Abstract
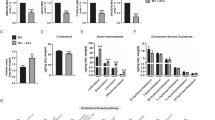
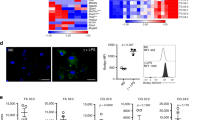
Macrophages have important roles in both lipid metabolism and inflammation and are central to the pathogenesis of atherosclerosis. The liver X receptors (LXRs) are established mediators of lipid-inducible gene expression, but their role in inflammation and immunity is unknown. We demonstrate here that LXRs and their ligands are negative regulators of macrophage inflammatory gene expression. Transcriptional profiling of lipopolysaccharide (LPS)-induced macrophages reveals reciprocal LXR-dependent regulation of genes involved in lipid metabolism and the innate immune response. In vitro, LXR ligands inhibit the expression of inflammatory mediators such as inducible nitric oxide synthase, cyclooxygenase (COX)-2 and interleukin-6 (IL-6) in response to bacterial infection or LPS stimulation. In vivo, LXR agonists reduce inflammation in a model of contact dermatitis and inhibit inflammatory gene expression in the aortas of atherosclerotic mice. These findings identify LXRs as lipid-dependent regulators of inflammatory gene expression that may serve to link lipid metabolism and immune functions in macrophages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Glass, C.K. & Witztum, J.L. Atherosclerosis. The road ahead. Cell 104, 503–516 (2001).
Lusis, A.J. Atherosclerosis. Nature 407, 233–241 (2000).
Febbraio, M. et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J. Clin. Invest. 105, 1049–1056 (2000).
Suzuki, H. et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386, 292–295 (1997).
Detmers, P.A. et al. Deficiency in inducible nitric oxide synthase results in reduced atherosclerosis in apolipoprotein E-deficient mice. J. Immunol. 165, 3430–3435 (2000).
Gu, L. et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 2, 275–281 (1998).
Kuhlencordt, P.J., Chen, J., Han, F., Astern, J. & Huang, P.L. Genetic deficiency of inducible nitric oxide synthase reduces atherosclerosis and lowers plasma lipid peroxides in apolipoprotein E-knockout mice. Circulation 103, 3099–3104 (2001).
Hansson, G.K. Inflammation and immune response in atherosclerosis. Curr. Atheroscler. Rep. 1, 150–155 (1999).
Wu, Z.L., Liang, M.Y. & Qiu, L.Q. Oxidized low-density lipoprotein decreases the induced nitric oxide synthesis in rat mesangial cells. Cell Biochem. Funct. 16, 153–158 (1998).
Liu, S.X., Chen, Y., Zhou, M. & Wan, J. Oxidized cholesterol in oxidized low density lipoprotein may be responsible for the inhibition of LPS-induced nitric oxide production in macrophages. Atherosclerosis 136, 43–49 (1998).
Nagy, L., Tontonoz, P., Alvarez, J.G., Chen, H. & Evans, R.M. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell 93, 229–240 (1998).
Tontonoz, P., Nagy, L., Alvarez, J.G., Thomazy, V.A. & Evans, R.M. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93, 241–252 (1998).
Jiang, C., Ting, A.T. & Seed, B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 391, 82–86 (1998).
Ricote, M., Li, A.C., Willson, T.M., Kelly, C.J. & Glass, C.K. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391, 79–82 (1998).
Chawla, A. et al. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nature Med. 7, 48–52 (2001).
Moore, K.J. et al. The role of PPAR-γ in macrophage differentiation and cholesterol uptake. Nature Med. 7, 41–47 (2001).
Repa, J.J. & Mangelsdorf, D.J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 16, 459–481 (2000).
Fu, X. et al. 27-hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J. Biol. Chem. 276, 38378–38387 (2001).
Laffitte, B.A. & Tontonoz, P. Orphan nuclear receptors find a home in the arterial wall. Curr. Atheroscler. Rep. 4, 213–221 (2002).
Repa, J.J. & Mangelsdorf, D.J. The liver X receptor gene team: Potential new players in atherosclerosis. Nature Med. 8, 1243–1248 (2002).
Joseph, S.B. et al. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc. Natl. Acad. Sci. USA 99, 7604–7609 (2002).
Tangirala, R.K. et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc. Natl. Acad. Sci. USA 99, 11896–11901 (2002).
Lowenstein, C.J. et al. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon γ and lipopolysaccharide. Proc. Natl. Acad. Sci. USA 90, 9730–9734 (1993).
Mestre, J.R. et al. Redundancy in the signaling pathways and promoter elements regulating cyclooxygenase-2 gene expression in endotoxin-treated macrophage/monocytic cells. J. Biol. Chem. 276, 3977–3982 (2001).
Sheu, M.Y. et al. Topical peroxisome proliferator activated receptor-α activators reduce inflammation in irritant and allergic contact dermatitis models. J. Invest. Dermatol. 118, 94–101 (2002).
Karin, M. & Chang, L. AP-1–glucocorticoid receptor crosstalk taken to a higher level. J. Endocrinol. 169, 447–451 (2001).
Herrlich, P. Cross-talk between glucocorticoid receptor and AP-1. Oncogene 20, 2465–2475 (2001).
Nissen, R.M. & Yamamoto, K.R. The glucocorticoid receptor inhibits NFκB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 14, 2314–2329 (2000).
Straus, D.S. et al. 15-Deoxy-δ 12,14-prostaglandin J2 inhibits multiple steps in the NF-κ B signaling pathway. Proc. Natl. Acad. Sci. USA 97, 4844–4849 (2000).
Li, M., Pascual, G. & Glass, C.K. Peroxisome proliferator-activated receptor γ-dependent repression of the inducible nitric oxide synthase gene. Mol. Cell. Biol. 20, 4699–4707 (2000).
Chawla, A. et al. A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol. Cell 7, 161–171 (2001).
Galis, Z.S. et al. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ. Res. 91, 852–859 (2002).
Galis, Z.S., Sukhova, G.K., Kranzhofer, R., Clark, S. & Libby, P. Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc. Natl. Acad. Sci. USA 92, 402–406 (1995).
Pasterkamp, G. et al. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis 150, 245–253 (2000).
Castrillo, A. et al. Inhibition of the nuclear factor κ B (NF-κ B) pathway by tetracyclic kaurene diterpenes in macrophages. Specific effects on NF-κ B-inducing kinase activity and on the coordinate activation of ERK and p38 MAPK. J. Biol. Chem. 276, 15854–15860 (2001).
Laffitte, B.A. et al. Autoregulation of the human liver X receptor alpha promoter. Mol. Cell. Biol. 21, 7558–7568 (2001).
Callejas, N.A., Bosca, L., Williams, C.S., Du, B.R. & Martin-Sanz, P. Regulation of cyclooxygenase 2 expression in hepatocytes by CCAAT/enhancer-binding proteins. Gastroenterology 119, 493–501 (2000).
Chen, L.M., Kaniga, K. & Galan, J.E. Salmonella spp. are cytotoxic for cultured macrophages. Mol. Microbiol. 21, 1101–1115 (1996).
Castrillo, A., Diaz-Guerra, M.J., Hortelano, S., Martin-Sanz, P. & Bosca, L. Inhibition of IκB kinase and IκB phosphorylation by 15-deoxy- Delta(12,14)-prostaglandin J(2) in activated murine macrophages. Mol. Cell. Biol. 20, 1692–1698 (2000).
Joseph, S.B. et al. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 277, 11019–11025 (2002).
Acknowledgements
We thank T.M. Willson and J. Collins for GW3965 and T1317, and L. Bosca and P. Martin for reagents and comments. P.T. is an Assistant Investigator of the Howard Hughes Medical Institute at the University of California, Los Angeles. This work was supported by grants from the NIH (HL 660881) the Human Frontier Science Project (RGY-021) to P.T.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Joseph, S., Castrillo, A., Laffitte, B. et al. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med 9, 213–219 (2003). https://doi.org/10.1038/nm820
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm820
This article is cited by
-
ASGR1 deficiency diverts lipids toward adipose tissue but results in liver damage during obesity
Cardiovascular Diabetology (2024)
-
Elder (Sambucus nigra), identified by high-content screening, counteracts foam cell formation without promoting hepatic lipogenesis
Scientific Reports (2024)
-
Risk of metabolic abnormalities in osteoarthritis: a new perspective to understand its pathological mechanisms
Bone Research (2023)
-
Protein biomarkers of disease progression in patients with systemic sclerosis associated interstitial lung disease
Scientific Reports (2023)
-
An epithelial cell-derived metabolite tunes immunoglobulin A secretion by gut-resident plasma cells
Nature Immunology (2023)