neuropeptides—an overview. Neuropharmacology 39, 1337–1356 (2000).
Dickinson, T. & Fleetwood-Walker, S.M. VIP and PACAP: very important in pain? Trends Pharmacol. Sci. 20, 324–329 (1999).
Lin, Q., Peng, Y.B. & Willis, W.D. Possible role of protein kinase C in the sensitization of primate spinothalamic tract neurons. J. Neurosci. 16, 3026–3034 (1996).
Willis, W.D. Role of neurotransmitters in sensitization of pain responses. Ann. NY Acad. Sci. 933, 142–156 (2001).
Mao, J., Price, D.D., Phillips, L.L., Lu, J. & Mayer, D.J. Increases in protein kinase C γ immunoreactivity in the spinal cord dorsal horn of rats with painful mononeuropathy. Neurosci. Lett. 198, 75–78 (1995).
Miletic, V., Bowen, K.K. & Miletic, G. Loose ligation of the rat sciatic nerve is accompanied by changes in the subcellular content of protein kinase C β II and γ in the spinal dorsal horn. Neurosci. Lett. 288, 199–202 (2000).
Contos, J.J., Fukushima, N., Weiner, J.A., Kaushal, D. & Chun, J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc. Natl. Acad. Sci. USA 97, 13384–13389 (2000).
Contos, J.J. et al. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol. Cell. Biol. 22, 6921–6929 (2002).
Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16, 109–110 (1983).
Adlkofer, K. et al. Heterozygous peripheral myelin protein 22-deficient mice are affected by a progressive demyelinating tomaculous neuropathy. J. Neurosci. 17, 4662–4671 (1997).
Inoue, M. et al. In vivo pain-inhibitory role of nociceptin/orphanin FQ in spinal cord. J. Pharmacol. Exp. Ther. 305, 495–501 (2003).
Acknowledgements
We thank T. Kawashima, S. Kondo, M. Matsumoto and T. Suematsu for technical help and behavioral studies, and S. Kozaki for BoTXC3. This study was supported by Special Coordination Funds of the Science and Technology Agency of the Japanese Government and Grants-in-Aid from the Ministry of Education, Science, Culture and Sports of Japan (H.U.) and by the National Institute of Mental Health, USA (J.C., J.J.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 3
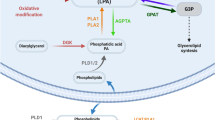
Schematic model showing role of LPA in development of neuropathic pain following peripheral nerve injury. (PDF 368 kb)
Rights and permissions
About this article
Cite this article
Inoue, M., Rashid, M., Fujita, R. et al. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med 10, 712–718 (2004). https://doi.org/10.1038/nm1060
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1060
This article is cited by
-
HSP27 Modulates Neuropathic Pain by Inhibiting P2X3 Degradation
Molecular Neurobiology (2024)
-
Different Profiles of the Triad of Lysophosphatidylcholine, Lysophosphatidic Acid, and Autotaxin in Patients with Neuropathic Pain Diseases: a Preliminary Observational Study
Pain and Therapy (2022)
-
LPA1 signaling drives Schwann cell dedifferentiation in experimental autoimmune neuritis
Journal of Neuroinflammation (2021)
-
Can a Western high-fat diet lead to painful neuropathy?
Nature Metabolism (2021)
-
Inhibition of autotaxin activity ameliorates neuropathic pain derived from lumbar spinal canal stenosis
Scientific Reports (2021)