Key Points
-
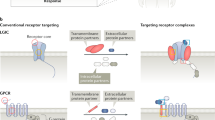
The human genome encodes more than 1,000 G-protein-coupled receptors (GPCRs), making these protein the largest class of drug targets, and it has been estimated that 50% of all modern drugs modulate GPCR activity. Despite the recent realization that these receptors form homo-oligomeric and hetero-oligomeric complexes, virtually all therapeutics directed towards GPCRs have been designed using assays that presume these receptors are monomeric, and this important aspect of GPCR biology remains largely unincorporated into schemes to search for new therapeutics.
-
Although it is not known how large GPCR oligomers are or whether receptors can exist in multiple oligomeric states, the assembly of correctly formed oligomers seems to be a requirement for proper cellular transport to the cell surface. Oligomerization of a receptor with mutant or variant forms of itself can result in attenuation of receptor expression, and might, therefore, represent a potential disease mechanism or a means of cellular self-regulatory modulation of receptor function.
-
Traditionally, signal amplification is generally thought to occur only at the level of the G protein or the effector, and not at the receptor level. However, both homo- and hetero-oligomerization might provide a means for signal amplification through the activation of many receptors by the action of a single ligand molecule.
-
GPCR hetero-oligomerization can result in the formation of receptor complexes that have ligand-binding and signalling properties distinct from their constituent receptors. It represents a novel aspect of GPCR biology that has exciting potential to generate new drug targets. Heteromeric interactions might also mask the individual properties of one of the constituent receptors.
-
Altered levels of GPCR hetero-oligomerization could represent the molecular basis of some clinical disorders.
-
Little is known about the dynamics and regulation of GPCR oligomer formation; that is, whether ligands promote association or dissociation of oligomers, or whether they bind to pre-formed oligomers and change the oligomeric receptor conformation. However, resonance-energy-transfer assays in live cells have indicated that agonist-induced oligomerization might occur.
-
There is evidence that the oligomeric nature of GPCRs can be exploited to improve drugs by developing dimeric ligands. Several dimeric ligands have been shown to have increased affinity and altered potency compared with their constituent ligands, potentially because dimeric ligands might more readily induce or stabilize the dimeric conformation of the receptors, which in some manner increases the efficacy of signal transduction.
-
Contemporary drug discovery for GPCRs has largely been based on using a single GPCR of interest expressed in a recombinant cell line. For future lead-compound identification, the current understanding of GPCR oligomerization mandates that hetero-oligomeric receptors must be considered as novel targets in the screening of compounds as drug candidates. Drugs that can enhance or disrupt GPCR oligomer formation as a means to regulate oligomerization-dependent functions will also have to be explored.
-
Given what is known about GPCR oligomerization so far, it is plausible that the development of 'new' therapies could be as simple as creating novel regimens with 'old' drugs.
-
The consideration of GPCR quaternary structure has been slow to permeate into the thinking of the drug discovery mainstream despite the potential to exploit it for improved therapies.
Abstract
G-protein-coupled receptors (GPCRs) represent by far the largest class of targets for modern drugs. Virtually all therapeutics that are directed towards GPCRs have been designed using assays that presume that these receptors are monomeric. The recent realization that these receptors form homo-oligomeric and hetero-oligomeric complexes has added a new dimension to rational drug design. However, this important aspect of GPCR biology remains largely unincorporated into schemes to search for new therapeutics. This review provides a synopsis of the current thinking surrounding GPCR homo-oligomerization and hetero-oligomerization and shows how new models point towards unexplored avenues in the development of new therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Howard, A. D. et al. Orphan G protein-coupled receptors and natural ligand discovery. Trends Pharmacol. Sci. 22, 132–140 (2001).
Spiegel, A. M. Defects in G protein-coupled signal transduction in human disease. Annu. Rev. Physiol. 58, 143–170 (1996).
Rana, B. K., Shiina, T. & Insel, P. A. Genetic variations and polymorphisms of G protein-coupled receptors: functional and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 41, 593–624 (2001).
Bailey, W. J. et al. Patent status of therapeutically important G protein-coupled receptors. Expert Opin. Ther. Patents 11, 1861–1887 (2001).
Bockaert, J. & Pin, J. P. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 18, 1723–1729 (1999).
Gether, U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 21, 90–113 (2000).References 5 and 6 are general reviews that, among other things, outline several key structural features of the different families of GPCRs.
Heldin, C. H. Dimerization of cell surface receptors in signal transduction. Cell 80, 213–223 (1995).
Salahpour, A., Angers, S. & Bouvier, M. Functional significance of oligomerization of G-protein-coupled receptors. Trends Endocrinol. Metab. 11, 163–168 (2000).
Lee, D. K., George, S. R., Evans, J. F., Lynch, K. R. & O'Dowd, B. F. Orphan G protein-coupled receptors in the CNS. Curr. Opin. Pharmacol. 1, 31–39 (2001).
Nimchinsky, E. A., Hof, P. R., Janssen, W. G., Morrison, J. H. & Schmauss, C. Expression of dopamine D3 receptor dimers and tetramers in brain and in transfected cells. J. Biol. Chem. 272, 29229–29237 (1997).
George, S. R. et al. A transmembrane domain-derived peptide inhibits D1 dopamine receptor function without affecting receptor oligomerization. J. Biol. Chem. 273, 30244–30248 (1998).
Lee, S. P. et al. Inhibition of cell surface expression by mutant receptors demonstrates that D2 dopamine receptors exist as oligomers in the cell. Mol. Pharmacol. 58, 120–128 (2000).
Chidiac, P., Green, M. A., Pawagi, A. B. & Wells, J. W. Cardiac muscarinic receptors. Cooperativity as the basis for multiple states of affinity. Biochemistry 36, 7361–7379 (1997).
Romano, C., Yang, W. L. & O'Malley, K. L. Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J. Biol. Chem. 271, 28612–28616 (1996).
Bai, M., Trivedi, S. & Brown, E. M. Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J. Biol. Chem. 273, 23605–23610 (1998).
Kunishima, N. et al. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407, 971–977 (2000).This paper details the crystal structure of the amino terminus of mGluR1, directly showing that this receptor is a covalently linked constitutive dimer.
Hebert, T. E. et al. A peptide derived from a β2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J. Biol. Chem. 271, 16384–16392 (1996).
Cvejic, S. & Devi, L. A. Dimerization of the δ-opioid receptor: implication for a role in receptor internalization. J. Biol. Chem. 272, 26959–26964 (1997).
Ng, G. Y. et al. Identification of a GABAB receptor subunit, GB2, required for functional GABAB receptor activity. J. Biol. Chem. 274, 7607–7610 (1999).
Rocheville, M. et al. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J. Biol. Chem. 275, 7862–7869 (2000).
Jordan, B. A. & Devi, L. A. G-protein-coupled receptor heterodimerization modulates receptor function. Nature 399, 697–700 (1999).
George, S. R. et al. Oligomerization of μ- and δ-opioid receptors. Generation of novel functional properties. J. Biol. Chem. 275, 26128–26135 (2000).
Schulz, A., Grosse, R., Schultz, G., Gudermann, T. & Schoneberg, T. Structural implication for receptor oligomerization from functional reconstitution studies of mutant V2 vasopressin receptors. J. Biol. Chem. 275, 2381–2389 (2000).
Mellado, M. et al. Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J. 20, 2497–2507 (2001).A study that demonstrates agonist-induced homo- and hetero-oligomerization of chemokine receptors. The formation of hetero-oligomers was detected at 100-fold lower concentration of agonist than required for the formation of homo-oligomers.
Casey, J. R. & Reithmeier, R. A. Detergent interaction with band 3, a model polytopic membrane protein. Biochemistry 32, 1172–1179 (1993).
Whitfield, G. K., Jurutka, P. W., Haussler, C. A. & Haussler, M. R. Steroid hormone receptors: evolution, ligands, and molecular basis of biologic function. J. Cell. Biochem. 33 (Suppl. 32), 110–122 (1999).
Jahangeer, S. & Rodbell, M. The disaggregation theory of signal transduction revisited: further evidence that G proteins are multimeric and disaggregate to monomers when activated. Proc. Natl Acad. Sci. USA 90, 8782–8786 (1993).
Yan, K., Greene, E., Belga, F. & Rasenick, M. M. Synaptic membrane G proteins are complexed with tubulin in situ. J. Neurochem. 66, 1489–1495 (1996).
Limbird, L. E., Meyts, P. D. & Lefkowitz, R. J. β-Adrenergic receptors: evidence for negative cooperativity. Biochem. Biophys. Res. Commun. 64, 1160–1168 (1975).
Henis, Y. I. & Sokolovsky, M. Muscarinic antagonists induce different receptor conformations in rat adenohypophysis. Mol. Pharmacol. 24, 357–365 (1983).
Hirschberg, B. T. & Schimerlik, M. I. A kinetic model for oxotremorine M binding to recombinant porcine M2 muscarinic receptors expressed in Chinese hamster ovary cells. J. Biol. Chem. 269, 26127–26135 (1994).
Mattera, R., Pitts, B. J., Entman, M. L. & Birnbaumer, L. Guanine nucleotide regulation of a mammalian myocardial muscarinic receptor system. Evidence for homo- and heterotropic cooperativity in ligand binding analyzed by computer-assisted curve fitting. J. Biol. Chem. 260, 7410–7421 (1985).
Boyer, J. L., Martinez-Carcamo, M., Monroy-Sanchez, J. A., Posadas, C. & Garcia-Sainz, J. A. Guanine nucleotide-induced positive cooperativity in muscarinic-cholinergic antagonist binding. Biochem. Biophys. Res. Commun. 134, 172–177 (1986).
Potter, L. T. et al. Evidence of paired M2 muscarinic receptors. Mol. Pharmacol. 39, 211–221 (1991).
Sinkins, W. G. & Wells, J. W. G protein-linked receptors labeled by [3H]histamine in guinea pig cerebral cortex. II. Mechanistic basis for multiple states of affinity [corrected]. Mol. Pharmacol. 43, 583–594 (1993).
Wreggett, K. A. & Wells, J. W. Cooperativity manifest in the binding properties of purified cardiac muscarinic receptors. J. Biol. Chem. 270, 22488–22499 (1995).
Chazenbalk, G. D., Kakinuma, A., Jaume, J. C., McLachlan, S. M. & Rapoport, B. Evidence for negative cooperativity among human thyrotropin receptors overexpressed in mammalian cells. Endocrinology 137, 4586–4591 (1996).
Armstrong, D. & Strange, P. G. Dopamine D2 receptor dimer formation: evidence from ligand binding. J. Biol. Chem. 276, 22621–22629 (2001).
Pizard, A., Marchetti, J., Allegrini, J., Alhenc-Gelas, F. & Rajerison, R. M. Negative cooperativity in the human bradykinin B2 receptor. J. Biol. Chem. 273, 1309–1315 (1998).
AbdAlla, S., Lother, H. & Quitterer, U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature 407, 94–98 (2000).
Hurtley, S. M. & Helenius, A. Protein oligomerization in the endoplasmic reticulum. Annu. Rev. Cell Biol. 5, 277–307 (1989).
Brown, D. & Breton, S. Sorting proteins to their target membranes. Kidney Int. 57, 816–824 (2000).
Karpa, K. D., Lin, R., Kabbani, N. & Levenson, R. The dopamine D3 receptor interacts with itself and the truncated D3 splice variant D3nf: D3–D3nf interaction causes mislocalization of D3 receptors. Mol. Pharmacol. 58, 677–683 (2000).
Benkirane, M., Jin, D. Y., Chun, R. F., Koup, R. A. & Jeang, K. T. Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by CCR5Δ32. J. Biol. Chem. 272, 30603–30606 (1997).
Zhu, X. & Wess, J. Truncated V2 vasopressin receptors as negative regulators of wild-type V2 receptor function. Biochemistry 37, 15773–15784 (1998).
Elmhurst, J. L., Xie, Z., O'Dowd, B. F. & George, S. R. The splice variant D3nf reduces ligand binding to the D3 dopamine receptor: evidence for heterooligomerization. Brain Res. Mol. Brain Res. 80, 63–74 (2000).
Okuda-Ashitaka, E. et al. Suppression of prostaglandin E receptor signaling by the variant form of EP1 subtype. J. Biol. Chem. 271, 31255–31261 (1996).
Osuga, Y. et al. Co-expression of defective luteinizing hormone receptor fragments partially reconstitutes ligand-induced signal generation. J. Biol. Chem. 272, 25006–25012 (1997).
Grosse, R., Schoneberg, T., Schultz, G. & Gudermann, T. Inhibition of gonadotropin-releasing hormone receptor signaling by expression of a splice variant of the human receptor. Mol. Endocrinol. 11, 1305–1318 (1997).
Kuner, R. et al. Role of heteromer formation in GABAB receptor function. Science 283, 74–77 (1999).
Kaupmann, K. et al. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687 (1998).
White, J. H. et al. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature 396, 679–682 (1998).
Couve, A. et al. Intracellular retention of recombinant GABAB receptors. J. Biol. Chem. 273, 26361–26367 (1998).
Margeta-Mitrovic, M., Jan, Y. N. & Jan, L. Y. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron 27, 97–106 (2000).
Levac, B. A., O'Dowd, B. F. & George, S. R. Oligomerization of opioid receptors: generation of novel signaling units. Curr. Opin. Pharmacol. 2, 76–81 (2002).
Pfeiffer, M. et al. Homo- and heterodimerization of somatostatin receptor subtypes. Inactivation of SST(3) receptor function by heterodimerization with SST(2A). J. Biol. Chem. 276, 14027–14036 (2001).
AbdAlla, S., Lother, H., el Massiery, A. & Quitterer, U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nature Med. 7, 1003–1009 (2001).It is shown that there is a potential relationship between the level of AT 1 – B 2 receptor hetero-oligomerization and pre-eclampsia.
Robbins, M. J. et al. GABA(B2) is essential for G-protein coupling of the GABA(B) receptor heterodimer. J. Neurosci. 21, 8043–8052 (2001).
Palczewski, K. et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289, 739–745 (2000).This landmark paper showing the crystal structure of rhodopsin represents the first and only determination of a high-resolution, three-dimensional structure for a family 1 GPCR.
Zeng, F. Y. & Wess, J. Identification and molecular characterization of M3 muscarinic receptor dimers. J. Biol. Chem. 274, 19487–19497 (1999).
Lee, S. P. et al. Oligomerization of dopamine and serotonin receptors. Neuropsychopharmacology 23, S32–S40 (2000).
Ng, G. Y. et al. Dopamine D2 receptor dimers and receptor-blocking peptides. Biochem. Biophys. Res. Commun. 227, 200–204 (1996).
Lemmon, M. A. et al. Glycophorin A dimerization is driven by specific interactions between transmembrane α-helices. J. Biol. Chem. 267, 7683–7689 (1992).
Fan, G. F., Ray, K., Zhao, X. M., Goldsmith, P. K. & Spiegel, A. M. Mutational analysis of the cysteines in the extracellular domain of the human Ca2+ receptor: effects on cell surface expression, dimerization and signal transduction. FEBS Lett. 436, 353–356 (1998).
Ward, D. T., Brown, E. M. & Harris, H. W. Disulfide bonds in the extracellular calcium-polyvalent cation-sensing receptor correlate with dimer formation and its response to divalent cations in vitro. J. Biol. Chem. 273, 14476–14483 (1998).
Tsuji, Y. et al. Cryptic dimer interface and domain organization of the extracellular region of metabotropic glutamate receptor subtype 1. J. Biol. Chem. 275, 28144–28151 (2000).A non-covalent dimerization interface between the amino termini of mGluR1 receptors revealed that an intermolecular disulphide bond might not be the only mechanism of interaction in family 3 receptor dimerization.
AbdAlla, S., Zaki, E., Lother, H. & Quitterer, U. Involvement of the amino terminus of the B2 receptor in agonist-induced receptor dimerization. J. Biol. Chem. 274, 26113–26119 (1999).
Bowery, N. G. et al. International Union of Pharmacology. XXXIII. Mammalian γ-aminobutyric acid(B) receptors: structure and function. Pharmacol. Rev. 54, 247–264 (2002).
Pagano, A. et al. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(B) receptors. J. Neurosci. 21, 1189–1202 (2001).
Ng, G. Y. et al. Resistance of the dopamine D2L receptor to desensitization accompanies the up-regulation of receptors on to the surface of Sf9 cells. Endocrinology 138, 4199–4206 (1997).
McVey, M. et al. Monitoring receptor oligomerization using time-resolved fluorescence resonance energy transfer and bioluminescence resonance energy transfer. The human δ-opioid receptor displays constitutive oligomerization at the cell surface, which is not regulated by receptor occupancy. J. Biol. Chem. 276, 14092–14099 (2001).
Himeno, A., Shigematsu, K., Taguchi, T. & Niwa, M. Endothelin-1 binding to endothelin receptors in the rat anterior pituitary gland: interaction in the recognition of endothelin-1 between ETA and ETB receptors. Cell. Mol. Neurobiol. 18, 447–452 (1998).
Angers, S. et al. Detection of β2-adrenergic receptor dimerization in living cells using bioluminescence resonance energy transfer (BRET). Proc. Natl Acad. Sci. USA 97, 3684–3689 (2000).
Kroeger, K. M., Hanyaloglu, A. C., Seeber, R. M., Miles, L. E. & Eidne, K. A. Constitutive and agonist-dependent homo-oligomerization of the thyrotropin-releasing hormone receptor. Detection in living cells using bioluminescence resonance energy transfer. J. Biol. Chem. 276, 12736–12743 (2001).
Rocheville, M. et al. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science 288, 154–157 (2000).
Cornea, A., Janovick, J. A., Maya-Nunez, G. & Conn, P. M. Gonadotropin-releasing hormone receptor microaggregation. Rate monitored by fluorescence resonance energy transfer. J. Biol. Chem. 276, 2153–2158 (2001).
Dupuis, D. S., Perez, M., Halazy, S., Colpaert, F. C. & Pauwels, P. J. Magnitude of 5-HT1B and 5-HT1A receptor activation in guinea-pig and rat brain: evidence from sumatriptan dimer-mediated [35S]GTPγS binding responses. Brain Res. Mol. Brain Res. 67, 107–123 (1999).
Halazy, S. et al. Serotonin dimers: application of the bivalent ligand approach to the design of new potent and selective 5-HT(1B/1D) agonists. J. Med. Chem. 39, 4920–4927 (1996).
Perez, M. et al. Dimerization of sumatriptan as an efficient way to design a potent, centrally and orally active 5-HT1B agonist. Bioorg. Med. Chem. Lett. 8, 675–680 (1998).Dimeric sumatriptan, a clinically used treatment for migraine headaches, was shown to be more potent than monomeric sumitriptan, possibly because it behaves as a bivalent ligand.
Yano, K., Kimura, S. & Imanishi, Y. Simultaneous activation of two different receptor systems by enkephalin/neurotensin conjugates having spacer chains of various lengths. Eur. J. Pharm. Sci. 7, 41–48 (1998).
Portoghese, P. S. Bivalent ligands and the message-address concept in the design of selective opioid receptor antagonists. Trends Pharmacol. Sci. 10, 230–235 (1989).The linking of opioid peptides with various chemical spacers generated opioid ligands with varying properties.
Hazum, E. & Keinan, D. Gonadotropin releasing hormone activation is mediated by dimerization of occupied receptors. Biochem. Biophys. Res. Commun. 133, 449–456 (1985).
Hillion, J. et al. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J. Biol. Chem. 277, 18091–18097 (2002).
Jordan, B. A., Trapaidze, N., Gomes, I., Nivarthi, R. & Devi, L. A. Oligomerization of opioid receptors with β2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc. Natl Acad. Sci. USA 98, 343–348 (2001).
Tarasova, N. I., Rice, W. G. & Michejda, C. J. Inhibition of G-protein-coupled receptor function by disruption of transmembrane domain interactions. J. Biol. Chem. 274, 34911–34915 (1999).
Yudt, M. R. & Koide, S. Preventing estrogen receptor action with dimer-interface peptides. Steroids 66, 549–558 (2001).
Salim, K. et al. Oligomerization of G-protein-coupled receptors shown by selective co-immunoprecipitation. J. Biol. Chem. 277, 15482–15485 (2002).
Pfeiffer, M. et al. Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J. Biol. Chem. 277, 19762–19772 (2002).
Rodriguez-Frade, J. M. et al. The chemokine monocyte chemoattractant protein-1 induces functional responses through dimerization of its receptor CCR2. Proc. Natl Acad. Sci. USA 96, 3628–3633 (1999).
Gines, S. et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc. Natl Acad. Sci. USA 97, 8606–8611 (2000).
Gouldson, P. R., Snell, C. R. & Reynolds, C. A. A new approach to docking in the β2-adrenergic receptor that exploits the domain structure of G protein-coupled receptors. J. Med. Chem. 40, 3871–3886 (1997).
Maggio, R., Vogel, Z. & Wess, J. Coexpression studies with mutant muscarinic/adrenergic receptors provide evidence for intermolecular 'cross-talk' between G-protein-linked receptors. Proc. Natl Acad. Sci. USA 90, 3103–3107 (1993).
Monnot, C. et al. Polar residues in the transmembrane domains of the type 1 angiotensin II receptor are required for binding and coupling. Reconstitution of the binding site by co-expression of two deficient mutants. J. Biol. Chem. 271, 1507–1513 (1996).
Baldwin, J. M. The probable arrangement of the helices in G protein-coupled receptors. EMBO J. 12, 1693–1703 (1993).
Hadac, E. M. et al. A peptide agonist acts by occupation of a monomeric G protein-coupled receptor: dual sites of covalent attachment to domains near TM1 and TM7 of the same molecule make biologically significant domain-swapped dimerization unlikely. J. Med. Chem. 42, 2105–2111 (1999).
Ciruela, F. et al. Immunological identification of A1 adenosine receptors in brain cortex. J. Neurosci. Res. 42, 818–828 (1995).
Vila-Coro, A. J. et al. HIV-1 infection through the CCR5 receptor is blocked by receptor dimerization. Proc. Natl Acad. Sci. USA 97, 3388–3393 (2000).
Vila-Coro, A. J. et al. The chemokine SDF-1α triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J. 13, 1699–1710 (1999).
Ng, G. Y. et al. Desensitization, phosphorylation and palmitoylation of the human dopamine D1 receptor. Eur. J. Pharmacol. 267, 7–19 (1994).
Fukushima, Y. et al. Oligomer formation of histamine H2 receptors expressed in Sf9 and COS7 cells. FEBS Lett. 409, 283–286 (1997).
Nguyen, T. et al. Discovery of a novel member of the histamine receptor family. Mol. Pharmacol. 59, 427–433 (2001).
Indrapichate, K. et al. Biological actions of monoclonal luteinizing hormone/human chorionic gonadotropin receptor antibodies. Biol. Reprod. 46, 265–278 (1992).
Ayoub, M. A. et al. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J. Biol. Chem. 277, 21522–21528 (2002).
Ng, G. Y. et al. Human serotonin1B receptor expression in Sf9 cells: phosphorylation, palmitoylation, and adenylyl cyclase inhibition. Biochemistry 32, 11727–11733 (1993).
Abe, J., Suzuki, H., Notoya, M., Yamamoto, T. & Hirose, S. Ig-hepta, a novel member of the G protein-coupled hepta-helical receptor (GPCR) family that has immunoglobulin-like repeats in a long N-terminal extracellular domain and defines a new subfamily of GPCRs. J. Biol. Chem. 274, 19957–19964 (1999).
Romano, C. et al. Covalent and noncovalent interactions mediate metabotropic glutamate receptor mGlu5 dimerization. Mol. Pharmacol. 59, 46–53 (2001).
Overton, M. C. & Blumer, K. J. G-protein-coupled receptors function as oligomers in vivo. Curr. Biol. 10, 341–344 (2000).
Yesilaltay, A. & Jenness, D. D. Homo-oligomeric complexes of the yeast α-factor pheromone receptor are functional units of endocytosis. Mol. Biol. Cell 11, 2873–2884 (2000).
Xie, Z., Lee, S. P., O'Dowd, B. F. & George, S. R. Serotonin 5-HT1B and 5-HT1D receptors form homodimers when expressed alone and heterodimers when co-expressed. FEBS Lett. 456, 63–67 (1999).
Ciruela, F. et al. Metabotropic glutamate 1α and adenosine A1 receptors assemble into functionally interacting complexes. J. Biol. Chem. 276, 18345–18351 (2001).
Yoshioka, K., Saitoh, O. & Nakata, H. Heteromeric association creates a P2Y-like adenosine receptor. Proc. Natl Acad. Sci. USA 98, 7617–7622 (2001).
AbdAlla, S., Lother, H., Abdel-Tawab, A. M. & Quitterer, U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J. Biol. Chem. 276, 39721–39726 (2001).
Scarselli, M. et al. D2/D3 dopamine receptor heterodimers exhibit unique functional properties. J. Biol. Chem. 276, 30308–30314 (2001).
Jones, K. A. et al. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature 396, 674–679 (1998).
Maggio, R. et al. G protein-linked receptors: pharmacological evidence for the formation of heterodimers. J. Pharmacol. Exp. Ther. 291, 251–257 (1999).
Nelson, G. et al. An amino-acid taste receptor. Nature 416, 199–202 (2002).
Nelson, G. et al. Mammalian sweet taste receptors. Cell 106, 381–390 (2001).
Perez, M., Jorand-Lebrun, C., Pauwels, P. J., Pallard, I. & Halazy, S. Dimers of 5HT1 ligands preferentially bind to 5HT1B/1D receptor subtypes. Bioorg. Med. Chem. Lett. 8, 1407–1412 (1998).
Jacobson, K. A., Xie, R., Young, L., Chang, L. & Liang, B. T. A novel pharmacological approach to treating cardiac ischemia. Binary conjugates of A1 and A3 adenosine receptor agonists. J. Biol. Chem. 275, 30272–30279 (2000).
Carrithers, M. D. & Lerner, M. R. Synthesis and characterization of bivalent peptide ligands targeted to G-protein-coupled receptors. Chem. Biol. 3, 537–542 (1996).
Moser, U. et al. Aliphatic and heterocyclic analogues of arecaidine propargyl ester. Structure–activity relationships of mono- and bivalent ligands at muscarinic M1 (M4), M2 and M3 receptor subtypes. Arzneimittelforschung 45, 449–455 (1995).
Portoghese, P. S. From models to molecules: opioid receptor dimers, bivalent ligands, and selective opioid receptor probes. J. Med. Chem. 44, 2259–2269 (2001).
Bolognesi, M. L. et al. Opioid antagonist activity of naltrexone-derived bivalent ligands: importance of a properly oriented molecular scaffold to guide 'address' recognition at κ opioid receptors. J. Med. Chem. 39, 1816–1822 (1996).
Portoghese, P. S. The bivalent ligand approach in the design of highly selective opioid receptor antagonists. NIDA Res. Monogr. 96, 3–20 (1990).
Lambrecht, G. et al. The novel heteromeric bivalent ligand SB9 potently antagonizes P2Y(1) receptor-mediated responses. J. Auton. Nerv. Syst. 81, 171–177 (2000).
Acknowledgements
The authors receive support from the Canadian Institutes of Health Research and the National Institute on Drug Abuse.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
gonadotropin-releasing hormone
gonadotropin-releasing-hormone receptor
α-melanocyte-stimulating-hormone receptor
thyrotropin-releasing-hormone receptor
Medscape DrugInfo
OMIM
nephrogenic diabetes insipidus
FURTHER INFORMATION
Encyclopedia of Life Sciences
Glossary
- POLYMORPHISM
-
The occurrence in a population of two or more variant alleles of a gene, for which the frequency of the rarer alleles is greater than can be explained by recurrent mutation alone.
- RADIATION INACTIVATION
-
A technique in which proteins are inactivated with high-energy particles to determine the molecular mass of functional oligomers.
- PALMITOYLATION
-
A post-translationlational modification in which palmitic acid, a fatty carbon chain, is attached to a cysteine residue by a thio-ester bond.
- ORPHAN RECEPTOR
-
A receptor for which no endogenous ligand has been identified.
- COOPERATIVITY
-
A property of receptors that have interacting binding sites, for which the binding of a ligand to one site modulates the binding of a second ligand to another site.
- CO-IMMUNOPRECIPITATION
-
A process that uses antibodies to isolate a protein that interacts with the protein of interest.
- DIFFERENTIALLY TAGGED
-
Having two unlike epitope tags.
- DESENSITIZATION
-
The mechanism by which a ligand becomes less effective at activating a receptor during prolonged application.
- ALTERNATIVE SPLICING
-
Different products can be generated from a single gene by, for example, combining alternative forms of particular exons.
- PERTUSSIS TOXIN
-
A toxin that ADP ribosylates the inhibitory G protein Gi, thereby causing it to uncouple from G-protein-coupled receptors.
- CROSS-TALK
-
An informal term that refers to the interaction or reciprocal modulation between two proteins.
- PRE-ECLAMPSIA
-
A hypertensive disorder of pregnancy for which the cause is unknown.
- CRYSTAL STRUCTURE
-
The three-dimensional arrangement of atoms in a protein that is determined by inducing the protein to form crystals.
- CROSSLINKING AGENT
-
A chemical compound that forms a covalent link between two closely associated proteins.
- DYSKINESIA
-
An impairment in the ability to control movements — characterized by spasmodic or repetitive motions or lack of coordination.
- PARKINSONISM
-
Any of a group of nervous disorders that are similar to Parkinson's disease — characterized by muscular rigidity, tremor and impaired motor control.
Rights and permissions
About this article
Cite this article
George, S., O'Dowd, B. & Lee, S. G-Protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov 1, 808–820 (2002). https://doi.org/10.1038/nrd913
Issue Date:
DOI: https://doi.org/10.1038/nrd913
This article is cited by
-
Digital nanoreactors to control absolute stoichiometry and spatiotemporal behavior of DNA receptors within lipid bilayers
Nature Communications (2023)
-
Insight into Evolution and Conservation Patterns of B1-Subfamily Members of GPCR
International Journal of Peptide Research and Therapeutics (2020)
-
GPCR drug discovery: integrating solution NMR data with crystal and cryo-EM structures
Nature Reviews Drug Discovery (2019)
-
Unique Roles of β-Arrestin in GPCR Trafficking Revealed by Photoinducible Dimerizers
Scientific Reports (2018)
-
Evidence of G-protein-coupled receptor and substrate transporter heteromerization at a single molecule level
Cellular and Molecular Life Sciences (2018)