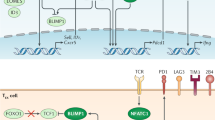
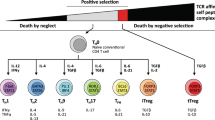
Key Points
-
Programmed cell death protein 1 (PD1) is an inhibitory receptor that is expressed by all T cells during activation. It regulates T cell effector functions during various physiological responses, including acute and chronic infection, cancer and autoimmunity, and in immune homeostasis.
-
PD1 often shows high and sustained expression levels during persistent antigen encounter, which can occur in the setting of chronic infections and cancer. In these settings, PD1 can limit protective immunity.
-
In addition to being expressed by conventional T cells, PD1 is expressed by regulatory T cells, B cells, natural killer cells and some myeloid cell populations. However, compared with conventional T cells, less is known about how PD1 inhibitory signals regulate these cell types.
-
Programmed cell death 1 ligand 1 (PDL1) shows broad expression on both haematopoietic and non-haematopoietic cells, positioning the PD1 pathway as a key regulator of immune cell functions in both secondary lymphoid organs and in non-lymphoid tissues.
-
PD1 limits the activation and function of potentially pathogenic self-reactive CD4+ and CD8+ T cells, and PDL1 can shield target organs from autoimmune attack.
-
Due to the diverse roles of the PD1 pathway in regulating host immunity, context is everything. In order to safely and effectively modulate the PD1 pathway therapeutically, the complex immunological status of the patient should be carefully considered.
Abstract
T cell activation is a highly regulated process involving peptide–MHC engagement of the T cell receptor and positive costimulatory signals. Upon activation, coinhibitory 'checkpoints', including programmed cell death protein 1 (PD1), become induced to regulate T cells. PD1 has an essential role in balancing protective immunity and immunopathology, homeostasis and tolerance. However, during responses to chronic pathogens and tumours, PD1 expression can limit protective immunity. Recently developed PD1 pathway inhibitors have revolutionized cancer treatment for some patients, but the majority of patients do not show complete responses, and adverse events have been noted. This Review discusses the diverse roles of the PD1 pathway in regulating immune responses and how this knowledge can improve cancer immunotherapy as well as restore and/or maintain tolerance during autoimmunity and transplantation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
13 November 2017
On page 5 of this manuscript, in the subsection titled 'PD1 ligands', the html and PDF versions originally contained the incorrect sentence "Cytokines are cru PDL2 expression (TABLE 1), with type I interferons and cial regulators of PDL1 and type II interferon being some of the most potent drivers of PDL1 expression6,48." This has now been corrected to "Cytokines are crucial regulators of PDL1 and PDL2 expression (TABLE 1), with type I interferons and type II interferon being some of the most potent drivers of PDL1 expression6,48." in the html and PDF versions of this manuscript.
References
Greenwald, R. J., Freeman, G. J. & Sharpe, A. H. The B7 family revisited. Annu. Rev. Immunol. 23, 515–548 (2005).
Dong, H., Zhu, G., Tamada, K. & Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 5, 1365–1369 (1999).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000). References 2 and 3 provide the first descriptions of PDL1; reference 3 also identified PDL1 as a binding partner for PD1.
Latchman, Y. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2, 261–268 (2001).
Tseng, S. Y. et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 193, 839–846 (2001). References 4 and 5 present the first descriptions of PDL2 as a second ligand for PD1. Reference 4 also provides one of the first descriptions of PDL1 and PDL2 expression by tumour cells.
Keir, M. E., Butte, M. J., Freeman, G. J. & Sharpe, A. H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Schildberg, F. A., Klein, S. R., Freeman, G. J. & Sharpe, A. H. Coinhibitory pathways in the B7-CD28 ligand-receptor family. Immunity 44, 955–972 (2016).
Riley, J. L. PD-1 signaling in primary T cells. Immunol. Rev. 229, 114–125 (2009).
Nishimura, H., Nose, M., Hiai, H., Minato, N. & Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11, 141–151 (1999). This study describes the development of the first Pdcd1 -knockout mouse. Characterization of Pdcd1 -knockout in the C57BL/6 background shows genetic loss of potentiated autoimmunity, as these mice developed a lupus-like disease.
Nishimura, H. et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291, 319–322 (2001).
Wang, J. et al. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc. Natl Acad. Sci. USA 102, 11823–11828 (2005).
Lucas, J. A. et al. Programmed death ligand 1 regulates a critical checkpoint for autoimmune myocarditis and pneumonitis in MRL mice. J. Immunol. 181, 2513–2521 (2008).
Pauken, K. E. & Wherry, E. J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 36, 265–276 (2015).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006). This study provides the first description of high and sustained expression of PD1 on exhausted T cells in chronic infection. The authors show that administering blocking antibodies against the PD1 pathway after the onset of exhaustion partially reverses this dysfunctional state. However, loss of PD1 signalling early (in Cd274 -knockout mice) results in lethal immunopathology.
Iwai, Y. et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA 99, 12293–12297 (2002). One of the first descriptions of loss of PD1 signalling promoting immunity to cancer in mice. Using both overexpression of PDL1 and blocking antibodies against PDL1, the authors show that perturbing the PD1 pathway can influence tumour growth in mice.
Iwai, Y., Terawaki, S. & Honjo, T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int. Immunol. 17, 133–144 (2005).
Curiel, T. J. et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 9, 562–567 (2003).
Hirano, F. et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 65, 1089–1096 (2005).
Strome, S. E. et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 63, 6501–6505 (2003).
Page, D. B., Postow, M. A., Callahan, M. K., Allison, J. P. & Wolchok, J. D. Immune modulation in cancer with antibodies. Annu. Rev. Med. 65, 185–202 (2014).
Topalian, S. L., Drake, C. G. & Pardoll, D. M. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015).
Wolchok, J. D. et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 369, 122–133 (2013). This is a key paper showing synergy with combination therapy (using PD1 and CTLA4 inhibitors) for the treatment of metastatic melanoma in patients. Compared with monotherapy targeting PD1 or CTLA4, combination therapy shows greater efficacy but also higher rates of adverse events.
Day, D. & Hansen, A. R. Immune-related adverse events associated with immune checkpoint inhibitors. BioDrugs 30, 571–584 (2016).
Duraiswamy, J. et al. Phenotype, function, and gene expression profiles of programmed death-1hi CD8 T cells in healthy human adults. J. Immunol. 186, 4200–4212 (2011).
Hellmann, M. D., Friedman, C. F. & Wolchok, J. D. Combinatorial cancer immunotherapies. Adv. Immunol. 130, 251–277 (2016).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Agata, Y. et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 8, 765–772 (1996).
Crawford, A. et al. Molecular and transcriptional basis of CD4+ T cell dysfunction during chronic infection. Immunity 40, 289–302 (2014).
Taylor, A. et al. Glycogen synthase kinase 3 inactivation drives T-bet-mediated downregulation of co-receptor PD-1 to enhance CD8+ cytolytic T cell responses. Immunity 44, 274–286 (2016).
Angelosanto, J. M., Blackburn, S. D., Crawford, A. & Wherry, E. J. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J. Virol. 86, 8161–8170 (2012).
Utzschneider, D. T. et al. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat. Immunol. 14, 603–610 (2013).
Pauken, K. E. et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016).
Youngblood, B. et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8+ T cells. Immunity 35, 400–412 (2011).
Sen, D. R. et al. The epigenetic landscape of T cell exhaustion. Science 354, 1165–1169 (2016).
McPherson, R. C. et al. Epigenetic modification of the PD-1 (Pdcd1) promoter in effector CD4+ T cells tolerized by peptide immunotherapy. eLife 3, e03416 (2014).
Staron, M. M. et al. The transcription factor FoxO1 sustains expression of the inhibitory receptor PD-1 and survival of antiviral CD8+ T cells during chronic infection. Immunity 41, 802–814 (2014).
Chang, C. H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Bengsch, B. et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8+ T cell exhaustion. Immunity 45, 358–373 (2016).
Scharping, N. E. et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity 45, 374–388 (2016).
Parry, R. V. et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 25, 9543–9553 (2005). This study presents a key comparison of PD1 and CTLA4 signalling, showing that these pathways are not redundant. Both PD1 and CTLA4 signalling can target the AKT pathway, although CTLA4 does so through PP2A regulating AKT, whereas PD1 targets the AKT pathway by inhibiting CD28-mediated activation of PI3K. The authors also show the importance of the immunoreceptor tyrosine-based switch motif of PD1 in mediating signalling.
Gubin, M. M. et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515, 577–581 (2014).
Patsoukis, N. et al. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun. 6, 6692 (2015).
Chamoto, K. et al. Mitochondrial activation chemicals synergize with surface receptor PD-1 blockade for T cell-dependent antitumor activity. Proc. Natl Acad. Sci. USA 114, E761–E770 (2017).
O'Sullivan, D. & Pearce, E. L. Targeting T cell metabolism for therapy. Trends Immunol. 36, 71–80 (2015).
Chang, C. H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
Ribas, A. & Hu-Lieskovan, S. What does PD-L1 positive or negative mean? J. Exp. Med. 213, 2835–2840 (2016).
Eppihimer, M. J. et al. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 9, 133–145 (2002).
Yokosuka, T. et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J. Exp. Med. 209, 1201–1217 (2012).
Hui, E. et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355, 1428–1433 (2017).
Patsoukis, N. et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci. Signal. 5, ra46 (2012).
Wherry, E. J., Barber, D. L., Kaech, S. M., Blattman, J. N. & Ahmed, R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl Acad. Sci. USA 101, 16004–16009 (2004).
Shin, H., Blackburn, S. D., Blattman, J. N. & Wherry, E. J. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204, 941–949 (2007).
Kamphorst, A. O. et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 355, 1423–1427 (2017).
Murali-Krishna, K. et al. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286, 1377–1381 (1999).
London, C. A., Lodge, M. P. & Abbas, A. K. Functional responses and costimulator dependence of memory CD4+ T cells. J. Immunol. 164, 265–272 (2000).
Kim, S. K., Schluns, K. S. & Lefrancois, L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J. Immunol. 163, 4125–4132 (1999).
Suresh, M. et al. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J. Immunol. 167, 5565–5573 (2001).
Eberlein, J. et al. Multiple layers of CD80/86-dependent costimulatory activity regulate primary, memory, and secondary lymphocytic choriomeningitis virus-specific T cell immunity. J. Virol. 86, 1955–1970 (2012).
Floyd, T. L. et al. Limiting the amount and duration of antigen exposure during priming increases memory T cell requirement for costimulation during recall. J. Immunol. 186, 2033–2041 (2011).
Xiao, Y. et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J. Exp. Med. 211, 943–959 (2014).
Butte, M. J., Keir, M. E., Phamduy, T. B., Sharpe, A. H. & Freeman, G. J. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27, 111–122 (2007).
Paterson, A. M. et al. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J. Immunol. 187, 1097–1105 (2011).
Park, J. J. et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood 116, 1291–1298 (2010).
Allie, S. R., Zhang, W., Fuse, S. & Usherwood, E. J. Programmed death 1 regulates development of central memory CD8 T cells after acute viral infection. J. Immunol. 186, 6280–6286 (2011).
Fuse, S. et al. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J. Immunol. 182, 4244–4254 (2009).
Honda, T. et al. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity 40, 235–247 (2014).
Lazar-Molnar, E. et al. The PD-1/PD-L costimulatory pathway critically affects host resistance to the pathogenic fungus Histoplasma capsulatum. Proc. Natl Acad. Sci. USA 105, 2658–2663 (2008).
Phares, T. W. et al. Target-dependent B7-H1 regulation contributes to clearance of central nervous system infection and dampens morbidity. J. Immunol. 182, 5430–5438 (2009).
Yao, S. et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood 113, 5811–5818 (2009).
Erickson, J. J. et al. Viral acute lower respiratory infections impair CD8+ T cells through PD-1. J. Clin. Invest. 122, 2967–2982 (2012).
Frebel, H. et al. Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J. Exp. Med. 209, 2485–2499 (2012).
Odorizzi, P. M., Pauken, K. E., Paley, M. A., Sharpe, A. & Wherry, E. J. Genetic absence of PD-1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 212, 1125–1137 (2015).
Rowe, J. H., Johanns, T. M., Ertelt, J. M. & Way, S. S. PDL-1 blockade impedes T cell expansion and protective immunity primed by attenuated Listeria monocytogenes. J. Immunol. 180, 7553–7557 (2008).
Talay, O., Shen, C. H., Chen, L. & Chen, J. B7-H1 (PD-L1) on T cells is required for T-cell-mediated conditioning of dendritic cell maturation. Proc. Natl Acad. Sci. USA 106, 2741–2746 (2009).
Sage, P. T. & Sharpe, A. H. T follicular regulatory cells. Immunol. Rev. 271, 246–259 (2016).
Mueller, S. N. et al. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J. Clin. Invest. 120, 2508–2515 (2010). This key report shows the unique biological roles for PDL1 in haematopoietic versus non-haematopoietic cells during chronic infection. PDL1 on haematopoietic cells limits CD8+ T cell functions, whereas PDL1 on non-haematopoietic cells regulates viral replication and immunopathology.
Gotsman, I. et al. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J. Clin. Invest. 117, 2974–2982 (2007).
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Buchholz, V. R. et al. Disparate individual fates compose robust CD8+ T cell immunity. Science 340, 630–635 (2013).
Gerlach, C. et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science 340, 635–639 (2013).
Keir, M. E., Latchman, Y. E., Freeman, G. J. & Sharpe, A. H. Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J. Immunol. 175, 7372–7379 (2005).
Nishimura, H., Honjo, T. & Minato, N. Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J. Exp. Med. 191, 891–898 (2000).
Ansari, M. J. et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 198, 63–69 (2003).
Reynoso, E. D. et al. Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J. Immunol. 182, 2102–2112 (2009).
Probst, H. C., McCoy, K., Okazaki, T., Honjo, T. & van den Broek, M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat. Immunol. 6, 280–286 (2005).
Fife, B. T. et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J. Exp. Med. 203, 2737–2747 (2006).
Fife, B. T. et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat. Immunol. 10, 1185–1192 (2009).
Guleria, I. et al. Mechanisms of PDL1-mediated regulation of autoimmune diabetes. Clin. Immunol. 125, 16–25 (2007).
Pauken, K. E., Jenkins, M. K., Azuma, M. & Fife, B. T. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during type 1 diabetes. Diabetes 62, 2859–2869 (2013).
Keir, M. E., Freeman, G. J. & Sharpe, A. H. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J. Immunol. 179, 5064–5070 (2007).
Rodig, N. et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 33, 3117–3126 (2003).
Pauken, K. E. et al. Cutting edge: identification of autoreactive CD4+ and CD8+ T cell subsets resistant to PD-1 pathway blockade. J. Immunol. 194, 3551–3555 (2015).
Liang, S. C. et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur. J. Immunol. 33, 2706–2716 (2003). One of the first studies to describe the tissue expression (both haematopoietic and non-haematopoietic) of PDL1 and PDL2.
Keir, M. E. et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 203, 883–895 (2006).
Scott-Browne, J. P. et al. Dynamic changes in chromatin accessibility occur in CD8+ T cells responding to viral infection. Immunity 45, 1327–1340 (2016).
Mognol, G. P. et al. Exhaustion-associated regulatory regions in CD8+ tumor-infiltrating T cells. Proc. Natl Acad. Sci. USA 114, E2776–E2785 (2017).
Schietinger, A., Delrow, J. J., Basom, R. S., Blattman, J. N. & Greenberg, P. D. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science 335, 723–727 (2012).
Bennett, F. et al. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J. Immunol. 170, 711–718 (2003).
Hirata, S. et al. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J. Immunol. 174, 1888–1897 (2005).
Ding, H. et al. Delivering PD-1 inhibitory signal concomitant with blocking ICOS co-stimulation suppresses lupus-like syndrome in autoimmune BXSB mice. Clin. Immunol. 118, 258–267 (2006).
Fuller, M. J. et al. Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1). Proc. Natl Acad. Sci. USA 110, 15001–15006 (2013).
Velu, V. et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature 458, 206–210 (2009).
Gardiner, D. et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PloS ONE 8, e63818 (2013).
Blackburn, S. D., Shin, H., Freeman, G. J. & Wherry, E. J. Selective expansion of a subset of exhausted CD8 T cells by αPD-L1 blockade. Proc. Natl Acad. Sci. USA 105, 15016–15021 (2008).
Im, S. J. et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016).
He, R. et al. Follicular CXCR5-expressing CD8+ T cells curtail chronic viral infection. Nature 537, 412–428 (2016).
Paley, M. A. et al. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 338, 1220–1225 (2012).
Spranger, S. et al. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8+ T cells directly within the tumor microenvironment. J. Immunother. Cancer 2, 3 (2014).
Juneja, V. R. et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Exp. Med. 214, 895–904 (2017).
Noguchi, T. et al. Temporally distinct PD-L1 expression by tumor and host cells contributes to immune escape. Cancer Immunol. Res. 5, 106–117 (2017).
Lau, J. et al. Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice. Nat. Commun. 8, 14572 (2017).
Masopust, D., Vezys, V., Wherry, E. J., Barber, D. L. & Ahmed, R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol. 176, 2079–2083 (2006).
Casey, K. A. et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 188, 4866–4875 (2012).
Masopust, D. & Schenkel, J. M. The integration of T cell migration, differentiation and function. Nat. Rev. Immunol. 13, 309–320 (2013).
Spitzer, M. H. et al. Systemic immunity Is iequired for effective cancer immunotherapy. Cell 168, 487–502.e15 (2017).
Ward, J. P., Gubin, M. M. & Schreiber, R. D. The role of neoantigens in naturally occurring and therapeutically induced immune responses to cancer. Adv. Immunol. 130, 25–74 (2016).
Woo, S. R. et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72, 917–927 (2012). This is a key paper showing potent synergy of combination therapy (targeting PD1 and LAG3) in mouse models of cancer. The authors show that loss of both PD1 and LAG3 has better antitumour efficacy than loss of either inhibitory receptor alone.
Shi, L. Z. et al. Interdependent IL-7 and IFN-γ signalling in T-cell controls tumour eradication by combined α-CTLA-4+α-PD-1 therapy. Nat. Commun. 7, 12335 (2016).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012). References 122 and 123 are the first reports to show the remarkable clinical efficacy of PD1 pathway blockade in clinical trials for cancer.
Robert, C. et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384, 1109–1117 (2014).
Ribas, A. et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA 315, 1600–1609 (2016).
Powles, T. et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515, 558–562 (2014).
Brahmer, J. R. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010).
Topalian, S. L. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 32, 1020–1030 (2014).
Hamid, O. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369, 134–144 (2013).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Taube, J. M. et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci.Transl Med. 4, 127ra37 (2012).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Chen, P. L. et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 6, 827–837 (2016).
Ascierto, M. L. et al. The intratumoral balance between metabolic and immunologic gene expression is associated with anti-PD-1 response in patients with renal cell carcinoma. Cancer Immunol. Res. 4, 726–733 (2016).
Hugo, W. et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 165, 35–44 (2016).
Roh, W. et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci. Transl Med. 9, eaah3560 (2017).
Twyman-Saint Victor, C. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015).
Vogelstein, B. et al. Cancer genome landscapes. Science 339, 1546–1558 (2013).
Rizvi, N. A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015). This study demonstrates that mutational burden may affect responsiveness to PD1 pathway inhibitors in cancer patients, noting that higher mutational burden in NSCLC correlates with better patient outcomes.
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Van Allen, E. M. et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211 (2015).
McGranahan, N. et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351, 1463–1469 (2016).
Yadav, M. et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515, 572–576 (2014).
Minn, A. J. Interferons and the immunogenic effects of cancer therapy. Trends Immunol. 36, 725–737 (2015).
Wilson, E. B. et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340, 202–207 (2013).
Teijaro, J. R. et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340, 207–211 (2013).
Benci, J. L. et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 167, 1540–1554.e12 (2016).
Zaretsky, J. M. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375, 819–829 (2016).
Gao, J. et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 167, 397–404.e9 (2016).
Shin, D. S. et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 7, 188–201 (2017).
Yang, W. et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 531, 651–655 (2016).
Lohmann, T., Leslie, R. D. & Londei, M. T cell clones to epitopes of glutamic acid decarboxylase 65 raised from normal subjects and patients with insulin-dependent diabetes. J. Autoimmun. 9, 385–389 (1996).
van Noort, J. M. et al. Minor myelin proteins can be major targets for peripheral blood T cells from both multiple sclerosis patients and healthy subjects. J. Neuroimmunol. 46, 67–72 (1993).
Singer, M. et al. A distinct gene module for dysfunction uncoupled from activation in tumor-infiltrating T cells. Cell 166, 1500–1511.e9 (2016).
Sage, P. T., Francisco, L. M., Carman, C. V. & Sharpe, A. H. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat. Immunol. 14, 152–161 (2013).
Francisco, L. M. et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206, 3015–3029 (2009).
Kean, L. S., Turka, L. A. & Blazar, B. R. Advances in targeting co-inhibitory and co-stimulatory pathways in transplantation settings: the Yin to the Yang of cancer immunotherapy. Immunol. Rev. 276, 192–212 (2017).
Blazar, B. R. et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-γ-dependent mechanism. J. Immunol. 171, 1272–1277 (2003).
Yang, J. et al. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J. Immunol. 187, 1113–1119 (2011).
Guleria, I. et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 202, 231–237 (2005).
Petroff, M. G. et al. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol. Reprod. 68, 1496–1504 (2003).
Habicht, A. et al. A link between PDL1 and T regulatory cells in fetomaternal tolerance. J. Immunol. 179, 5211–5219 (2007).
Kleffel, S. et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell 162, 1242–1256 (2015).
Yu, Y. et al. Single-cell RNA-seq identifies a PD-1hi ILC progenitor and defines its development pathway. Nature 539, 102–106 (2016).
Karakhanova, S., Bedke, T., Enk, A. H. & Mahnke, K. IL-27 renders DC immunosuppressive by induction of B7-H1. J. Leukocyte Biol. 89, 837–845 (2011).
Hirahara, K. et al. Interleukin-27 priming of T cells controls IL-17 production in trans via induction of the ligand PD-L1. Immunity 36, 1017–1030 (2012).
Severyn, C. J., Shinde, U. & Rotwein, P. Molecular biology, genetics and biochemistry of the repulsive guidance molecule family. Biochem. J. 422, 393–403 (2009).
Siebold, C., Yamashita, T., Monnier, P. P., Mueller, B. K. & Pasterkamp, R. J. RGMs: structural insights, molecular regulation, and downstream signaling. Trends Cell Biol. 27, 365–378 (2016).
Acknowledgements
The authors thank J. Schenkel for helpful discussions and critical reading of the manuscript. The authors apologize to colleagues whose work was not cited in this Review owing to space constraints. This work was supported by grants from the US National Institutes of Health (P01 AI56299, AI 40614) (to A.H.S.) and the Evergrande Center for Immunological Diseases at Harvard Medical School and Brigham and Women's Hospital.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to researching, writing and editing the review.
Corresponding author
Ethics declarations
Competing interests
A.H.S. is an inventor on patent numbers US 8552154 B2, US 8652465 B2 and US 9102727 B2, held by Emory University (Atlanta, Georgia, USA), Dana–Farber Cancer Institute (Boston, Massachusetts, USA), Brigham and Women's Hospital (Boston) and Harvard University (Cambridge, Massachusetts, USA), which cover the topic of PD1-directed immunotherapy.
Glossary
- Self-tolerance
-
Broadly refers to a series of mechanisms used by the body to limit the activation of self-reactive T cells and B cells to prevent these cells from targeting and destroying self tissues.
- Central tolerance
-
Mechanisms of tolerance that occur in the central lymphoid organs (thymus for T cells, bone marrow for B cells). Mechanisms include negative selection (for both T cells and B cells), receptor editing (for B cells) and lineage deviation (for T cells).
- Peripheral tolerance
-
Mechanisms of tolerance that occur in the periphery after full development of lymphocytes in the bone marrow or thymus and their egress from these sites. These mechanisms can occur during priming in the secondary lymphoid organs or in peripheral tissues.
- Immune checkpoints
-
An alternative term for coinhibitory molecules, generally referring to inhibitory signals that immune cells must overcome to perform full effector functions.
- Effector T cells
-
T cells that have recently encountered antigen and differentiated from a quiescent state to a fully activated state, a conversion that is accompanied by proliferation and acquisition of effector functions.
- T cell exhaustion
-
Caused by chronic antigenic stimulation and exposure to chronic inflammation, T cell exhaustion results in a progressive loss of effector functions and potential over time. There are subsets of exhausted T cells that differ in their functionality.
- Tolerant T cells
-
Self-reactive T cells that have been activated by cognate antigen but have been rendered hypofunctional to protect self tissues from destruction. Mechanisms include anergy, active suppression by regulatory T cells and suppression through programmed cell death protein 1 (PD1).
- Regulatory T (Treg) cells
-
Generally refers to a subset of CD4+ T cells that expresses the transcription factor forkhead box protein P3 (FOXP3) and actively inhibits immune responses (through immunosuppressive cytokine production, modulating dendritic cell function, metabolic disruption and/or production of adenosine). Additional populations of Treg cells include CD8+ Treg cells, RORγt+FOXP3+ Treg cells and T regulatory type 1 (TR1) cells.
- T follicular helper (TFH) cells
-
A subset of CD4+ T cells that expresses CXC-chemokine receptor 5 (CXCR5), BCL-6, programmed cell death protein 1 (PD1) and ICOS, localizes to the B cell follicle and provides help to B cells to generate productive humoral immune responses (through CD40 and IL-21).
- T follicular regulatory (TFR) cells
-
A subset of regulatory T cells that expresses forkhead box protein P3 (FOXP3), CXCR5, BCL-6, B lymphocyte-induced maturation protein 1 (BLIMP1), programmed cell death protein 1 (PD1) and ICOS and that attenuates humoral immunity by controlling TFH cell and B cell functions.
- Memory T cells
-
Long-lived populations of antigen-experienced T cells that persist after acute antigen is cleared. Compared with their naive counterparts, memory T cells are present in higher numbers, have a broader anatomical distribution and more rapidly differentiate into effector cells upon antigen re-encounter. Naive T cells are restricted to secondary lymphoid organs (SLOs), while central memory T cells can be found in SLOs, effector memory T cells circulate in blood and non-lymphoid tissues, and resident memory T cells permanently reside in either SLOs or non-lymphoid tissues.
- DNA methylation
-
An epigenetic modification that results in transcriptional repression.
- ATAC-seq
-
(Assay for transposase-accessible chromatin with high-throughput sequencing). A rapid and sensitive method of assaying chromatin accessibility that uses in vitro transposition of sequencing adaptors into open chromatin followed by high-throughput sequencing to determine the location of open chromatin.
- Epigenetic regulation
-
A broad set of heritable changes in gene expression that occur independently of changes to the DNA sequence (for example, DNA methylation, histone modifications) that broadly defines the transcriptional capacity of a cell, dictating cell lineage, fate and effector potential.
- Type I interferons
-
Type I interferons, such as IFNα and IFNβ, are generally produced in response to danger-associated signals such as Toll-like receptors and cytosolic nucleic acid sensors. Type I interferons are produced by most cells in the body and have a variety of immunostimulatory effects and innate antiviral effects during acute infection, including inhibition of translation. By contrast, persistent type I interferon signalling during chronic viral infection can promote immune dysfunction.
- Type II interferon
-
IFNγ, a key T cell and natural killer cell effector molecule that drives myeloid activation, MHC class I and II processing and presentation, leukocyte trafficking and pathogen replication inhibition.
- Adaptive resistance
-
Mechanisms by which a tumour adapts to tumour-specific immune responses, leading to the upregulation of immunosuppressive molecules, such as programmed cell death 1 ligand 1 (PDL1), in an attempt to evade host immunity.
- Anergic T cells
-
A form of peripheral tolerance induced at priming, generally resulting from high levels of antigen being recognized with inadequate amounts of costimulation and/or inflammatory cytokines. Anergic T cells persist in a functionally hyporesponsive state, but functionality can be restored if proper signals are provided.
- Neoantigen-specific T cell
-
A T cell that recognizes antigens in the tumour that have been mutated so that the antigen no longer resembles self antigens, theoretically making these antigens more immunogenic because they are less affected by central tolerance.
- Immunogenicity
-
The ability of an antigen to stimulate an immune response; highly immunogenic antigens are generally recognized by the immune system as foreign (or distinct from self) and their recognition is accompanied by inflammation.
- irRECIST
-
(Immune-related response evaluation criteria in solid tumours). Similar to RECIST, where a total of five malignant lesions, two per organ, are measured unidimensionally, with shared response criteria (complete response, partial response, stable disease and progressive disease defined), but the physician waits up to 12 weeks to confirm progressive disease to account for the flare effect.
Rights and permissions
About this article
Cite this article
Sharpe, A., Pauken, K. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 18, 153–167 (2018). https://doi.org/10.1038/nri.2017.108
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2017.108
This article is cited by
-
Emerging mechanisms of the unfolded protein response in therapeutic resistance: from chemotherapy to Immunotherapy
Cell Communication and Signaling (2024)
-
Novel nanotherapeutics for cancer immunotherapy by albumin nanoparticles functionalized with PD-1 and PD-L1 aptamers
Cancer Nanotechnology (2024)
-
Partial recovery of peripheral blood monocyte subsets in head and neck squamous cell carcinoma patients upon radio(chemo)therapy is associated with decreased plasma CXCL11
BMC Cancer (2024)
-
PD-1 signaling uncovers a pathogenic subset of T cells in inflammatory arthritis
Arthritis Research & Therapy (2024)
-
IGFBP3 induces PD-L1 expression to promote glioblastoma immune evasion
Cancer Cell International (2024)