Abstract
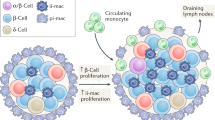
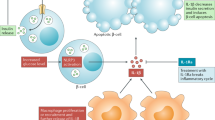
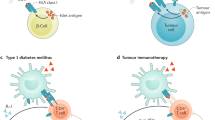
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease with a strong inflammatory component. The latest studies indicate that innate immunity and inflammatory mediators have a much broader role in T1DM than initially assumed. Inflammation might contribute to early induction and amplification of the immune assault against pancreatic β cells and, at later stages, to the stabilization and maintenance of insulitis. Inflammatory mediators probably contribute to the suppression of β-cell function and subsequent apoptosis; they may also inhibit or stimulate β-cell regeneration and might cause peripheral insulin resistance. The different effects of inflammation take place in different phases of the course of T1DM, and should be considered in the context of a 'dialog' between invading immune cells and the target β cells. This dialog is mediated both by cytokines and chemokines that are released by β cells and immune cells, and by putative, immunogenic signals that are delivered by dying β cells. In this Review, we divided the role of inflammation in T1DM into three arbitrary stages: induction, amplification and maintenance or resolution of insulitis. These stages, and their progression or resolution, might depend on a patient's genetic background, which contributes to disease heterogeneity.
Key Points
-
Innate immunity and inflammatory mediators have a broad and important role in the pathogenesis of type 1 diabetes mellitus
-
Activation of both endogenous and exogenous ligands of pattern-recognition receptors can induce islet inflammation and death of pancreatic β cells
-
Amplification of insulitis might depend on a 'dialog' between immune cells and β cells that is mediated by local production of chemokines and cytokines, and danger signals from dying β cells
-
After transition to adaptive immune response, inflammatory mediators, such as cytokines, might contribute to prolonged functional suppression and death of β cells, modulation of β-cell regeneration and insulin resistance
-
Some inflammatory mediators might promote the survival and proliferation of β cells, especially in the absence of autoimmune reaction
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Eizirik, D. L. & Mandrup-Poulsen, T. A choice of death—the signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 44, 2115–2133 (2001).
Cnop, M. et al. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54 (Suppl. 2), S97–S107 (2005).
Baccala, R. et al. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat. Med. 13, 543–551 (2007).
Zipris, D. Innate immunity and its role in type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 15, 326–231 (2008).
Vives-Pi, M. et al. Evidence of expression of endotoxin receptors CD14, Toll-like receptors TLR4 and TLR2 and associated molecule MD-2 and of sensitivity to endotoxin (LPS) in islet beta cells. Clin. Exp. Immunol. 133, 208–218 (2003).
Wen, L. et al. The effect of innate immunity on autoimmune diabetes and the expression of Toll-like receptors on pancreatic islets. J. Immunol. 172, 3173–3180 (2004).
Giarratana, N. et al. A vitamin D analog downregulates proinflammatory chemokine production by pancreatic islets inhibiting T-cell recruitment and type 1 diabetes development. J. Immunol. 173, 2280–2287 (2004).
Rasschaert, J. et al. Toll-like receptor 3 and STAT-1 contribute to double-stranded RNA + interferon-gamma-induced apoptosis in primary pancreatic beta-cells. J. Biol. Chem. 280, 33984–33991 (2005).
Ylipaasto, P. et al. Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia 48, 1510–1522 (2005).
Hultcrantz, M. et al. Interferons induce an antiviral state in human pancreatic islet cells. Virology 367, 92–101 (2007).
Liu, D. et al. Double-stranded ribonucleic acid (RNA) induces beta-cell Fas messenger RNA expression and increases cytokine-induced beta-cell apoptosis. Endocrinology 142, 2593–2599 (2001).
Liu, D. et al. Double-stranded RNA cooperates with interferon-gamma and IL-1-beta to induce both chemokine expression and nuclear factor-κΒ-dependent apoptosis in pancreatic beta-cells: potential mechanisms for viral-induced insulitis and beta-cell death in type 1 diabetes mellitus. Endocrinology 143, 1225–1234 (2002).
Dogusan, Z. et al. Double-stranded RNA induces pancreatic beta-cell apoptosis by activation of the TLR3 and IRF-3 pathways. Diabetes 57, 1236–1245 (2008).
Foulis, A. K. et al. Immunoreactive alpha-interferon in insulin-secreting beta cells in type 1 diabetes mellitus. Lancet 2, 1423–1427 (1987).
Devendra, D. et al. Interferon-alpha as a mediator of polyinosinic: polycytidylic acid-induced type 1 diabetes. Diabetes 54, 2549–2556 (2005).
Lang, K. S. et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat. Med. 11, 138–145 (2005).
Rasschaert, J. et al. Global profiling of double stranded RNA- and IFN-gamma-induced genes in rat pancreatic beta cells. Diabetologia 46, 1641–1657 (2003).
Gurzov, E. N. et al. JunB inhibits ER stress and apoptosis in pancreatic beta-cells. PLoS ONE 3, e3030 (2008).
Drescher, K. M. & Tracy, S. M. The CVB and etiology of type 1 diabetes. Curr. Top. Microbiol. Immunol. 323, 259–274 (2008).
Dotta, F. et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl Acad. Sci. USA 104, 5115–5120 (2007).
Christen, U. et al. A viral epitope that mimics a self-antigen can accelerate but not initiate autoimmune diabetes. J. Clin. Invest. 114, 1290–1298 (2004).
Filippi, C. M. & von Herrath, M. G. Viral trigger for type 1 diabetes: pros and cons. Diabetes 57, 2863–2871 (2008).
Kim, H. S. et al. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity 27, 321–333 (2007).
Goldberg, A. et al. Toll-like receptor 4 suppression leads to islet allograft survival. FASEB J. 21, 2840–2848 (2007).
Wen, L. et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature 455, 1109–1113 (2008).
Wenzlau, J. M. et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc. Natl Acad. Sci. USA 104, 17040–17045 (2007).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature 411, 599–603 (2001).
Hysi, P. et al. NOD1 variation, immunoglobulin E and asthma. Hum. Mol. Genet. 14, 935–941 (2005).
Kanneganti, T. D. et al. Intracellular NOD-like receptors in host defense and disease. Immunity 27, 549–559 (2007).
Bouskra, D. et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456, 507–510 (2008).
Charo, I. F. & Ransohoff, R. M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 354, 610–621 (2006).
Arimilli, S. et al. Chemokines in autoimmune diseases. Immunol. Rev. 177, 43–51 (2000).
Eizirik, D. L. et al. Use of microarray analysis to unveil transcription factor and gene networks contributing to beta-cell dysfunction and apoptosis. Ann. NY Acad. Sci. 1005, 55–74 (2003).
Shimada, A. et al. Elevated serum IP-10 levels observed in type 1 diabetes. Diabetes Care 24, 510–515 (2001).
Nicoletti, F. et al. Serum concentrations of the interferon-gamma-inducible chemokine IP-10/CXCL10 are augmented in both newly diagnosed type I diabetes mellitus patients and subjects at risk of developing the disease. Diabetologia 45, 1107–1110 (2002).
Hanifi-Moghaddam, P. et al. Altered chemokine levels in individuals at risk of type 1 diabetes mellitus. Diabet. Med. 23, 156–163 (2006).
Pfleger, C. et al. Relation of circulating concentrations of chemokine receptor CCR5 ligands to C-peptide, proinsulin and HbA1c and disease progression in type 1 diabetes. Clin. Immunol. 128, 57–65 (2008).
Wang, X. et al. Identification of a molecular signature in human type 1 diabetes mellitus using serum and functional genomics. J. Immunol. 180, 1929–1937 (2008).
Chen, M. C. et al. Monocyte chemoattractant protein-1 is expressed in pancreatic islets from prediabetic NOD mice and in interleukin-1beta exposed human and rat islet cells. Diabetologia 44, 325–332 (2001).
Cardozo, A. K. et al. IL-1beta and IFN-gamma induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from prediabetic NOD mice. Diabetologia 46, 255–266 (2003).
Martin, A. P. et al. Islet expression of M3 uncovers a key role for chemokines in the development and recruitment of diabetogenic cells in NOD mice. Diabetes 57, 387–394 (2008).
Martin, A. P. et al. Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis and diabetes. Diabetes 57, 3025–3033 (2008).
Piemonti, L. et al. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes 51, 55–65 (2002).
Cameron, M. J. et al. Differential expression of CC chemokines and the CCR5 receptor in the pancreas is associated with progression to type 1 diabetes. J. Immunol. 165, 1102–1110 (2000).
Bradley, L. M. et al. Islet-specific Th1, but not Th2, cells secrete multiple chemokines and promote rapid induction of autoimmune diabetes. J. Immunol. 162, 2511–2520 (1999).
Abdi, R. et al. The role of CC chemokine receptor 5 (CCR5) in islet allograft rejection. Diabetes 51, 2489–2495 (2002).
Christen, U. et al. Among CXCR3 chemokines, IFN-gamma-inducible protein of 10 kDa (CXC chemokine ligand (CXCL) 10) but not monokine induced by IFN-gamma (CXCL9) imprints a pattern for the subsequent development of autoimmune disease. J. Immunol. 171, 6838–6845 (2003).
Frigerio, S. et al. Beta cells are responsible for CXCR3-mediated T-cell infiltration in insulitis. Nat. Med. 8, 1414–1420 (2002).
Cardozo, A. K. et al. Identification of novel cytokine-induced genes in pancreatic beta-cells by high-density oligonucleotide arrays. Diabetes 50, 909–920 (2001).
Gysemans, C. A. et al. Dual role of interferon-[gamma] signaling pathway in sensitivity of pancreatic beta-cells to immune destruction. Diabetologia 44, 567–574 (2001).
Cardozo, A. K. et al. A comprehensive analysis of cytokine-induced and nuclear factor κB-dependent genes in primary rat pancreatic beta-cells. J. Biol. Chem. 276, 48879–48886 (2001).
Eldor, R. et al. Conditional and specific NF-κB blockade protects pancreatic beta–cell from diabetogenic agents. Proc. Natl Acad. Sci. USA 103, 5072–5077 (2006).
Gysemans, C. A. et al. Disruption of the gamma–interferon signaling pathway at the level of signal transducer and activator of transcription-1 prevents immune destruction of beta-cells. Diabetes 54, 2396–2403 (2005).
Callewaert, H. I. et al. Deletion of STAT-1 in pancreatic islets protects against streptozotocin-induced diabetes and early graft failure but not against late rejection. Diabetes 56, 2169–2173 (2007).
Eizirik, D. L. et al. Use of a systems biology approach to understand pancreatic beta-cell death in type 1 diabetes. Biochem. Soc. Trans. 36, 321–327 (2008).
Bettelli, E. et al. Induction and effector functions of TH17 cells. Nature 453, 1051–1057 (2008).
Jain, R. et al. Innocuous IFN-γ induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J. Exp. Med. 205, 207–218 (2008).
Filippi, C. M. & von Herrath, M. G. Islet beta-cell death—fuel to sustain autoimmunity? Immunity 27, 183–185 (2007).
Liadis, N. et al. Caspase-3-dependent beta-cell apoptosis in the initiation of autoimmune diabetes mellitus. Mol. Cell. Biol. 25, 3620–3629 (2005).
Cardozo, A. K. et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+ leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 54, 452–461 (2005).
Eizirik, D. L. et al. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29, 42–61 (2008).
Albert, M. L. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat. Rev. Immunol. 4, 223–231 (2004).
Blachere, N. E. et al. Apoptotic cells deliver processed antigen to dendritic cells for crosspresentation. PLoS Biol. 3, e185 (2005).
Scheuner, D. & Kaufman, R. J. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr. Rev. 29, 317–333 (2008).
Smith, J. A. et al. Pathogenesis of ankylosing spondylitis: current concepts. Best Pract. Res. Clin. Rheumatol. 20, 571–591 (2006).
Kent, S. C. et al. Expanded T cells from pancreatic lymph nodes of type 1 diabetic subjects recognize an insulin epitope. Nature 435, 224–228 (2005).
Nakayama, M. et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435, 220–223 (2005).
Wong, F. S. et al. Identification of an MHC class I-restricted autoantigen in type 1 diabetes by screening an organ-specific cDNA library. Nat. Med. 5, 1026–1031 (1999).
Skowera, A. et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J. Clin. Invest. 118, 3390–3402 (2008).
Klinke, D. J. Extent of beta cell destruction is important but insufficient to predict the onset of type 1 diabetes mellitus. PLoS ONE 3: e1374 (2008).
Steele, C. et al. Insulin secretion in type 1 diabetes. Diabetes 53, 426–433 (2004).
Strandell, E. et al. Reversal of beta-cell suppression in vitro in pancreatic islets isolated from nonobese diabetic mice during the phase preceding insulin-dependent diabetes mellitus. J. Clin. Invest. 85, 1944–1950 (1990).
Strandell, E. et al. Role of infiltrating T cells for impaired glucose metabolism in pancreatic islets isolated from nonobese diabetic mice. Diabetologia 35, 924–931 (1992).
Marchetti, P. et al. Function of pancreatic islets isolated from a type 1 diabetic patient. Diabetes Care 23, 701–703 (2000).
Koulmanda, M. et al. Modification of adverse inflammation is required to cure new-onset type 1 diabetic hosts. Proc. Natl Acad. Sci. USA 104, 13074–13079 (2007).
Sarvetnick, N. E. & Gu, D. Regeneration of pancreatic endocrine cells in interferon-gamma transgenic mice. Adv. Exp. Med. Biol. 321, 85–89 (1992).
Sreenan, S. et al. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes 48, 989–996 (1999).
Sherry, N. A. et al. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes 55, 3238–3245 (2006).
Ablamunits, V. et al. Autoimmunity and beta cell regeneration in mouse and human type 1 diabetes: the peace is not enough. Ann. NY Acad. Sci. 1103, 19–32 (2007).
Darville, M. I. & Eizirik, D. L. Notch signaling: a mediator of beta-cell dedifferentiation in diabetes? Biochem. Biophys. Res. Commun. 339, 1063–1068 (2006).
Kutlu, B. et al. Discovery of gene networks regulating cytokine-induced dysfunction and apoptosis in insulin-producing INS-1 cells. Diabetes 52, 2701–2719 (2003).
Tessem, J. S. et al. Critical roles for macrophages in islet angiogenesis and maintenance during pancreatic degeneration. Diabetes 57, 1605–1617 (2008).
Ehses, J. A. et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 56, 2356–2370 (2007).
Eizirik, D. L. et al. Repair of pancreatic beta-cells. A relevant phenomenon in early IDDM? Diabetes 42, 1383–1391 (1993).
In't Veld, P. et al. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes 56, 2400–2404 (2007).
Serhan, C. N. et al. Resolving inflammation: dual anti-inflammatory and proresolution lipid mediators. Nat. Rev. Immunol. 8, 349–361 (2008).
Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 449, 819–826 (2007).
Akira, S. et al. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Takeuchi, O. & Akira, S. MDA5/RIG-I and virus recognition. Curr. Opin. Immunol. 20, 17–22 (2008).
Ishii, K. J. et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 7, 40–48 (2006).
Schlee, M. et al. Beyond double-stranded RNA-type I IFN induction by 3pRNA and other viral nucleic acids. Curr. Top. Microbiol. Immunol. 316, 207–230 (2007).
Stetson, D. B. et al. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 134, 587–598 (2008).
Acknowledgements
This Review is partially based on a lecture given by DL Eizirik at the 13th European Association for the Study of Diabetes/Juvenile Diabetes Research Foundation Oxford Workshop, Oxford, UK, 8–11 August 2008. The concepts presented in this article were developed by the authors during research supported by the European Union (STREP SaveBeta, contract no. 036,903; Framework Program 6 of the European Community), the Fonds National de la Recherche Scientifique, Actions de Recherche Concerté de la Communauté Française, Belgium and the Belgium Program on Interuniversity Poles of Attraction initiated by the Belgian State (IUAP P5/17 and P6/40). M Colli is the recipient of a scholarship from the Brazilian Coordination for the Improvement of Higher Education Personnel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Eizirik, D., Colli, M. & Ortis, F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat Rev Endocrinol 5, 219–226 (2009). https://doi.org/10.1038/nrendo.2009.21
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2009.21
This article is cited by
-
Deficiency of Trex1 leads to spontaneous development of type 1 diabetes
Nutrition & Metabolism (2024)
-
Association of CIITA (rs8048002) and CLEC2D (rs2114870) gene variants and type 1 diabetes mellitus
Journal of Diabetes & Metabolic Disorders (2024)
-
The role of regulated necrosis in diabetes and its complications
Journal of Molecular Medicine (2024)
-
4-Octyl itaconate attenuates glycemic deterioration by regulating macrophage polarization in mouse models of type 1 diabetes
Molecular Medicine (2023)
-
Pre-treatment with IL-6 potentiates β-cell death induced by pro-inflammatory cytokines
BMC Molecular and Cell Biology (2023)