Abstract
In a rat model of drug craving and relapse, cue-induced drug seeking progressively increases after withdrawal from methamphetamine and other drugs, a phenomenon termed ‘incubation of drug craving’. However, current experimental procedures used to study incubation of drug craving do not incorporate negative consequences of drug use, which is a common factor promoting abstinence in humans. Here, we studied whether incubation of methamphetamine craving is observed after suppression of drug seeking by adverse consequences (punishment). We trained rats to self-administer methamphetamine or palatable food for 9 h per day for 14 days; reward delivery was paired with a tone-light cue. Subsequently, for one group within each reward type, 50% of the lever-presses were punished by mild footshock for 9–10 days, whereas for the other group lever-presses were not punished. Shock intensity was gradually increased over time. Next, we assessed cue-induced reward seeking in 1-h extinction sessions on withdrawal days 2 and 21. Response-contingent punishment suppressed extended-access methamphetamine or food self-administration; surprisingly, food-trained rats showed greater resistance to punishment than methamphetamine-trained rats. During the relapse tests, both punished and unpunished methamphetamine- and food-trained rats showed significantly higher cue-induced reward seeking on withdrawal day 21 than on day 2. These results demonstrate that incubation of both methamphetamine and food craving occur after punishment-induced suppression of methamphetamine or palatable food self-administration. Our procedure can be used to investigate mechanisms of relapse to drug and palatable food seeking under conditions that more closely approximate the human condition.
Similar content being viewed by others
INTRODUCTION
In drug addicts, relapse can occur after prolonged abstinence (Hunt et al, 1971) and is often precipitated by exposure to drug-associated cues that provoke drug craving (O'Brien et al, 1992). On the basis of clinical observations, Gawin and Kleber (1986) had hypothesized that cue-induced cocaine craving progressively increases over the first weeks of abstinence and remains high over extended periods. An analogous phenomenon, termed ‘incubation of drug craving’, was subsequently identified in rats, based on observations that time-dependent increases in cue-induced cocaine or heroin seeking occurred after withdrawal from these drugs (Grimm et al, 2001; Neisewander et al, 2000; Shalev et al, 2001). Subsequent studies have demonstrated that incubation of drug craving also occurs in rats with a history of nicotine, alcohol, or methamphetamine self-administration (Abdolahi et al, 2010; Bienkowski et al, 2004; Shepard et al, 2004).
In these and other studies, time-dependent increases in cue-induced drug seeking after withdrawal were examined in several established procedures to assess cue-induced relapse (Lu et al, 2004b; Marchant et al, 2013b; See, 2005). These include extinction tests in the presence of the drug-associated cues (Conrad et al, 2008; Lee et al, 2006; Lu et al, 2004a), discrete cue-induced reinstatement after extinction (Grimm et al, 2001; Mead et al, 2007), and acquisition of a new conditioned response (Di Ciano and Everitt, 2004). A common feature of these studies and related studies (Pacchioni et al, 2011; Van den Oever et al, 2008; Van den Oever et al, 2010) is that abstinence is forced either by removing the rats from the drug self-administration environment or by conducting extinction training. However, in humans, abstinence is typically self-imposed, despite drug availability, because the drug’s rewarding effects are outweighed by the aversive consequences of seeking or using them (Burman, 1997; Epstein and Preston, 2003; Klingemann, 1991; Marlatt, 1996).
This form of abstinence can be modeled in laboratory animals by punishment of the drug self-administration response, typically in the form of response-contingent presentation of mild intermittent footshock (Marchant et al, 2013a; Panlilio et al, 2003). Over the years, several studies have shown that punishment significantly decreases and even eliminates opiate and psychostimulant self-administration (Johanson, 1975; Panlilio et al, 2003; Pelloux et al, 2007; Smith and Davis, 1974). More recently, we and others have begun to use punishment-based procedures to study relapse to drug seeking induced by drug-priming injections (Panlilio et al, 2003; Panlilio et al, 2005) or drug cues and contexts (Cooper et al, 2007; Economidou et al, 2009; Marchant et al, 2013a; Peck et al, 2013).
In the present study, we used a punishment-based relapse procedure to determine whether cue-induced methamphetamine seeking (as assessed in extinction tests after 2 and 21 withdrawal days) progressively increases or incubates after punishment-induced suppression of extended-access drug self-administration. For comparison purposes, we also assessed incubation of methamphetamine craving under forced abstinence conditions like those typically used in incubation of drug craving studies (Lu et al, 2004b; Pickens et al, 2011). In addition, as incubation of craving has been demonstrated in rats trained to self-administer non-drug rewards (eg, sucrose) (Grimm et al, 2005; Grimm et al, 2002; Lu et al, 2004b), we also tested incubation of reward craving after both forced abstinence and punishment-induced suppression of extended-access palatable food self-administration.
MATERIALS AND METHODS
Subjects
We used male Sprague–Dawley rats (Charles River, Raleigh, NC, USA), weighing 350–400 g before surgery. We group-housed (two per cage) the rats for 1 week before surgery and housed them individually after surgery. We maintained the rats in the animal facility under a reversed 12 : 12 h light/dark cycle with regular (home-cage) food and water freely available, except during the first 1–2 days of food-pellet training, when regular home-cage food was limited to about 20 g/day. Our procedures followed the guidelines outlined in the Guide for the Care and Use of Laboratory Animals (eighth edition; http://grants.nih.gov/grants/olaw/Guide-for-the-Care-and-Use-of-Laboratory-Animals.pdf). Of the 97 rats we used in the study, we excluded three rats because of catheter problems and two rats because of failure to acquire stable methamphetamine self-administration.
Intravenous Surgery
We anesthetized rats with ketamine and xylazine (50 and 5 mg/kg, i.p., respectively) and inserted silastic catheters into the jugular vein, as described previously (Bossert et al, 2009; Theberge et al, 2013). We attached the catheters to a modified 22-gauge cannula that was mounted to the rats’ skulls with dental cement. We injected buprenorphine (0.1 mg/kg, s.c.) after surgery to relieve pain and allowed the rats to recover for 5 days before methamphetamine or food self-administration training. During the recovery, training and punishment phases, we flushed the catheters every 24–48 h with gentamicin (Butler Schein; 5 mg/ml) and sterile saline. We also performed the intravenous catheter surgery in the food-trained rats.
Apparatus
We trained rats in self-administration chambers located inside sound-attenuating cabinets and controlled by a Med Associates system. We equipped each chamber with two levers located 8.5 cm above the grid floor and connected the grid floors to electric shock generators; we also equipped the chambers of the food-trained rats with a pellet dispenser and receptacle located near the active lever. Presses on the retractable active lever activated the infusion pump or the pellet dispenser for the methamphetamine- and food-trained groups, respectively. Presses on the other inactive (stationary) lever had no reinforced consequences. We connected the catheters of rats in the methamphetamine self-administration group to a modified cannula (Plastics One) attached to a liquid swivel (Instech) via polyethylene-50 tubing that was protected by a metal spring. For rats in the food self-administration group, we covered the connecting 5-UP Plastics One cannula with dust caps and did not connect them to the intravenous line.
Training Phase
Methamphetamine
The training procedure for methamphetamine self-administration was similar to that used in our studies on incubation of cocaine, methamphetamine, and heroin craving (Koya et al, 2009; Shepard et al, 2004; Theberge et al, 2013). On the first day of training, we brought the rats to the self-administration room where we chronically housed them in the chambers. We trained rats to self-administer dl-methamphetamine HCl (NIDA) during three 3-h sessions/day (the sessions were separated by 30 min) over 14 days under a fixed-ratio-1 with 20-s timeout reinforcement schedule; lever presses were accompanied by a 5-s compound tone-light cue. These drug access and reinforcement schedule conditions are based on our previous incubation studies with heroin and methamphetamine (Theberge et al, 2012; Theberge et al, 2013). We trained the rats in seven cycles of 2 days of methamphetamine self-administration and one off day in order to prevent loss of body weight during the training phase (under our training conditions of 6 or 9 h daily sessions, rats lose weight after each training day and regain the lost weight during the off days (Shepard et al, 2004; Theberge et al, 2013)). Weight loss is a common side effect of methamphetamine use by humans (Mooney et al, 2009; Neale et al, 2009) or laboratory animals (Krasnova et al, 2010) because of the drug’s anorexic effects (Saito et al, 1995). We dissolved methamphetamine HCl in sterile saline and the rats self-administered the drug at a dose of 0.1 mg/kg/infusion over 3.5 s (0.10 ml/infusion) (Shepard et al, 2004; Theberge et al, 2013). To prevent overdose, we limited the number of infusions per 3-h session to 35. We started the self-administration sessions at the onset of the dark cycle and sessions began with the insertion of the active lever and the illumination of a red house-light that remained on for the duration of the session. At the end of each 3-h session, the house light was turned off, and the active lever was retracted.
Food pellets
Our food training procedure was similar to that used for methamphetamine, with the exception that lever-presses led to the delivery of five 45-mg ‘preferred’ food pellets (TestDiet, Catalog # 1811155, 12.7% fat, 66.7% carbohydrate, and 20.6% protein). In addition, before the formal training sessions, we gave the rats two to three 1-h magazine training sessions during which five pellets were delivered non-contingently every 5 min, accompanied by the 5-s tone-light cue. We have been using the ‘preferred’ TestDiet pellet type in our recent food reinstatement studies (Calu et al, 2013; Cifani et al, 2012; Pickens et al, 2012), because in food-preference tests we found that rats prefer it over other pellet types with different compositions of fat and carbohydrate, and different flavors (Calu et al, 2014). On the basis of pilot studies, we chose five pellets per reward delivery in order to roughly equate the number of rewards earned per day and the number of CS (tone-light cue)—UCS (methamphetamine, food) pairings during training for the two reward types. Because of an experimenter error, we gave 10 food-trained rats four pellets per reward delivery on the first two training days (the data for these rats for training days 1–2 are excluded from Figure 1, and for the repeated measures statistical analyses we used the group’s mean of the other food-trained rats to estimate the values of these rats).
Methamphetamine and food self-administration during the training phase. (a) Timeline of the experiment (see Materials and Methods for details). (b) Methamphetamine (n=46) and (c) food self-administration (n=46) training. Data are mean±SEM of number of methamphetamine infusions (0.1 mg/kg/infusion) or food rewards (five pellets/reward delivery) and of active and inactive lever-presses during the fourteen 9-h daily self-administration sessions. During training, active lever-presses were reinforced on an FR-1 20-s timeout reinforcement schedule; reward delivery was paired with a 5-s tone-light cue.
Punishment Phase
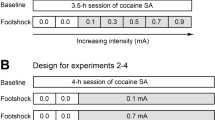
During this phase, the rats continued methamphetamine or food self-administration every day (9-h sessions) under the FR-1 20-s timeout reinforcement schedule that was used during training. For methamphetamine and food-trained rats in the punished groups, 50% of the reinforced lever-presses also resulted in the concurrent delivery of a 0.5-s footshock through the grid floor (Marchant et al, 2013a). We set the initial footshock at 0.12 mA and increased the shock intensity by 0.06 mA every day to a final value of 0.6 or 0.66 mA (a total of 9–10 punishment sessions). Punished responses continued to produce the tone-light cue and 0.1 mg/kg/infusion of methamphetamine or five food pellets. For rats in the unpunished groups, the conditions were identical to the previous training sessions. We gave the rats in the unpunished groups 9–10 methamphetamine or food self-administration sessions to match the duration of drug/food exposure to that of the punished groups. The n’s for each group were ‘METH Punished’=26 (9 rats were given 9 punishment sessions and 17 rats were given 10 punishment sessions); ‘METH Unpunished’=20; ‘Food Punished’=24 (all rats were given 10 punishment sessions); ‘Food Unpunished’=22.
Withdrawal Phase
At the end of the punishment phase, we brought the rats back to the animal colony room and handled them three times per week. We then brought them to the self-administration chambers on the morning of the extinction tests, which were conducted on withdrawal days 2 and 21 after the last punishment session or training session (for the unpunished groups).
Extinction Tests
The extinction tests in the presence of the methamphetamine or food-associated cues consisted of a single 1-h session on withdrawal days 2 and 21. Active lever-presses during testing resulted in contingent presentations of the tone-light cue that was previously paired with methamphetamine infusions or food rewards, but not methamphetamine or food pellets. We assessed cue-induced reward seeking in the extinction tests using both within-subject design and between-subject design (see Results), in which all rats tested on day 2 were also tested on day 21 and some rats were only tested on day 21 (Airavaara et al, 2011). We previously found that incubation of heroin, cocaine, and methamphetamine craving is observed using both within- and between-subject design (Marchant et al, 2013b; Pickens et al, 2011).
Statistical Analyses
We analyzed the data with the statistical program SPSS and followed significant effects (p<0.05) with SPSS post hoc contrasts within the repeated measures ANOVA module. For the training and punishment phases, the dependent variables were the number of methamphetamine infusions or food rewards during 14 training days and the first 9 punishment sessions. The dependent variables for the extinction tests were total (non-reinforced) active lever-presses and inactive lever-presses. We matched the punished and unpunished groups for their methamphetamine intake or food rewards during training, and used these values as a covariate in the analysis of the punishment data. Below, we only report interaction or main effects from the factorial ANOVAs that are critical for data interpretation.
RESULTS
Training Phase
The methamphetamine-trained (total n=46) and food-trained (n=46) rats increased their number of rewards per session earned during training (Figure 1). The repeated measures mixed ANOVA for rewards earned included the between-subject factor reward type (food, methamphetamine) and the within-subject factor of session (sessions 1–14). Analysis showed a significant effect of session × reward type (F(13,1170)=55.6, p<0. 01). The significant interaction reflects that for methamphetamine-trained rats reward intake continued to increase (escalate) for the first 10 training days, while for food-trained rats intake stabilized after 4 days.
Punishment Phase
As the intensity of footshock increased over days, both methamphetamine- and food-reinforced responding decreased. Surprisingly, the non-deprived food-trained rats were more resistant to punishment than the methamphetamine-trained rats (Figure 2). The statistical analysis of rewards earned included the between-subject factors of reward type and punishment condition (unpunished, punished), the within-subject factor of session (sessions 1–9), and the covariate of mean number of rewards earned per day during training. Analysis showed a significant effect of punishment condition × session (F(8,704)=104.5, p<0.01), indicating that the suppressing effect of increasing shock intensity becomes stronger over days, independent of the reward type. Surprisingly, this analysis also showed a significant interaction of punishment condition × reward type × session (F(8,704)=9.0, p<0.01), because the punished food-trained rats self-administered more rewards than the punished methamphetamine-trained rats, an effect that was most pronounced at moderate and high shock intensities (Figure 2).
Suppression of methamphetamine and food self-administration by punishment. Reward delivery (a) and percent suppression from the last training day (b) in the punished (left panel, n=24–26 per group) and unpunished (right panel, n=20–22 per group) methamphetamine and food self-administration groups. Data are mean±SEM of infusions/rewards or percent suppression. During the punishment phase, shock intensity was gradually increased from 0.12 mA to 0.66 mA over 9-10 days, and 50% of rewarded lever presses in the punished groups were accompanied by concurrent footshock. *Different from the methamphetamine punished group, p<0.05.
Extinction Tests (Incubation of Reward Craving Tests)
In the methamphetamine-trained rats, we found time-dependent increases in cue-induced reward seeking in the extinction tests (the operantional measure of incubation of reward craving) in both the punished and unpunished conditions, using both the within-subject and between-subject assessment (Figure 3). In the food-trained rats, we found time-dependent increases in cue-induced reward seeking in punished and unpunished food-trained rats, using the between-subject but not within-subject assessment (Figure 3).
Time-dependent increases in cue-induced reward seeking in the extinction tests (incubation of reward craving) in the punished and unpunished methamphetamine-trained and food-trained rats after withdrawal. Data are mean±SEM responses on the previously active and on the inactive lever in the unpunished and punished methamphetamine-trained rats (a, within-subjects, b, between-subjects) and food-trained rats (c, within-subjects, d, between-subjects) during the 1-h extinction tests on withdrawal days 2 and 21 (n=9–17 per time point). During the extinction tests, lever-presses led to contingent presentations of the tone-light cue previously paired with methamphetamine infusions or food reward during training and punishment. *Different from Day 2, p<0.05. (Note: the data for day 2 in (a, within-subject) and (b, between-subject) are identical, because all rats tested on day 2 were also tested on day 21 (within-subject assessment), while other rats were only tested on day 21 and their data were compared with those tested on day 2 (between-subject assessment)).
Within-subject incubation of reward craving
The statistical analyses of active lever-presses included the between-subject factors of reward type and punishment condition, the within-subject factor of withdrawal day, and the covariate of inactive lever-presses. This analysis showed significant effects of reward type (F(1,50)= 9.8, p<0.01), punishment condition (F(1,50)=13.7, p<0.01), and reward type × withdrawal day (F(1,50)=5.9, p<0.05). The reward type effect reflects that across punishment conditions and withdrawal days, active lever-pressing during testing was overall higher in the food-trained rats. The punishment condition effect reflects that across reward types and withdrawal days, active lever-pressing was higher in the unpunished rats. The reward type × withdrawal day interaction effect reflects that, independent of the punishment conditions, time-dependent increases in cue-induced reward seeking in the extinction tests were only observed in the methamphetamine-trained rats.
Between-subject analysis
The statistical analyses of active lever-presses included the between-subject factors of reward type, punishment condition, and withdrawal day, and the covariate of inactive lever-presses. This analysis showed significant effects of reward type (F(1,83)= 35.0, p<0.01) and punishment condition (F(1,83)=10.5, p<0.01), but not reward type × withdrawal day (p>0.05). The significant effect of reward type reflects that across punishment conditions and withdrawal days, active lever-pressing during testing was overall higher in the food-trained rats. The significant effect of punishment condition reflects that, across reward types and withdrawal days, active lever-pressing was higher in the unpunished rats than in the punished rats.
DISCUSSION
We studied the time course of cue-induced methamphetamine or palatable-food seeking after self-administration of these rewards was suppressed by response-contingent punishment. In rats with a history of methamphetamine self-administration, we observed incubation of methamphetamine craving using both the within-subject and between-subject assessment. In rats with a history of palatable food self-administration, we observed incubation of food craving after punishment using the between-subject but not within-subject assessment. We also observed time-dependent increases in cue-induced methamphetamine and food seeking after withdrawal, confirming previous reports (Lu et al, 2004b; Pickens et al, 2011). Unexpectedly, we also found that food-trained rats were more resistant to punishment than methamphetamine-trained rats.
Incubation of Methamphetamine and Food Craving after Punishment
Our finding of incubation of methamphetamine craving after punishment extend previous reports in which investigators used response-contingent shock- or shock-barrier-based conflict procedures to study drug relapse induced by exposure to drug priming (Panlilio et al, 2005), drug-associated cues (Cooper et al, 2007; Economidou et al, 2009; Peck et al, 2013), or drug-associated contexts (Marchant et al, 2013a).
An unexpected finding was that incubation of food craving after punishment was procedure-dependent and was only observed in the between-subject assessment. The reasons for these experimental-design-specific differences in the food-trained rats are unknown. The negative data from the within-subject design are in agreement with the early punishment literature (Azrin and Holz, 1966). For example, Estes (1944) reported that, when food-reinforced responding in rats had been suppressed by punishment, there was no increase in responding during extinction tests performed 1, 8, 9, and 29 days after the last punishment session. We speculate that extinction learning that had occurred on day 2 test may have resulted in decreased responding to the food cues on day 21 test. However, this explanation cannot account for the within-subject incubation after punishment in methamphetamine-trained rats. In this case, however, methamphetamine-induced memory impairments (Marshall and O'Dell, 2012) may have interfered with extinction learning on day 2, resulting in a lesser impact of day 2 test on day 21 test.
A consistent finding in our study was lower extinction responding in the punished vs unpunished methamphetamine- and food-trained rats (Figure 3). One reason for this is that the pairing of shock with the self-administration chamber may have made the chamber a conditioned inhibitor of operant responding during the extinction tests. Another reason is that pairing of contingent shock with lever-pressing for methamphetamine or food should have led to devaluation of not only the rewards but also of the discrete cue associated with their delivery. Thus, cue-induced reward seeking in the punished groups could have been suppressed because the original reward cue became a reliable predictor of shock.
Barnea-Ygael et al (2012) used a conflict-based relapse model (Cooper et al, 2007) to assess the time course of cue-induced cocaine seeking after suppression of cocaine self-administration by an electric barrier (Warden, 1931). During tests for cue-induced relapse 1 or 14 days after the last electric barrier session, rats were tested in the presence of the barrier. They found that cue-induced cocaine seeking was higher after 1 day than after 14 days, a pattern of results opposite to our results. A likely reason for the discrepant results is that we tested our rats shock-free, whereas Barnea-Ygael et al (2012) tested their rats with the electric barrier. Under these conditions, an incubation of reward craving process could be masked by time-dependent stress sensitization processes (Antelman et al, 2000), resulting in increased reactivity to the electric barrier after 14 shock-free days.
Incubation of Methamphetamine and Food Craving in Unpunished Rats
In agreement with previous studies (Shepard et al, 2004; Theberge et al, 2013), we observed incubation of methamphetamine craving after withdrawal in unpunished rats. The mechanisms underlying this incubation are unknown, but we believe there are at least two possible reasons. The first is development of drug-induced neuroadaptations that progressively increase after withdrawal, leading to higher cue-induced drug seeking during late withdrawal (Loweth et al, 2014; Pickens et al, 2011). Alternatively, incubation of drug craving involves certain drug-induced neuroadaptations that suppress cue-induced drug seeking during early withdrawal; these suppressor mechanisms dissipate over time, leading to higher cue-induced drug seeking during late withdrawal (Pickens et al, 2011).
We suspect that the latter mechanism accounts for incubation of methamphetamine craving. This is because extinction responding on day 2 in unpunished methamphetamine-trained rats was almost as low as that of punished rats in which drug-taking behavior was suppressed by shock (Figure 3). One possible reason for the low responding in methamphetamine-trained unpunished rats on day 2 is the induction of early withdrawal depressive-like states by extended access methamphetamine (Jang et al, 2013; McGregor et al, 2005), resulting in decreased responding to the drug-associated cues. Additional support for the idea that incubation of drug craving involves early withdrawal neuroadaptations that decrease cue-induced drug seeking comes from cocaine studies in which the duration of the training session and number of training days were manipulated (Pacchioni et al, 2011; Sorge et al, 2005). These studies indicate that extended cocaine exposure, which presumably leads to more profound neuroadaptations (Ahmed et al, 2005; Ben-Shahar et al, 2009), does not increase cue-induced drug seeking during late withdrawal but instead decreases cocaine seeking during early withdrawal. This idea is also supported by recent molecular studies that have documented differential transcriptional and translational adaptations at early and late withdrawal times after methamphetamine self-administration in rats (Krasnova et al, 2013).
Consistent with previous studies on incubation of non-drug sucrose craving (Grimm et al, 2005; Li and Frantz, 2010; Uejima et al, 2007), we observed incubation of palatable food craving after withdrawal using between-subject assessment. An unexpected finding was a lack of incubation of food craving using the within-subject assessment. The negative findings with the within-subject assessment may be due to extinction learning that had occurred during day 2 test, resulting in decreased lever-presses on day 21 test (see above).
Resistance to Punishment after Methamphetamine and Food Self-Administration
An unexpected finding was the higher resistance to punishment in food-trained rats than in methamphetamine-trained rats (Figure 2). Early (Johanson, 1975; Smith and Davis, 1974) and more recent (Negus, 2005; Panlilio et al, 2003; Pelloux et al, 2007) studies reported similar punishment sensitivity for drugs and non-drug rewards. The reasons for the differences between these studies and our study are unknown, and are particularly unexpected because our rats were not food-restricted. One potential reason is the drug type (cocaine vs methamphetamine). Another potential reason is the different food types: oral sucrose vs our 45 mg pellets that rats strongly prefer over sucrose pellets (Calu et al, 2014). Higher resistance to punishment in our food-trained rats may be due to the higher rewarding efficacy of the food pellets. Indeed, we recently found that most rats trained to self-administer both food and methamphetamine prefer the food pellets (Caprioli and Shaham, unpublished data). These data confirm previous results of Ahmed and Lenoir that the majority of rats trained for cocaine self-administration strongly prefer an alternative non-drug food reward (saccharin solution) (Ahmed et al, 2013; Cantin et al, 2010; Lenoir et al, 2007). A question for future research is whether both resistance to punishment and preference for food over methamphetamine would be reversed in rats classified as ‘addicts’ following months of drug self-administration in the DSM-based addiction model developed in Bordeaux (Deroche-Gamonet and Piazza, 2014; Piazza and Deroche-Gamonet, 2013).
Two other issues should be considered in interpreting our resistance to punishment data. The first is that investigators divided rats into punishment-sensitive vs punishment-resistant in some previous studies (Pelloux et al, 2007; Vanderschuren and Everitt, 2004), while all rats were eventually punishment-sensitive in our study. The main reason for this difference is that other investigators had used a single pre-set low to mid-range shock intensity to determine punishment resistance in these previous studies, whereas we employed a psychometric approach of gradually increasing shock sensitivity to determine punishment resistance (Panlilio et al, 2003; Panlilio et al, 2005). Another issue is that shock intensity increases over days in our procedure. Thus, it is unknown whether shock suppression is due to increased shock intensities or days of shock exposure. However, it is most likely that shock intensity is the critical parameter, because we have not observed additional decreases in operant responding when shock intensity remains constant over days (Marchant et al, 2013a).
Concluding Remarks
We demonstrated that ‘incubation of methamphetamine craving’ occurs after punishment-induced abstinence. This experimental condition mimics the self-imposed abstinence that occurs in some humans, despite the continued availability of drugs, when the aversive consequences of drug seeking and taking outweigh the drug rewarding effects. However, a note of caution is that the punishment-based procedure does not fully capture abstinence in humans (Marchant et al, 2013a). This is because human abstinence also involves addicts considering delayed long-term negative consequences of drug use (eg, loss of employment, marital problems, loss of social status), psychological processes that cannot be modeled in laboratory rodents (Catania, 1992). Nonetheless, we propose that our punishment-based procedure can be used to investigate mechanisms of relapse to drug and food seeking under conditions that more closely approximate the human condition.
FUNDING AND DISCLOSURE
Research was supported by the National Institute on Drug Abuse, Intramural Research Program. The authors declare no conflict of interests (financial or otherwise) related to the data presented in this manuscript.
References
Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ (2010). Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci 31: 733–741.
Ahmed SH, Guillem K, Vandaele Y (2013). Sugar addiction: pushing the drug-sugar analogy to the limit. Curr Opin Clin Nutr Metab Care 16: 434–439.
Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, Morales M et al (2005). Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci USA 102: 11533–11538.
Airavaara M, Pickens CL, Stern AL, Wihbey KA, Harvey BK, Bossert JM et al (2011). Endogenous GDNF in ventral tegmental area and nucleus accumbens does not play a role in the incubation of heroin craving. Addiction Biol 16: 261–272.
Antelman SM, Levine J, Gershon S (2000). Time-dependent sensitization: the odyssey of a scientific heresy from the laboratory to the door of the clinic. Mol Psychiatry 5: 350–356.
Azrin NH, Holz WC (1966). Punishment. In: Honig WK (ed) Operant Behavior: Areas of Research and Application. Prentice-Hall: Englewood Cliffs, NJ, USA. pp 380–447.
Barnea-Ygael N, Yadid G, Yaka R, Ben-Shahar O, Zangen A (2012). Cue-induced reinstatement of cocaine seeking in the rat "conflict model": effect of prolonged home-cage confinement. Psychopharmacology 219: 875–883.
Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL et al (2009). Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse 63: 598–609.
Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L et al (2004). Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur Neuropsychopharmacol 14: 355–360.
Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y (2009). Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology 206: 51–60.
Burman S (1997). The challenge of sobriety: natural recovery without treatment and self-help groups. J Subst Abuse 9: 41–61.
Calu DJ, Chen YW, Kawa AB, Nair SG, Shaham Y (2014). The use of the reinstatement model to study relapse to palatable food seeking during dieting. Neuropharmacology 76: 395–406.
Calu DJ, Kawa AB, Marchant NJ, Navarre BM, Henderson MJ, Chen B et al (2013). Optogenetic inhibition of dorsal medial prefrontal cortex attenuates stress-induced reinstatement of palatable food seeking in female rats. J Neurosci 33: 214–226.
Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F et al (2010). Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS ONE 5: e11592.
Catania CA (1992) Learning 3 edn. Prentice-Hall: Englewood Cliffs, NJ, USA.
Cifani C, Koya E, Navarre BM, Calu DJ, Baumann MH, Marchant NJ et al (2012). Medial prefrontal cortex neuronal activation and synaptic alterations after stress-induced reinstatement of palatable food seeking: a study using c-fos-GFP transgenic female rats. J Neurosci 32: 8480–8490.
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y et al (2008). Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454: 118–121.
Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A (2007). A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology 194: 117–125.
Deroche-Gamonet V, Piazza PV (2014). Psychobiology of cocaine addiction: Contribution of a multi-symptomatic animal model of loss of control. Neuropharmacology 76: 437–449.
Di Ciano P, Everitt BJ (2004). Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: implications for the persistence of addictive behaviour. Neuropharmacology 47: 202–213.
Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ (2009). High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry 65: 851–856.
Epstein DH, Preston KL (2003). The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology 168: 31–41.
Estes WK (1944). An experimental study of punishment. Psychol Monographs 57: 1–39.
Gawin FH, Kleber HD (1986). Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry 43: 107–113.
Grimm JW, Fyall AM, Osincup DP (2005). Incubation of sucrose craving: effects of reduced training and sucrose pre-loading. Physiol Behav 84: 73–79.
Grimm JW, Hope BT, Wise RA, Shaham Y (2001). Incubation of cocaine craving after withdrawal. Nature 412: 141–142.
Grimm JW, Shaham Y, Hope BT (2002). Effect of the cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol 13: 379–388.
Hunt WA, Barnett LW, Branch LG (1971). Relapse rates in addiction programs. J Clin Psychol 27: 455–456.
Jang CG, Whitfield T, Schulteis G, Koob GF, Wee S (2013). A dysphoric-like state during early withdrawal from extended access to methamphetamine self-administration in rats. Psychopharmacology 225: 753–763.
Johanson CE (1975). Pharmacological and environmental variables affecting drug preference in rhesus monkeys. Pharmacol Rev 27: 343–355.
Klingemann HKH (1991). The motivation for change from problem alcohol and heroin use. Br J Addict 86: 727–744.
Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y (2009). Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology 56: 177–185.
Krasnova IN, Chiflikyan M, Justinova Z, McCoy MT, Ladenheim B, Jayanthi S et al (2013). CREB phosphorylation regulates striatal transcriptional responses in the self-administration model of methamphetamine addiction in the rat. Neurobiol Dis 58: 132–143.
Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C et al (2010). Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS ONE 5: e8790.
Lee JL, Milton AL, Everitt BJ (2006). Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci 26: 5881–5887.
Lenoir M, Serre F, Cantin L, Ahmed SH (2007). Intense sweetness surpasses cocaine reward. PLoS One 2: e698.
Li C, Frantz KJ (2010). Time-dependent increases in cue-induced reinstatement of sucrose seeking after sucrose self-administration in adolescence. Behav Brain Res 213: 109–112.
Loweth JA, Tseng KY, Wolf ME (2014). Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology 76: 287–300.
Lu L, Grimm JW, Dempsey J, Shaham Y (2004a). Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology 176: 101–108.
Lu L, Grimm JW, Hope BT, Shaham Y (2004b). Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47: 214–226.
Marchant NJ, Khuc TN, Pickens CL, Bonci A, Shaham Y (2013a). Context-induced relapse to alcohol seeking after punishment in a rat model. Biol Psychiatry 73: 256–262.
Marchant NJ, Li X, Shaham Y (2013b). Recent developments in animal models of drug relapse. Curr Opin Neurobiol 23: 675–683.
Marlatt AG (1996). Models of relapse and relapse prevention: a commentary. Exp Clin Psychopharmacol 4: 55–60.
Marshall JF, O'Dell SJ (2012). Methamphetamine influences on brain and behavior: unsafe at any speed? Trends Neurosci 35: 536–545.
McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM (2005). The nature, time course and severity of methamphetamine withdrawal. Addiction 100: 1320–1329.
Mead AN, Zamanillo D, Becker N, Stephens DN (2007). AMPA-receptor GluR1 subunits are involved in the control over behavior by cocaine-paired cues. Neuropsychopharmacology 32: 343–353.
Mooney ME, Herin DV, Schmitz JM, Moukaddam N, Green CE, Grabowski J (2009). Effects of oral methamphetamine on cocaine use: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 101: 34–41.
Neale A, Abraham S, Russell J (2009). "Ice" use and eating disorders: a report of three cases. Int J Eat Disord 42: 188–191.
Negus SS (2005). Effects of punishment on choice between cocaine and food in rhesus monkeys. Psychopharmacology 181: 244–252.
Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF (2000). Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci 20: 798–805.
O'Brien CP, Childress AR, McLellan AT, Ehrman R (1992). Classical conditioning in drug-dependent humans. Ann N Y Acad Sci 654: 400–415.
Pacchioni AM, Gabriele A, See RE (2011). Dorsal striatum mediation of cocaine-seeking after withdrawal from short or long daily access cocaine self-administration in rats. Behav Brain Res 218: 296–300.
Panlilio L, Thorndike E, Schindler C (2003). Reinstatement of punishment-suppressed opioid self-administration in rats: an alternative model of relapse to drug abuse. Psychopharmacology 168: 229–235.
Panlilio LV, Thorndike EB, Schindler CW (2005). Lorazepam reinstates punishment-suppressed remifentanil self-administration in rats. Psychopharmacology 179: 374–382.
Peck JA, Wercberger R, Kariyeva E, Ranaldi R (2013). Cue-induced resumption of heroin and cocaine seeking in rats using a conflict model of abstinence and relapse. Psychopharmacology (Berl) 228: 651–658.
Pelloux Y, Everitt BJ, Dickinson A (2007). Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology 194: 127–137.
Piazza PV, Deroche-Gamonet V (2013). A multistep general theory of transition to addiction. Psychopharmacology 229: 387–413.
Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011). Neurobiology of the incubation of drug craving. Trends Neurosci 34: 411–420.
Pickens CL, Cifani C, Navarre BM, Eichenbaum H, Theberge FR, Baumann MH et al (2012). Effect of fenfluramine on reinstatement of food seeking in female and male rats: implications for the predictive validity of the reinstatement model. Psychopharmacology 221: 341–353.
Saito M, Terada M, Saito TR, Takahashi KW (1995). Effects of the long-term administration of methamphetamine on body weight, food intake, blood biochemistry and estrous cycle in rats. Exp Anim 43: 747–754.
See RE (2005). Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol 526: 140–146.
Shalev U, Morales M, Hope B, Yap J, Shaham Y (2001). Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology 156: 98–107.
Shepard JD, Bossert JM, Liu SY, Shaham Y (2004). The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry 55: 1082–1089.
Smith SG, Davis WM (1974). Punishment of amphetamine and morphine self-administration behavior. Psychol Rec 24: 477–480.
Sorge RE, Rajabi H, Stewart J (2005). Rats maintained chronically on buprenorphine show reduced heroin and cocaine seeking in tests of extinction and drug-induced reinstatement. Neuropsychopharmacology 30: 1681–1692.
Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM et al (2013). Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry 73: 729–737.
Theberge FR, Pickens CL, Goldart E, Fanous S, Hope BT, Liu QR et al (2012). Association of time-dependent changes in mu opioid receptor mRNA, but not BDNF, TrkB, or MeCP2 mRNA and protein expression in the rat nucleus accumbens with incubation of heroin craving. Psychopharmacology (Berl) 224: 559–571.
Uejima JL, Bossert JM, Poles GC, Lu L (2007). Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of sucrose craving in rats. Behav Brain Res 181: 292–296.
Van den Oever MC, Goriounova NA, Li KW, Van der Schors RC, Binnekade R, Schoffelmeer AN et al (2008). Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci 11: 1053–1058.
Van den Oever MC, Lubbers BR, Goriounova NA, Li KW, Van der Schors RC, Loos M et al (2010). Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology 35: 2120–2133.
Vanderschuren LJ, Everitt BJ (2004). Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305: 1017–1019.
Warden CJ (1931) Animal motivation: experimental studies on the albino rat. Columbia University Press: New York, NY, USA.
Acknowledgements
We thank Eric Thorndike for his technical (computer programming) help with self-administration experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krasnova, I., Marchant, N., Ladenheim, B. et al. Incubation of Methamphetamine and Palatable Food Craving after Punishment-Induced Abstinence. Neuropsychopharmacol 39, 2008–2016 (2014). https://doi.org/10.1038/npp.2014.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2014.50
This article is cited by
-
Preventing incubation of drug craving to treat drug relapse: from bench to bedside
Molecular Psychiatry (2023)
-
Acute food deprivation-induced relapse to heroin seeking after short and long punishment-imposed abstinence in male rats
Psychopharmacology (2023)
-
Increased responsiveness to punishment of cocaine self-administration after experience with high punishment
Neuropsychopharmacology (2022)
-
Footshock-Induced Abstinence from Compulsive Methamphetamine Self-administration in Rat Model Is Accompanied by Increased Hippocampal Expression of Cannabinoid Receptors (CB1 and CB2)
Molecular Neurobiology (2022)
-
Effects of heroin self-administration and forced withdrawal on the expression of genes related to the mTOR network in the basolateral complex of the amygdala of male Lewis rats
Psychopharmacology (2022)