Abstract
Orexins have a role in sleep regulation, and orexin receptor antagonists are under development for the treatment of insomnia. We conducted a randomised, double-blind, placebo-controlled, four-period crossover study to investigate the effect of single doses of the dual orexin receptor antagonist SB-649868 (10 or 30 mg) and a positive control zolpidem (10 mg), an allosteric modulator of GABAA receptors. Objective and subjective sleep parameters and next-day performance were assessed in 51 healthy male volunteers in a traffic noise model of situational insomnia. Compared with placebo, SB-649868 10 and 30 mg increased total sleep time (TST) by 17 and 31 min (p<0.001), whereas after zolpidem TST was increased by 11.0 min (p=0.012). Wake after sleep onset was reduced significantly by 14.7 min for the SB–6489698 30 mg dose (p<0.001). Latency to persistent sleep was significantly reduced after both doses of SB–6489698 (p=0.003), but not after zolpidem. Slow wave sleep (SWS) and electroencephalogram (EEG) power spectra in non-REM sleep were not affected by either dose of SB-640868, whereas SWS (p< 0.001) and low delta activity (<=1.0 Hz) were increased, and 2.25–11.0 Hz activity decreased after zolpidem. REM sleep duration was increased after SB-649868 30 mg (p=0.002) and reduced after zolpidem (p=0.049). Latency to REM sleep was reduced by 20.1 (p=0.034) and 34.0 min (p<0.001) after 10 and 30 mg of SB-649868. Sleep-onset REM episodes were observed. SB-649868 was well tolerated. This dual orexin receptor antagonist exerts hypnotic activity, with effects on sleep structure and the EEG that are different from those of zolpidem.
Similar content being viewed by others
INTRODUCTION
Insomnia is a serious health problem with an estimated prevalence ranging from approximately 4 to 22% (Roth et al, 2011) and associated costs, which are significant (Sarsour et al, 2011). Current pharmacological treatments of insomnia are based primarily on positive allosteric modulators of several GABAA receptor subtypes, that is, benzodiazepines and non-benzodiazepines such as zolpidem (Nutt and Stahl, 2010; Winsky-Sommerer, 2009). Several novel pharmacological treatments for insomnia are under development, including antagonists of the orexin 1 and orexin 2 receptors (OX1R, OX2R) (Scammell and Winrow, 2011; Sullivan and Guilleminault, 2009). The development of OXR antagonists for the treatment of insomnia is based on the large body of evidence supporting a role for orexin neuropeptides (also known as hypocretins) in the control of arousal and sleep/wake states (Brown et al, 2001; Eriksson et al, 2001; Eriksson et al, 2004; Eriksson et al, 2010; Hagan et al, 1999; Piper et al, 2000; Sakurai and Mieda, 2011). In particular, (1) Orexin-producing neurons in the lateral hypothalamus exhibit high-firing rates during wakefulness, which dissipate during non-REM and REM sleep (Lee et al, 2005), and orexins activate the key neurotransmitter systems implicated in maintaining wakefulness, such as histamine, serotonin, and noradrenaline, as well as cholinergic neurons (Sakurai and Mieda, 2011); (2) deficiency in the orexinergic system is associated with disruption of the sleep–wake cycle. This is observed in human narcolepsy–cataplexy, in canine and rodent animal models of human narcolepsy (ie, in knock out, in mice devoid of either the prepro-orexin precursor or the orexin receptors, and in transgenic mice and rats with selective postnatal degeneration of orexin-expressing neurons; Chemelli et al, 1999; Mignot, 2004; niz Behn et al, 2010; Sakurai and Mieda, 2011); (3) orexin concentrations in CSF fluctuate with a circadian rhythm, which is synchronised to the wake–sleep cycle, with peaks during the day in the day-active squirrel monkey (Zeitzer et al, 2003), and during the night in the nocturnal rat (Deboer et al, 2004); (4) the circadian variation in orexins is abolished by lesioning of the suprachiasmatic nuclei, the locus of the circadian pacemaker in mammals, which drives the circadian variation in sleep propensity and structure (Deboer et al, 2004).
To our knowledge, until recently, the only data available on effects of dual orexin receptor antagonists on sleep in humans are those recently published by Brisbare-Roch et al (2007). The authors reported on the effect of almorexant on sleep propensity and the electroencephalogram (EEG) in sleep recorded during two 25-min sessions during the daytime in healthy volunteers. Thus, there are currently only very limited data available on the effects of dual orexin receptor antagonists on sleep propensity and structure, as well as on the sleep EEG in humans. Furthermore, it is unknown how such effects compare with the effects of allosteric modulators of GABAA receptors, which are currently the most commonly prescribed hypnotics for the treatment of insomnia.
SB-649868 is a potent, orally acting, selective OX1/OX2 receptor antagonist under investigation for the treatment of insomnia. Studies in rodent and primate models have demonstrated sleep-promoting effects and lack of motor impairment following administration of SB-649868 (Gerrard et al, 2009). In healthy volunteers, SB-649868 was shown to be safe and well tolerated at doses up to 80 mg, with mechanism-related adverse events (eg, somnolence and fatigue) observed in a majority of subjects after 60 and 80 mg single doses. Evening administration of doses up to 60 mg to healthy volunteers without sleep complaint and under normal sleeping conditions resulted in significant dose-dependent decrease in latency to persistent sleep (LPS), and wake after sleep onset (WASO), and increase in total sleep time (TST) as measured by polysomnography (PSG; Bettica et al, 2011).
We here assess and compare the effect of SB-649868 on sleep structure and the spectral composition of the sleep EEG and those of zolpidem, a non-benzodiazepine allosteric modulator of GABAA receptor subtypes, relative to placebo, in a validated model of situational insomnia.
MATERIALS AND METHODS
This was a randomised, double-blind, double-dummy, placebo-controlled, four-period crossover study (study code: GSK104094; ID number NCT00440323 on http://ClinicalTrials.gov) to investigate the effect of single oral doses of SB-649868 (10 or 30 mg) and of a positive control (zolpidem, 10 mg) in a model of noise-induced situational insomnia in healthy male volunteers (Cluydts et al, 1995). In this model, disruption of sleep maintenance and initiation is induced by standardised traffic noise, and efficacy of hypnotics can be assessed reliably (Cluydts et al, 1995; Dijk et al, 2007; Dijk et al, 2011).
The study was conducted at a single site (Surrey Clinical Research Centre, University of Surrey), and the protocol was approved by the Ravencourt Ethics Committee, which is a Phase 1 Research Ethics committee. The primary objective of the study was to determine the effect of SB-649868 on TST, a measure of hypnotic efficacy comprising both sleep initiation and sleep maintenance elements, in healthy volunteers undergoing a noise-disturbed sleep model. Secondary objectives were to study: a) the changes induced by SB-649868 and zolpidem on other PSG sleep parameters such as the non-REM sleep stages 1, 2, 3, 4, REM sleep, the number of awakenings (NAWs), as well as the spectral composition of the sleep EEG; b) to investigate the effects of SB-649868 and zolpidem on subjective sleep quality, on the morning after dosing and on the following 3 days; c) to investigate the effects of SB-649868 and zolpidem on daytime cognitive functioning on the morning following dosing; and d) to investigate the safety and the pharmacokinetic profile of SB-649868 and zolpidem in healthy volunteers. Under the conditions and dose range used in the present study, SB-649868 has been reported to have a half-life in the range of 3.4–3.9 h (Bettica et al, 2011). In this report, we will focus on the primary outcome measures, as a prime indicator of hypnotic efficacy, as well as on the sleep related secondary outcome variables.
Population
All subjects provided written informed consent to participate in the study and were screened approximately 28 days before treatment. A total of 51 subjects entered the study; 44 subjects completed the study, whereas 7 subjects prematurely discontinued. Subjects were healthy adult males aged 18–55 years inclusive, with a body weight ⩾50 kg and a body mass index within the range 18.5–29.9 kg/m2. Subjects were to have a history of going to bed from 2200 to 0000 h on at least 5–7 nights per week, with a reported nightly sleep duration of 6.5–8.5 h over the previous 3 months or more at screening. Subjects who had consumed beverages and medications that could interfere with the effects of treatments or study assessments were excluded. Other key exclusion criteria were sleep apnoea and disorders of periodic limb movements and other sleep disturbances, as assessed during a clinical PSG screening, sleep complaints, and a recent history of or current shift work. Subjects who were not prepared to use protocol-specified methods of contraception or sexual abstinence as appropriate, could also not be included. All subjects had to have laboratory values, vital signs and electrocardiographic (ECG) values within the normal reference range.
Study Design and Treatments
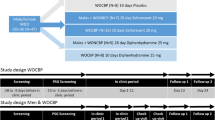
The study consisted of four treatment sessions. Each treatment session consisted of two nights (Figure 1). Subjects were admitted to the unit on Day 1 at approximately 1600 h in the afternoon before the first night (Night 1) and underwent admission procedures. At approximately 1800 h, subjects were served a standard light snack, and at approximately 2100 h, a standardised dinner was served. At approximately 2230 h, they retired to bed with lights off at 2300 h and lights on at 0700 h. During Night 1, study medication was not administered and subjects underwent PSG monitoring only (no noise disturbance).
Schematic representation of the protocol. This was a four-period crossover study, each period consisting of two consecutive nights (Night 1: adaptation; Night 2: treatment). During Night 2 for all sessions, randomised subjects were administered two double-blinded doses of study medication (ie, either placebo or SB-649868 (10 or 30 mg); or either placebo or zolpidem 10 mg), while exposed to the traffic-noise model of situational insomnia.
During the second night (Night 2) for all sessions, after a standard light snack at approximately 1800 h and a standardised dinner at approximately 2100 h, randomised subjects were administered two double-blinded doses of study medication. Thirty minutes after a standard dinner, at approximately 2130 h, subjects received either placebo or SB-649868 (10 or 30 mg). This timing of administration was chosen, because within the used dose range and administered in the fed state, average tmax is approximately in the 2.5–2.75 h range (Bettica et al, 2011). At approximately 2230 h, subjects received either placebo or zolpidem (10 mg). Subjects retired to bed at 2230 h with lights off at 2300 h (Figure 1).
During Night 2, subjects underwent PSG monitoring, blood sampling for pharmacokinetic analysis (before lights off) and were exposed to the pre-recorded traffic noise. Calibrated noise lasted from lights off (2300 h) continuously until lights on (0700 h).
All subjects remained in the unit during each of the treatment sessions. Subjects were discharged at the end of each treatment session when all study procedures had been completed at the discretion of the investigator, depending on the nature of any ongoing adverse events. During the study, each single-dose treatment was separated by a 7-day washout period (±1 day), and preferably occurred on the same day of the week, or were consistently held on either workdays or days of rest, for any given subject. Subjects were instructed to maintain a normal pattern of sleep between 2300 h and 0700 h, when outside the unit between treatment sessions. Compliance with the maintenance of a regular sleep–wake cycle instruction was monitored by actigraphy recordings for the duration of the study. Subjects were instructed to return to the unit within 7–14 days of the last dose of study treatment for the follow-up visit.
EEG/PSG Recordings
Nine EEG channels (C4-A1, C3-A2, O2-A1, O1-A2, Fpz-A1, Fz-A1, Cz-A1, Oz-A1, Pz-A1), two electrooculogram (EOG) channels, and one submental electromyogram (EMG) channel, using gold electrodes, were recorded onto a Compumedics Siesta digital EEG machine. Signals were digitised at 256 (EEG, EMG) or 128 Hz (EOG/ECG), and stored at 128 Hz for the EEG and EOG/EMG. The low- and high-frequency filters were set at 0.3 and 70 Hz for the EEG/EOG, and at 10 and 70 Hz for the EMG. A single-channel ECG was also recorded. For the PSG screening night, this montage was complemented by recordings from the left and right anterior tibialis muscle, recording of nasal/oral airflow, thoracic and abdominal effort, body position, tracheal microphone and oximetry. All PSG were scored according to the standard criteria of Rechtschaffen and Kales (1968). Epochs annotated as artefacts were excluded from the analyses of the EEG power spectra by visual inspection of the records, whereas blind to treatment. Nights were only included in the spectral analysis if at least 5 h of artefact-free data were available. EEG spectral analysis was performed on the C3-A2 and C4-A1 channel using a fast Fourier transform, as previously described (Dijk et al, 2010). Briefly, EEG power spectra were computed per 4-s epochs and the 0.25 Hz spectra were collapsed into 1-Hz bins. Average spectra were computed separately for non-REM sleep (encompassing stages 1–4) and REM sleep for C3-A2 or, if this channel was not available, C4-A1.
Assessments
Treatment effects on sleep duration, maintenance, and initiation were assessed by quantifying TST, WASO, NAWs, sleep efficiency (SE), LPS and latency to REM sleep as measured with PSG. In addition, we assessed effects on sleep structure, that is, REM sleep and non-REM sleep and its sub-stages 1, 2, 3, 4, as well as slow wave sleep (SWS), which is the sum of stage 3 and 4. Sleep questionnaires were used to assess subjective TST, subjective WASO, subjective NAW, subjective sleep latency, and sleep quality. A computerised battery of standard tests was used to assess treatment effects on subjective alertness, mood, working/short-term memory, long-term memory, attention/executive functions, and motor control. The battery, comprising three blocks of tests, was used on the morning after dosing, beginning approximately 30 min after waking (ie, 10 h post-dose for SB-649868 and 9 h post-dose for zolpidem) and ending approximately 90 min later. Although the battery enables assessment of a wide range of functions, here we report the effects on two widely used tests of residual effects: psychomotor vigilance task (PVT), which lasted for 10 min, and digit-symbol substitution (DSST), which was administered in each of the three test blocks.
Treatment safety was assessed by collecting adverse events, vital signs, 12-lead ECG, and clinical laboratory assessments. Plasma concentrations were assessed at different time points after the administration of both SB-649868 and zolpidem.
Statistical Analysis
A sample size of 46 subjects completing the study was planned, as it would have provided 80% power to detect a difference between SB-649868 and placebo of at least 22 min in TST, assuming a within-subject SD of about 36.8 min. A pre-specified interim analysis was performed when approximately half of the subjects had completed the study and showed that no sample size adjustment was required because the variability of the data collected was in line with that expected.
Available data from withdrawals were used for all statistical analyses, as planned in the protocol and as properly managed by the mixed models applied. For the final analysis, a mixed-effects model was applied on PSG data of the second night, with period and treatment as fixed effects and subject as random effect. Estimates for mean treatment differences of SB-649868 compared with placebo and with zolpidem were derived. Similar estimates were provided for zolpidem compared with placebo. Tests of significance were performed at the 5% level.
Subjective Sleep Questionnaire data were also analysed in this mixed model with the pre-dose’ values included as a ‘baseline’ covariate.
Measures of DSST and PVT underwent an exploratory analysis of covariance (ANCOVA). Cognitive performance after the night with no-noise exposure (baseline) was used as a covariate, period and treatment as fixed effects, and subject as random effect. Estimates for mean treatment differences of SB-649868 compared with placebo were derived.
In an exploratory analysis of the effects of treatment on EEG power spectra, non-REM and REM sleep spectra in each of the three treatments were expressed as a percentage of the placebo condition for each individual, and geometric means and 95% confidence intervals were computed for each of the conditions.
Safety data were summarised by means of descriptive statistics.
RESULTS
Subject Disposition
Fifty-one healthy men were recruited, and received at least one treatment. Forty-four subjects completed the study as planned. Baseline characteristics of the subjects are reported in Table 1.
Effects on Sleep Maintenance and Initiation
A summary of the effects of placebo, of SB-649868 (10 and 30 mg) and of zolpidem (10 mg) is presented in Table 2.
Although a baseline no-noise condition was not recorded in this study, sleep in the placebo condition showed the disruptive effects of the model on sleep, that is, a long LPS and relatively low SE (87%), reflecting a TST of only 419 min. When compared with placebo, both doses of SB-649868 significantly increased TST by 17 and 31 min for the 10 and 30 mg dose, respectively. A significant increase in TST was also observed after zolpidem, although to a lower extent (+11 min). The increase in TST after SB-649868 10 mg was not significantly different from zolpidem, but the increase after SB-649868 30 mg was statistically significantly greater than after zolpidem. The significant changes in TST were accompanied by significant changes in SE (%). WASO was significantly reduced for the SB-648969 30 mg dose, but not so for the other active treatments, although the improvement of around 7 min obtained with the 10 mg dose was already of the same extent of that estimated for zolpidem. The NAWs was not significantly different from placebo for any of the treatments. LPS was significantly reduced after both doses of SB–648969 (by 8.5 and 17.4 min after 10 and 30 mg, respectively), whereas the effect of zolpidem on LPS was not statistically significant.
Effects on Sleep Structure and the EEG
All-night measures
SB-649868 and zolpidem had different effects on sleep architecture. When analysed as percentage of sleep-period time, SB-649868 did not affect non-REM sleep, with the exception of a significant prolongation of Stage 1 observed for SB-649868 10 mg. By contrast, zolpidem significantly increased the percentage of sleep-period time spent in Stage 3 and 4, and SWS. Furthermore, with regard to REM sleep SB-640868 30 mg significantly increased the percentage of sleep-period time spent in REM sleep, whereas a significant reduction in percentage sleep-period time spent in REM sleep was observed with zolpidem 10 mg. Latency to REM sleep was reduced after both doses of SB-649868. Analysis of the distribution of REM latencies is presented in Figure 2. Sleep onset REM latencies, that is, intervals between sleep onset and the first occurrence of REM sleep, less than or equal to 15 min, were observed for SB-640868 10 (n=1) and 30 mg (n=2), but not for placebo (n=0) or zolpidem (n=0).
Distribution of latency to REM sleep during the four conditions.
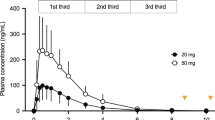
Analyses per third of sleep episode
The effects of the treatment on REM sleep and SWS were also analysed per third of the sleep episode. REM sleep increased from the first to the final third, whereas SWS decreased in all conditions (Figure 3). In the first third, REM sleep was increased compared with placebo for both SB-649868 10 and 30 mg. In the second third, REM sleep a significant increase compared with placebo was only observed after SB-649868 30 mg, and no significant differences from placebo were observed for REM sleep in the final third.
REM sleep and slow wave sleep (SWS) per third of sleep episode during noise exposure after treatment with placebo, 10 mg SB-649868, 30 mg SB-649868, and 10 mg zolpidem. Error bars indicate one SEM. *P<0.05; §P<0.01; #P<0.001, compared with placebo.
In the first third of the sleep episode, SWS was enhanced compared with placebo, after both SB-649868 30 mg and zolpidem. In the second third, a significant increase of SWS was only observed after zolpidem, and in the final third, no statistically significant differences from placebo were present.
SB-649868 did not alter the EEG power density in either non-REM sleep (Figure 4) or REM sleep (data not shown), that is, all values were close to placebo. In contrast, during non-REM sleep, zolpidem increased EEG power density in the 0.25–1.0 Hz bin, whereas a decrease was observed for frequencies between 2.25 and 11 Hz (Figure 4).
Effect of SB-649868 (10 and 30 mg) and zolpidem (10 mg) on electroencephalogram (EEG) power density spectra during non-REM sleep. All data are geometric means expressed relative to placebo (=100%). Vertical bars indicate 95% confidence intervals. A total of 36–38 subjects contributed to each of the comparisons for frequencies up to 15 Hz. Data are plotted at the upper limits of the frequency bins. Thus, values plotted at 1 Hz represent the 0.25–1.0 Hz bin.
Subjective Sleep Measures
Subjective sleep measure assessed in the morning following baseline sleep (ie, no noise) and the morning following noise exposure are presented separately for placebo, SB-649868 (10 and 30 mg) and of zolpidem (10 mg) in Table 3. Exposure to the noise model was associated with an increase in subjective sleep latency and the number and duration of night awakenings and a reduction in subjective TST, in particular in the placebo condition. The effects of the treatments on subjective sleep assessments were quite variable, although all treatments appeared to counter the negative effects of the noise model to some extent. Significant improvements compared with placebo were only observed for subjective sleep latency (p=0.029) and subjective TST (p=0.008) after treatment with the SB-649868 30 mg dose.
Performance
Performance across all tests was consistently high, indicating that participants were well motivated.
Digit-symbol substitution test
Performance on the DSST is summarised by presenting the number of correct responses during the three assessments at baseline and following noise exposure, separately for the three treatments (Table 4). Mean and median numbers of correct responses were consistently high. Median correct responses varied from 14–15, whereas the median number of attempted response varied from 15–16. ANCOVA did not detect any significant differences from placebo.
Psychomotor vigilance task
Performance on the PVT was summarised by presenting the number of lapses (response time >500 ms) and the median response times (Table 5). For lapses, some indication of an effect of treatment was present in the SB-649868 30 mg condition, in which according to the ANCOVA, the mean number of lapses was significantly greater than in the placebo condition (p=0.012). The median number of lapses was one in all conditions. The average median response time was short in all conditions, with some indication of an increase in the average median response time in the SB-649868 30 mg condition (p=0.013 vs placebo). However, also in the SB-649868 30 mg condition, the median of the median response time was identical to placebo.
Adverse events
Overall, the frequency of adverse events was similar for the active treatment groups and placebo. Table 6 reports the most frequent adverse events, that is, those reported by at least 5% of the subjects. More than twice as many subjects reported somnolence and disturbance of attention after SB-649868 30 mg compared with placebo. On the other hand, headache was reported in more than twice as many subjects after placebo compared with the other treatments, and insomnia was reported in more than twice as many subjects after placebo compared with SB-649868 30 mg. Most of the adverse events were mild or moderate in intensity. Only one subject experienced an adverse event of severe intensity (severe somnolence after receiving SB–649868 30 mg, which was judged by the investigator to be related to investigational product). One subject reported a mild hallucination, which started 90 min after the administration of SB-649868 30 mg and lasted 2 h; the adverse event recovered and the subject continued the study. There were no clinically relevant abnormalities in urinalysis, 12-lead ECG and vital signs parameters.
DISCUSSION
This is a first report of the effects of the orexin receptor 1 and 2 antagonist SB-649868 on sleep and the EEG in a traffic-noise model of situational insomnia. Sleep under placebo conditions showed the characteristic disruptive effect of this validated model (Cluydts et al, 1995) on PSG assessed sleep initiation and TST, as well as subjective measures of sleep initiation. These disruptive effects were countered to some extent by the active treatments. Both doses (10 and 30 mg) of SB-649868 showed significant hypnotic efficacy as indexed by a significant increase in TST and a reduction in the LPS. The effects on TST are not only due to the reduction in the latency to sleep onset, but also because wakefulness after sleep onset was reduced after SB-649868 30 mg. Thus, these data indicate that SB-649868 has positive effects on both sleep initiation, as well as sleep maintenance, as indexed by WASO. The NAWs, on the other hand, was not affected by SB-649868. The effects observed in this study are in line with those observed in healthy volunteers in normal sleeping conditions (Bettica et al, 2011). Also in that case, SB-649868 did not affect the NAWs. As we cannot say whether noise disturbance significantly disrupted NAWs, we cannot conclude an SB-649868 effect on NAWs. The positive control (zolpidem, 10 mg) showed its well-established hypnotic efficacy as indexed by an increase in TST. Interestingly, the effect on TST was greater for SB-649868 30 mg than zolpidem. Zolpidem did not improve sleep initiation in this study, but compared with SB-649868 30 mg led to a greater reduction in the NAWs. The effects of dual orexin receptor antagonists on sleep initiation and TST are likely to be mediated by a reduced orexinergic drive on several neuronal populations known to have a key role in the transitions between wakefulness and sleep, and to be densely innervated by the orexin-producing neurons. These include the monoaminergic noradrenergic neurons of the locus coeruleus and serotonergic neurons of the raphe nuclei, expressing, respectively, OX1R and OX1R/OX2R, as well as the histaminergic neurons located in the tuberomammillary nucleus via OX2R (Fort et al, 2009; Mieda et al, 2011). Thus, dual orexin receptor antagonists may exert their effects by reducing the excitatory action of orexins on these wake-promoting neurostransmitter systems, resulting in a decreased monoaminergic tone, which in turn will reduce cortical activation and an increased sleep propensity. In addition, dual orexin receptor antagonists may exert their effects on cholinergic neurons of the basal forebrain and of the latero-dorsal tegmental (LDT) and pedunculopontine tegmental nuclei that are part of the arousal-promoting circuits (Fort et al, 2009). Zolpidem acts through a positive allosteric modulation of GABAA receptors, showing a preferential affinity for the α1-GABAA receptor subtype and lower affinity to α2- and α3-GABAA receptor subtypes (Mohler, 2006; Winsky-Sommerer, 2009). Whether and how these differential effects on TST and NAWs relate to the different roles of these specific GABAA subtypes and the orexinergic system in ‘sleep-state switching’ (Fort et al, 2009; Saper et al, 2010) cannot be determined from our data.
The effects on sleep structure differed between the two compounds such that the percentage of sleep period spent in the SWS stage was enhanced after zolpidem and not affected by SB-649868, whereas the percentage of sleep period spent in REM sleep was enhanced after SB-649868 and reduced after zolpidem. SB-649868 enhanced REM sleep propensity as evidenced by both an increase in REM sleep duration and a reduction in REM latency. Enhancement of visually scored SWS by zolpidem has been reported previously (Kanno et al, 2000). In this study, zolpidem led to an increase in EEG activity in the 0.25–1.0 Hz range and a reduction in higher delta and theta frequencies, consistent with previous reports (Brunner et al, 1991; Dijk et al, 2010). The lack of an effect of SB-649868 on EEG power spectra in non-REM sleep in the present study can therefore not be attributed to a lack of sensitivity of spectral measures in this model of situational insomnia. Suppression of REM sleep by benzodiazepines is also well documented, although for zolpidem and other non-benzodiazepine allosteric modulators of the GABAA receptor, this effect is not always observed (Lancel, 1999; Mohler, 2006). Effects of dual orexin receptor antagonists on REM sleep have been previously shown in animals (eg, Brisbare-Roch et al, 2007; Gerrard et al, 2009) and humans, possibly mediated at least in part by their effects on LDT/pedunculopontine tegmental cholinergic neurons implicated in REM sleep regulation (Sakurai and Mieda, 2011). This would be in accordance with previous studies in cats showing that microinjection of orexin-A in the LDT significantly suppressed REM sleep (Xi et al, 2001). In addition, REM-off noradrenergic and serotonergic neurons, located respectively in the locus coeruleus and the raphe nuclei, are known to be implicated in the control of the gating to REM sleep (Luppi et al, 2011), and to display high expression levels of OX1R and OX1R/OX2R, respectively (Mieda et al, 2011). Thus, SB-649868 is likely to suppress serotonergic and noradrenergic transmission that would counteract REM sleep suppression by, in part, reducing the noradrenergic excitation of REM-off neurons in the dorsal deep mesencephalic reticular nucleus (Crochet et al, 2006). Furthermore, a recent study suggested that suppression of REM sleep by orexin-A involves both O1X and OX2 receptors (Mieda et al, 2011). Thus, the effects of the dual orexin receptor antagonist on sleep structure as assessed by visual scoring of PSG records, is very different from the effects of zolpidem, even though both compounds have hypnotic effects. Quantitative EEG analysis of the non-REM EEG further differentiated the effects of these compounds. Although zolpidem altered the non-REM sleep EEG in accordance with the extensively described benzodiazepine EEG fingerprint shared by non-benzodiazepine compounds, SB-649868 did not induce noticeable changes in the EEG power density.
Overall, our results suggest that the dual orexin antagonist receptor SB-649868 exerts direct or indirect effects on several transmitter systems involved in the transitions between vigilance states, whereas it does not—at least at the 10 and 30 mg doses—alter neuronal networks involved in the generation of EEG rhythms.
The characteristic dynamics of sleep structure across the sleep episode when sleep occurs during the night, that is, a decline of SWS and an increase in REM sleep (Dijk and Czeisler, 1995) was preserved in all conditions, indicating that major regulatory process underlying sleep are not significantly disrupted by either the traffic-noise model or the pharmacological treatments.
The distribution of REM sleep latencies as observed after SB-649868 is reminiscent of the bimodal distribution, which has been observed in disorders such as depression, and has been successfully simulated on the basis of mathematical models of REM sleep regulation (Beersma et al, 1984).
Some neuronal pathways involved in the control of autonomic function, as well as several regions of the limbic system, associated with stress, emotions, and anxiety, provide neuronatomical and physiological input onto orexinergic neurons (Yoshida et al, 2006), and may contribute to hyper-arousal associated with insomnia, and orexin antagonists may reduce this arousal. We note that a limitation of the traffic-noise insomnia model is that some of the arousal circuits activated may be different from those activated in insomnia.
The DSST is a sensitive task to assess residual effects (eg, Boyle et al, 2009; Stone et al, 2002). In the present study, neither of the two doses of SB-649868, nor 10 mg of zolpidem induced residual effects on the DSST. The absence of an effect of zolpidem is in accordance with previous studies (Hindmarch et al, 2001). Performance on the PVT, a task used extensively to document the negative effects of sleep loss (eg, Lim and Dinges, 2008), also did not indicate major residual effects, although for the SB-649868 30 mg dose, some indication for reduced performance on this task was observed.
Both SB-649868 and zolpidem were well tolerated. Adverse events, which were most frequent and which occurred more often after SB-649868, were related to its pharmacological effect. This result is in line with what was previously reported in healthy volunteers who received SB-649868 in the morning, after lunch, or after dinner (Bettica et al, 2011). Somnolence was reported by 29% of subjects treated with SB-649868 30 mg. This is not unexpected considering the potent sleep-inducing effect of SB-649868, the fact that dosing was timed to maximise the hypnotic effect, that is, subjects were dosed 90 min before bedtime, and that circulating levels of SB-649868 were still relatively high at lights on (data not shown). Future studies should explore different doses and dosing regimens of the drug, both in healthy subjects and in the target patient population.
Narcolepsy/cataplexy could be a possible consequence of orexin antagonism, based on preclinical and human data, suggesting a deficiency of the orexinergic system in narcolepsy (Chen et al, 2005). Symptoms common in narcolepsy/cataplexy are hallucinations, sleep paralysis, cataplexy, and sleep onset REM episodes (SOREM; Narcolepsy fact sheet, National Institute of Neurological Disorders and Stroke. http://www.ninds.nih.gov/disorders). One mild hallucination was reported by a subject treated with SB-649868 30 mg, and SOREM episodes were reported only after the administration of SB-649868. It should be noted that also in this case, the timing of drug administration, 2 h before lights out, may have artificially increased the risk of hallucinations and SOREM episodes. Future studies in patients and with different timing of dosing will be needed to assess the risk of SB-649868 to induce narcolepsy/cataplexy.
Overall, these data indicate that the dual orexin receptor antagonist SB-649868 has significant hypnotic effects in a model of situational insomnia, with effects on REM sleep, SWS, and the EEG, which are different from those of zolpidem. These differences may be related to the different mechanisms of action of these two compounds.
References
Beersma DG, Daan S, Van den Hoofdakker RH (1984). Distribution of REM latencies and other sleep phenomena in depression as explained by a single ultradian rhythm disturbance. Sleep 7: 126–136.
Bettica P, Nucci G, Pyke C, Squassante L, Zamuner S, Ratti E et al (2011). Phase I studies on the safety, tolerability, pharmacokinetics and pharmacodynamics of SB-649868, a novel dual orexin receptor antagonist. J Psychopharmacol; e-pub ahead of print 5 July 2011.
Boyle J, Wolford D, Gargano C, McCrea J, Cummings C, Cerchio K et al (2009). Next-day residual effects of gaboxadol and flurazepam administered at bedtime: a randomized double-blind study in healthy elderly subjects. Hum Psychopharmacol 24: 61–71.
Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S et al (2007). Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med 13: 150–155.
Brown RE, Sergeeva O, Eriksson KS, Haas HL (2001). Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology 40: 457–459.
Brunner DP, Dijk DJ, Munch M, Borbely AA (1991). Effect of zolpidem on sleep and sleep EEG spectra in healthy young men. Psychopharmacology (Berl) 104: 1–5.
Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C et al (1999). Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98: 437–451.
Chen W, Zeitzer JM, Mignot E . The hypocretins and narcolepsy. In: Lecea L, Sutcliffe JG (eds). Hypocretins: Integrators of Physiological Functions. Springer: New York; 2005, pp 233–252.
Cluydts R, De Roeck J, Cosyns P, Lacante P (1995). Antagonizing the effects of experimentally induced sleep disturbance in healthy volunteers by lormetazepam and zolpidem. J Clin Psychopharmacol 15: 132–137.
Crochet S, Onoe H, Sakai K (2006). A potent non-monoaminergic paradoxical sleep inhibitory system: a reverse microdialysis and single-unit recording study. Eur J Neurosci 24: 1404–1412.
Deboer T, Overeem S, Visser NA, Duindam H, Frolich M, Lammers GJ et al (2004). Convergence of circadian and sleep regulatory mechanisms on hypocretin-1. Neuroscience 129: 727–732.
Dijk DJ, Czeisler CA (1995). Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci 15: 3526–3538.
Dijk DJ, James LM, Peters S, Walsh JK, Deacon S (2010). Sex differences and the effect of gaboxadol and zolpidem on EEG power spectra in NREM and REM sleep. J Psychopharmacol 24: 1613–1618.
Dijk DJ, Stanley N, Groeger JA, Legters A, Deacon S (2007). Gaboxadol reduces the detrimental effects of traffic noise on sleep: A randomised, double-blind, placebo-controlled, parallel group study in healthy subjects. Sleep 30: A253.
Dijk DJ, Stanley N, Lundahl J, Groeger JA, Legters A, Trap Huusom AK et al (2011). Enhanced slow wave sleep and improved sleep maintenance after gaboxadol administration during seven nights of exposure to a traffic noise model of transient insomnia. J Psychopharmacol; e-pub ahead of print 13 October 2011.
Eriksson KS, Sergeeva O, Brown RE, Haas HL (2001). Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci 21: 9273–9279.
Eriksson KS, Sergeeva OA, Haas HL, Selbach O (2010). Orexins/hypocretins and aminergic systems. Acta Physiol (Oxf) 198: 263–275.
Eriksson KS, Sergeeva OA, Selbach O, Haas HL (2004). Orexin (hypocretin)/dynorphin neurons control GABAergic inputs to tuberomammillary neurons. Eur J Neurosci 19: 1278–1284.
Fort P, Bassetti CL, Luppi PH (2009). Alternating vigilance states: new insights regarding neuronal networks and mechanisms. Eur J Neurosci 29: 1741–1753.
Gerrard PA, Porter RA, Holland V, Massagrande M, Poffe A, Piccoli L et al (2009). Preclinical pharmacology of SB-649868: a novel orexin OX1/OX2 receptor antagonist possessing potent hypnotic activity in rodents and primates. Sleep 32: A42.
Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S et al (1999). Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA 96: 10911–10916.
Hindmarch I, Patat A, Stanley N, Paty I, Rigney U (2001). Residual effects of zaleplon and zolpidem following middle of the night administration five hours to one hour before awakening. Hum Psychopharmacol 16: 159–167.
Kanno O, Sasaki T, Watanabe H, Takazawa S, Nakagome K, Nakajima T et al (2000). Comparison of the effects of zolpidem and triazolam on nocturnal sleep and sleep latency in the morning: a cross-over study in healthy young volunteers. Prog Neuropsychopharmacol Biol Psychiatry 24: 897–910.
Lancel M (1999). Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep 22: 33–42.
Lee MG, Hassani OK, Jones BE (2005). Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci 25: 6716–6720.
Lim J, Dinges DF (2008). Sleep deprivation and vigilant attention. Ann NY Acad Sci 1129: 305–322.
Luppi PH, Clement O, Sapin E, Gervasoni D, Peyron C, Leger L et al (2011). The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med Rev 15: 153–163.
Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T (2011). Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J Neurosci 31: 6518–6526.
Mignot E (2004). Sleep, sleep disorders and hypocretin (orexin). Sleep Med 5 (Suppl 1): S2–S8.
Mohler H (2006). GABA(A) receptor diversity and pharmacology. Cell Tissue Res 326: 505–516.
niz Behn CG, Klerman EB, Mochizuki T, Lin SC, Scammell TE (2010). Abnormal sleep/wake dynamics in orexin knockout mice. Sleep 33: 297–306.
Nutt DJ, Stahl SM (2010). Searching for perfect sleep: the continuing evolution of GABAA receptor modulators as hypnotics. J Psychopharmacol 24: 1601–1612.
Piper DC, Upton N, Smith MI, Hunter AJ (2000). The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. Eur J Neurosci 12: 726–730.
Rechtschaffen A, Kales A . A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. US Government Printing Office: Washington, DC; 1968.
Roth T, Coulouvrat C, Hajak G, Lakoma MD, Sampson NA, Shahly V et al (2011). Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry 69: 592–600.
Sakurai T, Mieda M (2011). Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci 32: 451–462.
Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE (2010). Sleep state switching. Neuron 68: 1023–1042.
Sarsour K, Kalsekar A, Swindle R, Foley K, Walsh JK (2011). The Association between Insomnia Severity and Healthcare and Productivity Costs in a Health Plan Sample. Sleep 34: 443–450.
Scammell TE, Winrow CJ (2011). Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol 51: 243–266.
Stone BM, Turner C, Mills SL, Paty I, Patat A, Darwish M et al (2002). Noise-induced sleep maintenance insomnia: hypnotic and residual effects of zaleplon. Br J Clin Pharmacol 53: 196–202.
Sullivan SS, Guilleminault C (2009). Emerging drugs for insomnia: new frontiers for old and novel targets. Expert Opin Emerg Drugs 14: 411–422.
Winsky-Sommerer R (2009). Role of GABAA receptors in the physiology and pharmacology of sleep. Eur J Neurosci 29: 1779–1794.
Xi MC, Morales FR, Chase MH (2001). Effects on sleep and wakefulness of the injection of hypocretin-1 (orexin-A) into the laterodorsal tegmental nucleus of the cat. Brain Res 901: 259–264.
Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE (2006). Afferents to the orexin neurons of the rat brain. J Comp Neurol 494: 845–861.
Zeitzer JM, Buckmaster CL, Parker KJ, Hauck CM, Lyons DM, Mignot E (2003). Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci 23: 3555–3560.
Acknowledgements
We thank the staff of the Surrey Clinical Research Centre at the University of Surrey for the conduct of the study, Dr Sigurd Johnsen for summary statistics for the EEG power spectra, and Giuseppe Atzori with help in preparing some of the figures. Supportive editorial services were contributed by Gary Evoniuk, PhD, GlaxoSmithKline. This study was registered on ClinicalTrials.gov with the following identifier: NCT00440323. This study was sponsored by GlaxoSmithKline.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Paolo Bettica and Lisa Squassante were full-time employees at GlaxoSmithKline at the time of the design conduct and reporting of this study. Dr Winsky-Sommerer has no conflict of interest. Dr Groeger has contributed to research projects funded by Road Safety Authority (Irl), Science Foundation, Ireland, US Air Force Office of Scientific Research, Eisai, Glaxo Smith Kline (GSK), Eli Lilly, and has been compensated directly, or through the University of Surrey, for his engagement by the latter four sponsors. Dr Gennery was an employee of the University of Surrey to which GSK provided funds to conduct this study. Dr Dijk has contributed to research projects funded by GSK, Eli Lilly, Merck, Ono Pharma, Philips, Airforce Office of Scientific Research, Biotechnology and the Biological Sciences Research Council. He has provided consulting services to Johnson and Johnson, Sanofi-Aventis, Metro Naps, Pepsi Co, Actelion, Cephalon, GSK, Eli Lilly, Merck, Ono Pharma, UCB, and CHDI.
Rights and permissions
About this article
Cite this article
Bettica, P., Squassante, L., Groeger, J. et al. Differential Effects of a Dual Orexin Receptor Antagonist (SB-649868) and Zolpidem on Sleep Initiation and Consolidation, SWS, REM Sleep, and EEG Power Spectra in a Model of Situational Insomnia. Neuropsychopharmacol 37, 1224–1233 (2012). https://doi.org/10.1038/npp.2011.310
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2011.310
Keywords
This article is cited by
-
An examination of sleep spindle metrics in the Sleep Heart Health Study: superiority of automated spindle detection over total sigma power in assessing age-related spindle decline
BMC Neurology (2023)
-
Doxepin is more effective than zolpidem in improving executive function in patients with insomnia disorder
Sleep and Breathing (2023)
-
Effects of the selective orexin-2 receptor antagonist JNJ-48816274 on sleep initiated in the circadian wake maintenance zone: a randomised trial
Neuropsychopharmacology (2022)
-
Dual Orexin Receptor Antagonists (DORAs) as an Adjunct Treatment for Smoking Cessation
CNS Drugs (2022)
-
Nonclinical pharmacology of daridorexant: a new dual orexin receptor antagonist for the treatment of insomnia
Psychopharmacology (2021)