Abstract
A 20-minute session of 10 Hz repetitive transcranial magnetic stimulation (rTMS) of Brodmann Area (BA) nine of the left dorsolateral prefrontal cortex (DLPFC) can produce analgesic effects on postoperative and laboratory-induced pain. This analgesia is blocked by pretreatment with naloxone, a μ-opioid antagonist. The purpose of this sham-controlled, double-blind, crossover study was to identify the neural circuitry that underlies the analgesic effects of left DLPFC rTMS, and to examine how the function of this circuit, including midbrain and medulla, changes during opioid blockade. Fourteen healthy volunteers were randomized to receive intravenous saline or naloxone immediately before sham and real left DLPFC rTMS on the same experimental visit. One week later, each participant received the novel pretreatment but the same stimulation paradigm. Using short sessions of heat on capsaicin-sensitized skin, hot allodynia was assessed during 3 Tesla functional magnetic resonance imaging (fMRI) scanning at baseline, post-sham rTMS, and post-real rTMS. Data were analyzed using whole-brain voxel-based analysis, as well as time series extractions from anatomically-defined regions of interest representing midbrain and medulla. Consistent with previous findings, real rTMS significantly reduced hot allodynia pain ratings. This analgesia was associated with elevated blood oxygenation-level dependent (BOLD) signal in BAs 9 and 10, and diminished BOLD signal in the anterior cingulate, thalamus, midbrain, and medulla during pain. Naloxone pretreatment largely abolished rTMS-induced analgesia, as well as rTMS-induced attenuation of BOLD signal response to painful stimuli throughout pain processing regions, including midbrain and medulla. These preliminary results suggest that left DLPFC rTMS drives top-down opioidergic analgesia.
Similar content being viewed by others
INTRODUCTION
Non-invasive forms of brain stimulation such as transcranial direct current stimulation and transcranial magnetic stimulation (TMS) are currently being investigated as alternative or adjunctive therapies for pain. Clinical interest in these techniques continues to grow because of rising opiate abuse and inadequate pain management strategies (McCarberg, 2011; Warner et al, 2009). Despite this enthusiasm, studies on the efficacy of repetitive TMS (rTMS) for pain have produced mixed results (O'Connell et al, 2011). Some of the most promising and informative research has focused on rTMS for perioperative pain. In two different postoperative studies, a single session of left dorsolateral prefrontal cortex (DLPFC) rTMS after gastric bypass surgery reduced morphine self-administration by 40% when compared with sham stimulation (Borckardt et al, 2008; Borckardt et al, 2006b). These data are particularly fascinating given the role of the DLPFC in top-down pain processing (Lorenz et al, 2003).
Centered at the juncture of Brodmann Areas (BAs) 9 and 46 (Rajkowska and Goldman-Rakic, 1995a, 1995b), the DLPFC remains a popular therapeutic target for rTMS, given its accessible location and presumed role in high-order cognition and emotional valence (Fuster, 2001; Hains et al, 2009; Kober et al, 2008). Animal and human studies suggest that cingulofrontal regions like DLPFC may modulate pain perception via recruitment of opioidergic midbrain and brainstem structures like the periaqueductal gray (PAG) and the rostroventromedial medulla, respectively (Bingel et al, 2006; Hardy and Haigler, 1985; Lorenz et al, 2003; Valet et al, 2004; Wager et al, 2004). These data outline the functional circuitry that might be involved in the analgesic effects of DLPFC rTMS.
Although many studies aim to evaluate the clinical efficacy of DLPFC rTMS for pain management, few have examined how it affects pain processing. Imaging the cerebral signature of pain (Tracey and Mantyh, 2007) before and after left DLPFC rTMS might reveal information about pain circuitry, and help to elucidate the mechanism by which prefrontal rTMS may produce analgesia. Previous studies suggest that opioid blockade abolishes left but not right DLPFC rTMS-induced analgesia (de Andrade et al, 2011; Taylor et al, 2012). In this study, our a priori hypothesis was that left DLPFC rTMS would attenuate blood oxygenation-level dependent (BOLD) signal response to painful stimuli in pain processing regions. More specifically, we anticipated that midbrain and medulla BOLD signal changes induced by left DLPFC rTMS would be abolished by pretreatment with the μ-opioid antagonist naloxone.
MATERIALS AND METHODS
The Institutional Review Board of the Medical University of South Carolina approved this sham-controlled, double-blind, crossover study. Fifteen healthy volunteers signed a written informed consent. One participant withdrew from the study without completing the experiments. Thus, 14 healthy volunteers participated in the study.
Screening Procedures
Prospective participants were interviewed over the phone. In order to qualify for the study, each right-handed healthy control had to be 18–45 years of age without a history of seizures, depression, or pain conditions. Stimulants or other medications that lower seizure threshold were also part of the exclusion criteria. Qualified individuals were invited to a screening visit during which risks and benefits were explained. All participants were tested for opiate use and females were also tested for pregnancy hormones.
Study Design
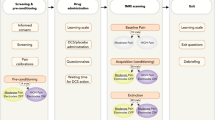
Outside of the magnetic resonance imaging (MRI) scanner, participants underwent resting motor threshold (rMT) assessment, left DLPFC localization and preliminary pain testing (Figure 1). Next, participants were placed in a 3 Tesla MRI scanner for baseline pain testing. At the conclusion of baseline testing, participants were asked to rate the pain that they had experienced. Participants were subsequently removed from the scanner and randomized to receive ∼10 ml intravenous (I.V.) naloxone (0.1 mg/kg) or saline immediately before 20 min of sham left DLFPC rTMS. Following sham rTMS, participants returned to the scanner for the same block testing performed at baseline. After rating their pain, participants were removed from the scanner for 20 min of real left DLPFC rTMS. Participants then returned to the scanner for the final block test. The order of rTMS treatments was not counterbalanced because administering real rTMS first might preclude our ability to cleanly evaluate the effects of sham rTMS on pain ratings and BOLD signal in pain processing regions.
Schematic representation of study methodology. The vertical line separates preparatory steps (left) from experimental steps (right). After rMT assessment via parameter estimation by sequential testing and BA 9 localization via BEAM F3 method, participants underwent preliminary pain testing on capsaicin-sensitized skin. The temperature reported as ‘7 out of 10’ on a VAS during preliminary testing was used during fMRI scanning at baseline, post-sham rTMS and post-real rTMS (gray boxes). Participants were randomized to I.V. saline on one visit and naloxone on the other visit.
Motor Threshold Assessment and Prefrontal Localization
A Neuronetics Model 2100 Therapy System with an iron-core, solid-state figure-of-8 coil (Neuronetics, Inc.; Malvern, PA) was used to assess rMT. The TMS machine was initially set to 50% of its maximal output. Single pulses were administered near the primary motor cortex until the area on the scalp that produced contraction of adbuctor pollicis brevis was identified. Custom-developed software that use adaptive parameter estimation by sequential testing data was used to determine rMT, or the minimum machine output necessary for visible adbuctor pollicis brevis contraction 50% of the time that pulses were delivered (Borckardt et al, 2006a). Once rMT was determined, the location on the scalp that corresponds to BA 9 of the left DLPFC was found using the Beam F3 method (Beam et al, 2009). A marker was used to denote this location for subsequent rTMS sessions.
Pain Measures
Pain assessments were performed using cutaneous hot stimuli via a 30 × 30 mm ATS thermode on the Medoc Pathway System (Israel). Block testing was done with a slightly adapted model of hot allodynia (Petersen and Rowbotham, 1999). First, 0.1% capsaicin cream was applied to a 40 × 40 mm region of skin 12 cm away from the wrist on the right volar forearm. After 30 min, the capsaicin was cleaned off, and cutaneous heat stimuli were applied to the capsaicin-treated skin via the ATS thermode. Twenty-two second blocks of fixed temperatures were used to assess pain. After each trial, participants rated pain unpleasantness and intensity using an 11-point (0–10) rating scale.
During preliminary testing, multiple trials were conducted to determine the temperature that each participant consistently rated as ‘7 out of 10’ in intensity. Studies have shown that stimuli rated as ‘7 out of 10’ produce reproducible pain ratings and functional MRI (fMRI) activation without posing significant risk to participants (deCharms et al, 2005). The temperature rated as ‘7 out of 10’ during preliminary testing was used as the test temperature for the remainder of that participant’s experimental visit.
Structural and Functional Imaging
All scanning was performed on a 3 Tesla Siemens TIM Trio (Siemens, Erlangen, Germany). High-resolution T1-weighted anatomical images were acquired for each participant (TR=1750 ms, TE=4 ms, voxel dimensions 1.0 × 1.0 × 1.0 mm, 160 slices). BOLD functional imaging was carried out using a gradient echo based echo-planar imaging scan sequence (TR=2520 ms, TE=25 ms, TA=9:08, voxel dimensions 3.0 × 3.0 × 3.0 mm, 44 slices). Each BOLD pain scan (baseline, post-sham rTMS, and post-real rTMS) contained 12 22 s blocks of heat alternating with 12 22 s blocks of rest (Figure 1). Pain ratings were acquired immediately upon completion of each functional run.
Intravenous Drug Infusion and rTMS Treatments
Immediately before sham rTMS, a bolus of ∼10 ml 0.1 mg/kg naloxone or saline was infused intravenously into the participant’s left arm. Neither the participant nor the person administering the I.V. knew which clear liquid was being injected. I.V. naloxone was chosen over oral μ-opioid antagonists like naltrexone because of its consistent bioavailability and immediate onset. Moreover, the biological half-life of I.V. naloxone (60–90 min) was sufficiently long for experimental testing (Berkowitz, 1976; Fishman et al, 1973). Naloxone has previously been shown to be safe to administer to healthy volunteers and drug-naïve subjects (Borras et al, 2004; Jefferys and Volans, 1983).
Immediately following naloxone or saline infusion, participants received 20 min of left DLPFC sham rTMS. The eSham system was implemented in conjunction with a specialized Neuronetics sham TMS coil. Two Thymapad stimulus electrodes (Somatics, LLC; Lake Bluff, IL) were placed on the scalp location that corresponded to left DLPFC. Studies have shown that the eSham system effectively blinds participants to TMS treatment (active vs sham) (Borckardt et al, 2011a; Taylor et al, 2012). The eSham system was only active during sham rTMS although electrodes were placed in the appropriate position during subsequent real rTMS (20 min of 10 Hz, 5 s on, 10 s off, 110% RMT).
Blinding
Unblinded nurses who were not present during TMS administration, data acquisition or data analysis prepared the saline or naloxone syringes according to a randomization chart. These individuals had minimal interaction with study participants. This arrangement allowed the Principal Investigator (J.J.T.), who ran all study sessions, to remain blind to rTMS pretreatment. Study participants, by contrast, were blind to both pretreatment and rTMS allocation. At the conclusion of each rTMS session, participants were asked to guess whether they had received real or sham stimulation, and to rate the confidence of their guess on a scale from 0–10.
Data Analysis
Pain Ratings
Pain ratings data were processed using IBM SPSS Statistics Version 20 (New York, NY). All data were reviewed for quality before being locked for statistical analysis. Results are expressed as mean intensity rating±SE. Analysis of variance (ANOVA) tests were used to examine effects with Bonferroni adjustments for post hoc analyses.
fMRI Preprocessing
Functional images were preprocessed using Statistical Parametric Mapping 8 software (SPM8; London, UK), which was implemented in MATLAB (Natick, MD). Preprocessing steps included realignment to the first volume in the run, coregistration of the mean realigned image with individual structural images, normalization to the Montreal Neurological Institute average echo-planar imaging template, spatial smoothing with an 8 mm3 Gaussian kernel (full-width at half-maximum), and high-pass filtering (128 s) to remove low frequency noise.
fMRI Whole-Brain Analyses
A general linear model approach was used to examine BOLD signal changes during pain relative to rest blocks. For each participant, first-level contrasts for pain greater than rest were created. The resulting contrast images were entered into a random effects 2 × 3 full factorial model to examine group-level differences. The first factor was pretreatment (saline or naloxone) and the second factor was pain block (baseline, post-sham rTMS, and post-real rTMS). Thermode ramp times were subtracted from data before specifying the timing of pain and rest blocks. The baseline pain contrast was used as a comprehensive (P<0.05 uncorrected voxel level threshold) inclusive mask for evaluation of post-sham and post-real pain contrasts. Significant clusters comprised of five or more voxels were reported (P<0.005 uncorrected voxel level threshold; P<0.005 cluster level threshold). Montreal Neurological Institute coordinates were converted to Talairach coordinates using nonlinear registration (Lacadie et al, 2008). The Talairach Daemon was used for region identification and MRIcron was used for data visualization.
fMRI Region of Interest Analyses
An anatomically-defined region of interest (ROI) analysis was used to address our a priori hypothesis about BOLD signal changes in the midbrain and medulla for the pain minus rest contrast during pain at baseline, pain post-sham rTMS, and pain post-real rTMS. ROIs were defined using a Talairach atlas overlay in MANGO (Research Imaging Institute, UT Health Science Center at San Antonio, TX, USA; http://ric.uthscsa.edu/mango/) and covered all structures located in the midbrain and medulla. Time courses from both ROIs were extracted from the preprocessed time series for each individual using MarsBaR (Brett et al, 2002). Percent signal change (PSC) was calculated based on the rest block immediately preceding each pain block. ANOVAS were run in SPSS. Results are expressed as mean PSC±SE.
RESULTS
Pretreatment Baseline Comparisons
There were no significant differences between the saline and naloxone pretreatment visits in terms of rMT assessment (P=0.68; Student’s t-tests, two-tailed) or block pain test temperature (P=0.84).
TMS Blind Integrity
As a group, participants were successfully blind to rTMS treatment condition. Participants successfully identified 13 out of 28 (46%) sham treatments and 14 out of 28 (50%) real treatments (χ2 (1)=0.02, not significant). The mean confidence rating for correct and incorrect guesses was 4.93 (±0.95) and 4.34 (±0.81), respectively. Confidence ratings between correct and incorrect guesses were not significantly different (P=0.29). Thus, even participants who guessed correctly were no more or less confident than participants who guessed incorrectly.
Pain Ratings Results
A two-way repeated measures ANOVA (pretreatment: saline, naloxone; rTMS treatment: sham, real rTMS) revealed a significant effect for rTMS treatment (F(3,11)= 27, P<0.001) and a significant pretreatment*rTMS effect (F(3,11)=4, P=0.04) for pain intensity. To probe the interaction, two (pretreatment condition) one-way ANOVAs were conducted.
During the saline visit, participants rated the mean temperature of 45.51 °C (±0.98 °C) at an intensity of 7.13 (±0.11) during preliminary testing outside of the scanner, 7.46 (±0.26) during baseline testing, 7.36 (±0.24) following sham rTMS, and 5.61 (±0.26) following real rTMS (Figure 2). A one-way ANOVA revealed a significant effect for rTMS treatment (F(3,30)=16, P<0.001). Bonferroni corrected pairwise comparisons identified significant differences between preliminary pain ratings vs pain ratings after real rTMS (P<0.001), baseline pain ratings vs pain ratings after real rTMS (P<0.001), and pain ratings following sham rTMS vs pain ratings following real rTMS (P<0.001).
Comparison of block pain intensity ratings (mean±SE). Twelve 22-second blocks of a fixed temperature reported as ‘7 out of 10’ on a VAS during preliminary testing were applied to capsaicin-sensitized skin during fMRI scanning at baseline, post-sham rTMS, and post-real rTMS. Asterisks (*P<0.05, **P<0.001) indicate Bonferroni corrected significant within-group differences between ratings after real rTMS and all prior ratings. The asterisks to the right of the bar indicate a Bonferroni corrected significant between-group difference after real rTMS.
During the naloxone visit, participants rated the mean temperature of 45.25 °C (±0.89 °C) at an intensity of 7.26 (±0.13) during preliminary testing outside of the scanner, 7.18 (±0.18) during baseline testing, 7.54 (±0.15) following sham rTMS, and 6.75 (±0.21) following real rTMS. A one-way ANOVA revealed a significant effect for rTMS treatment (F(3,4)=3, P=0.04). Bonferroni corrected pairwise comparisons revealed a significant difference between pain ratings following sham rTMS vs pain ratings following real rTMS (P=0.023).
fMRI Whole-Brain Results
Baseline Pain
A full factorial analysis revealed significant BOLD signal increases in a number of pain processing areas during baseline scanning when rest was subtracted from pain. Significant clusters were found in regions such as anterior cingulate cortex (ACC), insula, thalamus, and DLPFC (Figure 3a).
Visual of whole-brain analysis. Participants received 12 22 s blocks of rest alternating with 12 22 s blocks of a fixed temperature reported as ‘7 out of 10’ on a VAS during preliminary pain testing at baseline, post-sham rTMS, and post-real rTMS. Rest block data were subtracted from pain block data for all analyses. Panel (a) shows significant BOLD signal elevations during baseline testing before any intervention. This baseline pain contrast was used as a comprehensive (P<0.05 uncorrected voxel level threshold) inclusive mask for evaluation of post-sham and post-real pain contrasts (P<0.005 uncorrected voxel level threshold; P<0.005 cluster level threshold; cluster size of five or greater). Panel (b) shows the brain regions that exhibit significantly less BOLD signal activation during pain after real rTMS than during pain after sham rTMS with saline pretreatment. Panel (c) shows how naloxone pretreatment largely abolishes the BOLD signal decreases driven by real rTMS shown in Panel (b).
Pain Processing Following Real or Sham rTMS
With saline pretreatment, a number of cortical and subcortical regions exhibited lower BOLD signal during pain post-real rTMS relative to post-sham rTMS (Figure 3b). These regions included ACC, thalamus, midbrain, and medulla. When naloxone pretreatment was administered, however, the rTMS-induced attenuation of BOLD signal during pain post-real rTMS was largely abolished (Figure 3c). There were also regions of the PFC (BA9 and BA 10) that exhibited higher BOLD signal exclusively post-real rTMS during pain (Table 1).
fMRI ROI Results
Midbrain
A repeated measures ANOVA revealed a significant effect for rTMS (F(1,13)=9. P=0.011) and a significant rTMS*pretreatment effect (F(1,13)=6, P=0.033). To probe the interaction, two (pretreatment condition) one-way ANOVAS and a t-test were conducted.
During the saline visit, the average PSC in the midbrain for the pain minus rest contrast was 0.16% (±0.04%) at baseline, 0.15% (±0.03%) post-sham rTMS, and 0.00% (±0.04%) post-real rTMS. A one-way ANOVA revealed a significant effect for rTMS after saline pretreatment (F(2,39)=7, P=0.003; Figure 4a). Bonferroni corrected pairwise comparisons identified significant differences in signal change at baseline vs post-real rTMS with saline pretreatment (P=0.009) and post-sham rTMS with saline pretreatment vs post-real rTMS with saline pretreatment (P=0.009).
Graphs of midbrain and medulla PSC (mean±SEM). Midbrain (Panel (a): green) and medulla (Panel (b); purple) ROIs were extracted from a Talairach atlas overlay and used for time series extractions via MarsBaR. An asterisk (* P<0.05) above a bar indicates a significant difference as measured via ANOVA.
During the naloxone visit, the average PSC in the midbrain for the pain minus rest contrast was 0.13% (±0.04%) at baseline, 0.10% (±0.03%) post-sham rTMS, and 0.13% (±0.05%) post-real rTMS. A one-way ANOVA did not reveal a significant effect for rTMS after naloxone pretreatment (F(2,39)=0.232, P=0.794).
A t-test comparing PSC in the midbrain for the pain minus rest contrast revealed a significant difference between post-real rTMS with saline pretreatment vs post-real rTMS with saline pretreatment (P=0.037; Figure 4a).
Medulla
A repeated measures ANOVA revealed a significant effect for rTMS (F(1,13)=6, P=0.031) and a significant rTMS*pretreatment effect (F(1,13)=5, P=0.047). To probe the interaction, two (pretreatment condition) one-way ANOVAS and a t-test were conducted.
During the saline visit, the average PSC in the medulla for the pain minus rest contrast was 0.18% (±0.03%) at baseline, 0.18% (±0.04%) post-sham rTMS, and 0.01% (±0.05%) post-real rTMS (Figure 4b). A one-way ANOVA revealed a significant effect for rTMS after saline pretreatment (F(2,39)=13, P<0.001; Figure 4b). Bonferroni corrected pairwise comparisons identified significant differences in signal change at baseline vs post-real rTMS with saline pretreatment (P<0.001) and post-sham rTMS with saline pretreatment vs post-real rTMS with saline pretreatment (P<0.001).
During the naloxone visit, the average PSC in the medulla for the pain minus rest contrast was 0.23% (±0.04%) at baseline, 0.19% (±0.07%) post-sham rTMS, and 0.16% (±0.04%) post-real rTMS. A one-way ANOVA did not reveal a significant effect for rTMS after naloxone pretreatment (F(2,39)=0.57, P=0.945).
A t-test comparing PSC in the medulla for the pain minus rest contrast revealed a significant difference between post-real rTMS with saline pretreatment vs post-real rTMS with saline pretreatment (P=0.006; Figure 4b).
DISCUSSION
rTMS Induces Naloxone-Reversible BOLD Signal Changes in Pain Processing Regions
This study combined neuropharmacologic manipulation with neuroimaging to examine how neuromodulation of DLPFC with rTMS affects the cerebral signature of pain. Consistent with our a priori hypothesis, real DLPFC rTMS with saline pretreatment was associated with decreased pain ratings, and modestly decreased BOLD signal response to painful stimuli in pain processing regions. Midbrain and medulla were among the regions affected by rTMS, exhibiting significant PSC decreases that were largely abolished by naloxone pretreatment. These results support and extend previous studies, in which naloxone pretreatment significantly diminished the analgesic effects of rTMS (Taylor et al, 2012). To our knowledge, this is the first study to show that a single session of left DLPFC rTMS has the capacity to alter BOLD signal response to noxious stimuli in pain processing areas. It is also the first study to image the circuitry that might underlie the opioidergic basis of left prefrontal rTMS-induced analgesia.
Evidence for a Top-Down Analgesic Circuit
This study builds upon animal studies that show a top-down functional circuit between PFC, midbrain, and medulla. Reciprocal anatomical connections between PFC, PAG, and rostroventromedial medulla have been documented using retrograde tracing (An et al, 1998; Bragin et al, 1984) and c-fos immmunoreactivity (Lim et al, 2009). These projections have been shown to have physiological relevance for nociception and pain (Condes-Lara et al, 1989). In rats and cats alike, electrical stimulation of various cingulofrontal regions produces behaviorally relevant analgesia that is correlated with reduced thalamic and midbrain spiking activity in response to noxious stimuli (Andersen, 1986; Hardy and Haigler, 1985; Hutchison et al, 1996). There is also evidence from learned helplessness models of depression and PTSD that PFC modulates the protective effects of perceived control via top-down regulation of the dorsal raphe nucleus and its serotonergic projections (Hammack et al, 2012; Robbins, 2005). This circuit could explain why pain and depression are highly comorbid disorders (Bair et al, 2003; Emptage et al, 2005) that may both respond to prefrontal rTMS (George and Aston-Jones, 2010).
Although the mechanism remains unclear, human data suggest that prefrontal inhibition of functional coupling within the circuit from midbrain to thalamus to ACC or PFC is critical for endogenous pain relief (Akitsuki and Decety, 2009; Lorenz et al, 2003; Tracey et al, 2002). In one PET experiment, elevated left DLPFC activity was associated with diminished interregional correlation between midbrain and medial thalamus. These network changes correlated negatively with perceived stimulus intensity and unpleasantness during hot allodynia (Lorenz et al, 2003). The existence of a top-down circuit is further evidenced by a meta-analysis of 162 imaging studies, in which coactivation of BA 9 and midbrain regions like PAG was found to be essential for assigning emotional valence, a process that likely contributes to noxious stimulus interpretation (Kober et al, 2008). A functional connection between the highly evolved PFC (Fuster, 2001; Hains et al, 2009) and the phylogenetically conserved opioid circuit (Eippert et al, 2009; Zambreanu et al, 2005) may be the evolutionary substrate for low fidelity pain perception and adaptive stress management.
The Role of TMS in Elucidating Pain Circuitry
In order to move beyond functional connectivity based on time correlations, it is necessary to develop focal stimulation techniques like TMS to map and modulate circuitry. Placebo analgesia, for example, has previously been shown to be associated with naloxone-reversible BOLD signal changes in DLPFC, ACC, and PAG (Eippert et al, 2009; Wager et al, 2004). There is now evidence to suggest that reducing DLPFC activity with low-frequency rTMS blocks opiate-mediated analgesia (Krummenacher et al, 2010), while enhancing DLPFC activity with high-frequency rTMS induces opiate-mediated analgesia (Taylor et al, 2012). Left DLPFC rTMS has also been shown to suppress the analgesic effects of perceived noxious stimulus controllability, findings that corroborate the animal literature on a prefrontal control circuit (Borckardt et al, 2011b).
Limitations of the Present Study
There are a number of limitations that should be considered when interpreting this study. First, participants received sham rTMS before real rTMS, because a reversal of this order would have made it impossible to cleanly measure the effects of sham rTMS. Although the rationale for administering sham rTMS before real rTMS is sound, it is plausible that order of treatments confounded our results.
Second, the precise level of naloxone blockade is unknown. The dose of naloxone used in the study was identical to the dose used in our previous study (Taylor et al, 2012) and similar to doses used in studies examining BOLD signal changes in pain processing regions (Borras et al, 2004; Willer et al, 1981). The biological half-life of naloxone has historically been reported as 60–90 min (Berkowitz, 1976; Fishman et al, 1973). The fact that behavioral testing occurred within 60 min of pretreatment suggests that the majority of naloxone had not yet lost its pharmacologic activity. Nevertheless, there is a possibility that reduced opioid blockade explains the significant decrease in block ratings after real TMS with naloxone pretreatment. A more likely explanation for this finding is that opioids are necessary, but not sufficient for prefrontal rTMS-induced analgesia. This explanation is validated by the fact that the average pain rating following real TMS with saline pretreatment (5.61±0.24) was significantly lower than the average pain rating following real TMS with naloxone pretreatment (6.75±0.21). Similar results were found in our last study (Taylor et al, 2012).
A third limitation is that all fMRI analyses were run using a pain minus rest contrast. It is important to note that capsaicin cream, even without thermode heat, can be interpreted as a painful stimulus. Thus, rest blocks likely contained some BOLD signal changes in pain processing areas. This design limitation raises the possibility that the results would have been more robust had the rest block been free of capsaicin.
One final limitation of note is that we were unable to use high-resolution scanning for midbrain and medulla because of our decision to get full brain coverage during pain. This decision prevented us from investigating BOLD signal changes in small, poorly differentiated gray matter regions like the PAG (Linnman et al, 2012). Studies with high-resolution scanning are needed to replicate and refine our findings.
Summary
A single session of left DLPFC rTMS produced an opioid-dependent analgesia that was associated with augmented BOLD signal in BA 9 and 10 during pain and attenuated BOLD signal in ACC, thalamus, midbrain, and medulla during pain. It is difficult to interpret the directionality of BOLD signal changes in midbrain and medulla because these structures may exhibit signal increases or decreases during pain (Becerra et al, 2006). Mu-opioid receptor agonism has traditionally been linked with the inhibition of synaptic activity (Standifer and Pasternak, 1997). This mechanism may explain why I.V. morphine administration diminishes BOLD signal in midbrain and various pain processing structures (Becerra et al, 2006). From this perspective, morphine and left DLPFC rTMS have similar directional effects on BOLD signal in pain processing areas. With future studies aimed at revealing mechanisms and maximizing analgesia with serial rTMS doses, prefrontal TMS may eventually become clinically useful for chronic and perioperative pain management.
References
Akitsuki Y, Decety J (2009). Social context and perceived agency affects empathy for pain: an event-related fMRI investigation. Neuroimage 47: 722–734.
An X, Bandler R, Ongur D, Price JL (1998). Prefrontal cortical projections to longitudinal columns in the midbrain periaqueductal gray in macaque monkeys. J Comp Neurol 401: 455–479.
Andersen E (1986). Periaqueductal gray and cerebral cortex modulate responses of medial thalamic neurons to noxious stimulation. Brain Res 375: 30–36.
Bair MJ, Robinson RL, Katon W, Kroenke K (2003). Depression and pain comorbidity: a literature review. Arch Intern Med 163: 2433–2445.
Beam W, Borckardt JJ, Reeves ST, George MS (2009). An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul 2: 50–54.
Becerra L, Harter K, Gonzalez RG, Borsook D (2006). Functional magnetic resonance imaging measures of the effects of morphine on central nervous system circuitry in opioid-naive healthy volunteers. Anesth Analg 103: 208–216.
Berkowitz BA (1976). The relationship of pharmacokinetics to pharmacological activity: morphine, methadone and naloxone. Clin Pharmacokinet 1: 219–230.
Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C (2006). Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 120: 8–15.
Borckardt JJ, Nahas Z, Koola J, George MS (2006a). Estimating resting motor thresholds in transcranial magnetic stimulation research and practice: a computer simulation evaluation of best methods. J ECT 22: 169–175.
Borckardt JJ, Reeves ST, Beam W, Jensen MP, Gracely RH, Katz S et al (2011a). A randomized, controlled investigation of motor cortex transcranial magnetic stimulation (TMS) effects on quantitative sensory measures in healthy adults: evaluation of TMS device parameters. Clin J Pain 27: 486–494.
Borckardt JJ, Reeves ST, Frohman H, Madan A, Jensen MP, Patterson D et al (2011b). Fast left prefrontal rTMS acutely suppresses analgesic effects of perceived controllability on the emotional component of pain experience. Pain 152: 182–187.
Borckardt JJ, Reeves ST, Weinstein M, Smith AR, Shelley N, Kozel FA et al (2008). Significant analgesic effects of one session of postoperative left prefrontal cortex repetitive transcranial magnetic stimulation: a replication study. Brain Stimul 1: 122–127.
Borckardt JJ, Weinstein M, Reeves ST, Kozel FA, Nahas Z, Smith AR et al (2006b). Postoperative left prefrontal repetitive transcranial magnetic stimulation reduces patient-controlled analgesia use. Anesthesiology 105: 557–562.
Borras MC, Becerra L, Ploghaus A, Gostic JM, DaSilva A, Gonzalez RG et al (2004). fMRI measurement of CNS responses to naloxone infusion and subsequent mild noxious thermal stimuli in healthy volunteers. J Neurophysiol 91: 2723–2733.
Bragin EO, Yeliseeva ZV, Vasilenko GF, Meizerov EE, Chuvin BT, Durinyan RA (1984). Cortical projections to the periaqueductal grey in the cat: a retrograde horseradish peroxidase study. Neurosci Lett 51: 271–275.
Brett M, Jean-Luc A, Valabregue R, Poline J (2002) Region of interest analysis using an SPM toolbox 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan.
Condes-Lara M, Omana Zapata I, Leon-Olea M, Sanchez-Alvarez M (1989). Dorsal raphe and nociceptive stimulations evoke convergent responses on the thalamic centralis lateralis and medial prefrontal cortex neurons. Brain Res 499: 145–152.
de Andrade DC, Mhalla A, Adam F, Texeira MJ, Bouhassira D (2011). Neuropharmacological basis of rTMS-induced analgesia: the role of endogenous opioids. Pain 152: 320–326.
deCharms RC, Maeda F, Glover GH, Ludlow D, Pauly JM, Soneji D et al (2005). Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci USA 102: 18626–18631.
Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J et al (2009). Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63: 533–543.
Emptage NP, Sturm R, Robinson RL (2005). Depression and comorbid pain as predictors of disability, employment, insurance status, and health care costs. Psychiatr Serv 56: 468–474.
Fishman J, Roffwarg H, Hellman L (1973). Disposition of naloxone-7,8,3H in normal and narcotic-dependent men. J Pharmacol Exp Ther 187: 575–580.
Fuster JM (2001). The prefrontal cortex—an update: time is of the essence. Neuron 30: 319–333.
George MS, Aston-Jones G (2010). Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 35: 301–316.
Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF (2009). Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl AcadSci USA 106: 17957–17962.
Hammack SE, Cooper MA, Lezak KR (2012). Overlapping neurobiology of learned helplessness and conditioned defeat: implications for PTSD and mood disorders. Neuropharmacology 62: 565–575.
Hardy SG, Haigler HJ (1985). Prefrontal influences upon the midbrain: a possible route for pain modulation. Brain Res 339: 285–293.
Hutchison WD, Harfa L, Dostrovsky JO (1996). Ventrolateral orbital cortex and periaqueductal gray stimulation-induced effects on on- and off-cells in the rostral ventromedial medulla in the rat. Neuroscience 70: 391–407.
Jefferys DB, Volans GN (1983). An investigation of the role of the specific opioid antagonist naloxone in clinical toxicology. Hum Toxicol 2: 227–231.
Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD (2008). Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage 42: 998–1031.
Krummenacher P, Candia V, Folkers G, Schedlowski M, Schonbachler G (2010). Prefrontal cortex modulates placebo analgesia. Pain 148: 368–374.
Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X (2008). More accurate Talairach coordinates for neuroimaging using non-linear registration. Neuroimage 42: 717–725.
Lim LW, Temel Y, Visser-Vandewalle V, Blokland A, Steinbusch H (2009). Fos immunoreactivity in the rat forebrain induced by electrical stimulation of the dorsolateral periaqueductal gray matter. J Chem Neuroanat 38: 83–96.
Linnman C, Moulton EA, Barmettler G, Becerra L, Borsook D (2012). Neuroimaging of the periaqueductal gray: state of the field. Neuroimage 60: 505–522.
Lorenz J, Minoshima S, Casey KL (2003). Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 126: 1079–1091.
McCarberg BH (2011). Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion. Postgrad Med 123: 119–130.
O'Connell NE, Wand BM, Marston L, Spencer S, Desouza LH (2011). Non-invasive brain stimulation techniques for chronic pain. A report of a Cochrane systematic review and meta-analysis. Eur J Phys Rehabil Med 47: 309–326.
Petersen KL, Rowbotham MC (1999). A new human experimental pain model: the heat/capsaicin sensitization model. Neuroreport 10: 1511–1516.
Rajkowska G, Goldman-Rakic PS (1995a). Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex 5: 307–322.
Rajkowska G, Goldman-Rakic PS (1995b). Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex 5: 323–337.
Robbins TW (2005). Controlling stress: how the brain protects itself from depression. Nat Neurosci 8: 261–262.
Standifer KM, Pasternak GW (1997). G proteins and opioid receptor-mediated signalling. Cell Signal 9: 237–248.
Taylor JJ, Borckardt JJ, George MS (2012). Endogenous opioids mediate left dorsolateral prefrontal cortex rTMS-induced analgesia. Pain 153: 1219–1225.
Tracey I, Mantyh PW (2007). The cerebral signature for pain perception and its modulation. Neuron 55: 377–391.
Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS et al (2002). Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci 22: 2748–2752.
Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B et al (2004). Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain–an fMRI analysis. Pain 109: 399–408.
Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ et al (2004). Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303: 1162–1167.
Warner M, Chen LH, Makuc DM (2009). Increase in fatal poisonings involving opioid analgesics in the USA, 1999–2006. NCHS Data Brief 1–8.
Willer JC, Dehen H, Cambier J (1981). Stress-induced analgesia in humans: endogenous opioids and naloxone-reversible depression of pain reflexes. Science 212: 689–691.
Zambreanu L, Wise RG, Brooks JC, Iannetti GD, Tracey I (2005). A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain 114: 397–407.
Acknowledgements
We thank Kathryn Beaver, RN and Tracey Greene, RN for their assistance in conducting this study. JJT is funded by 1F30DA033748-01 (NIDA) and a Grant-In-Kind from the Center for Biomedical Imaging (CBI) at the Medical University of South Carolina. MC is funded by T32DA007288. CH is funded by K01DA0267756. A Clinical Trail Agreement with Neuronetics provided the TMS treatment cards used to drive laboratory owned TMS machines in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Taylor, J., Borckardt, J., Canterberry, M. et al. Naloxone-Reversible Modulation of Pain Circuitry by Left Prefrontal rTMS. Neuropsychopharmacol 38, 1189–1197 (2013). https://doi.org/10.1038/npp.2013.13
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.13
Keywords
This article is cited by
-
The role of endogenous opioids in mindfulness and sham mindfulness-meditation for the direct alleviation of evoked chronic low back pain: a randomized clinical trial
Neuropsychopharmacology (2023)
-
New Developments in Non-invasive Brain Stimulation in Chronic Pain
Current Physical Medicine and Rehabilitation Reports (2020)
-
Can neuroimaging help combat the opioid epidemic? A systematic review of clinical and pharmacological challenge fMRI studies with recommendations for future research
Neuropsychopharmacology (2019)
-
Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism
Molecular Psychiatry (2019)
-
Role of the Prefrontal Cortex in Pain Processing
Molecular Neurobiology (2019)