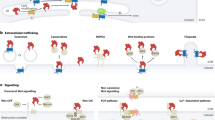
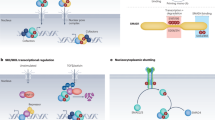
Key Points
-
Development is a progressive, dynamic process that involves coordination between cell signalling and gene expression. Heterotrimeric G proteins are well-known mediators of various cell signalling pathways, but their roles in development have been poorly understood.
-
G proteins have been identified in some plants and in all animals from Drosophila melanogaster to humans, including roundworms, the sea urchin, zebrafish, frogs and rodents. G proteins have been implicated as essential transducers in all main models of development.
-
Many heptahelical cell-membrane-localized receptors have been implicated in development, including the Frizzled proteins, the methuselah gene product and perhaps Smoothened. Use of knockout mice and knock-down technologies in cells enables the elucidation of the precise identity of the G proteins that transduce the binding of agonists (for example, Wnts) to the receptor (Frizzled) into effector activation.
-
The superfamily of G-protein-coupled receptors (GPCRs) functions through a smaller family of G proteins that can signal both by their activated, GTP-bound Gα subunits as well as their Gβγ-subunit complexes, which activates G-protein-coupled effectors. Effectors of G proteins include the well-known adenylyl cyclases, phosphodiesterases, phospholipase Cβs and various ion channels.
-
GPCRs can activate several G proteins, which in turn can activate several effectors that are linked to downstream signalling cascades, such as the mitogen-activated-protein-kinase cascades. These cascades link G-protein activation to changes in gene transcription and expression and to rearrangement of the cytoskeleton.
-
G-protein-based signalling complexes make use of scaffold molecules, such as the A-kinase anchoring proteins (AKAPs), which provide a mobile toolbox of protein kinases, protein phosphatases and adaptor molecules to modulate signals from the GPCRs to the signalling cascades. Scaffolds confer spatial localization on signalling complexes in cells, an important facet of early development in which axis and morphogen gradients function.
-
G proteins are regulated not only by activated GPCRs, but also by members of several classes of newly discovered modulators, including regulators of G-protein signalling (RGS proteins), activators of G-protein signalling (AGS proteins), and partners of Inscuteable (Pins). G proteins have essential roles in the formation and orientation of mitotic spindles, in planar cell polarity, and in Frizzled signalling.
Abstract
The focus of developmental biologists has expanded from the analysis of gene expression to include the analysis of cell signalling. Heterotrimeric G proteins (G proteins) mediate signalling from a superfamily of heptahelical receptors (G-protein-coupled receptors) to a smaller number of effector units that include adenylyl cyclases, phospholipase C and various ion channels. The convergence of developmental biology with cell signalling has now revealed overlaps in which G proteins mediate complex pathways in embryonic development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Morris, A. J. & Malbon, C. C. Physiological regulation of G protein-linked signalling. Physiol. Rev. 79, 1373–1430 (1999).
Katanaev, V. L., Ponzielli, R., Semeriva, M. & Tomlinson, A. Trimeric G protein-dependent Frizzled signalling in Drosophila. Cell 120, 111–122 (2005). First demonstration of an obligate role for the heterotrimeric G protein (G o ) in Wnt–β-catenin and Wnt-planar-cell-polarity pathways in D. melanogaster.
Casey, P. J. Mechanisms of protein prenylation and role in G protein function. Biochem. Soc. Trans. 23, 161–166 (1995).
Weston, C. R., Lambright, D. G. & Davis, R. J. Signal transduction. MAP kinase signalling specificity. Science 296, 2345–2347 (2002).
Weng, Z. et al. A DNA damage and stress inducible G protein-coupled receptor blocks cells in G2/M. Proc. Natl Acad. Sci. USA 95, 12334–12339 (1998).
Lyons, J. et al. Two G protein oncogenes in human endocrine tumours. Science 249, 655–659 (1990).
Spiegel, A. M. Inborn errors of signal transduction: mutations in G proteins and G protein-coupled receptors as a cause of disease. J. Inherit. Metab. Dis. 20, 113–121 (1997).
Malbon, C. C. Heterotrimeric G-proteins and development. Biochem. Pharmacol. 53, 1–4 (1997).
Lin, Y. J., Seroude, L. & Benzer, S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science 282, 943–946 (1998). Identification of the product of the methuselah gene in D. melanogaster as a GPCR that controls the lifespan of the organism.
Pupillo, M. et al. cAMP receptor and G-protein interactions control development in Dictyostelium. Cold Spring Harb. Symp. Quant. Biol. 53, 657–665 (1988).
Strittmatter, S. M., Valenzuela, D., Kennedy, T. E., Neer, E. J. & Fishman, M. C. Go is a major growth cone protein subject to regulation by GAP-43. Nature 344, 836–841 (1990).
Parks, S. & Wieschaus, E. The Drosophila gastrulation gene concertina encodes a Gα- like protein. Cell 64, 447–458 (1991).
Wang, H. Y., Watkins, D. C. & Malbon, C. C. Antisense oligodeoxynucleotides to GS protein α-subunit sequence accelerate differentiation of fibroblasts to adipocytes. Nature 358, 334–337 (1992).
Moxham, C. M., Hod, Y. & Malbon, C. C. Induction of Gαi2-specific antisense RNA in vivo inhibits neonatal growth. Science 260, 991–995 (1993).
Yu, S. et al. Paternal versus maternal transmission of a stimulatory G-protein α subunit knockout produces opposite effects on energy metabolism. J. Clin. Invest. 105, 615–623 (2000).
Wettschureck, N., Moers, A. & Offermanns, S. Mouse models to study G-protein-mediated signalling. Pharmacol. Ther. 101, 75–89 (2004).
Spiegel, A. M. G protein defects in signal transduction. Horm. Res. 53, 17–22 (2000).
Liu, T. et al. G protein signalling from activated rat frizzled-1 to the β-catenin–Lef-Tcf pathway. Science 292, 1718–1722 (2001). First publication to show the obligate role for heterotrimeric G proteins in the Wnt–β-catenin pathway of development.
Slusarski, D. C., Corces, V. G. & Moon, R. T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 390, 410–413 (1997). Early demonstration in zebrafish embryos that specific inhibitors of heterotrimeric G-protein subunits (for example, pertussis toxin and GDP-β-S) inhibit the Ca2+ release triggered by Xwnt5a and the rat Fz2.
Wolfgang, W. J. et al. Signalling through Gsα is required for the growth and function of neuromuscular synapses in Drosophila. Dev. Biol. 268, 295–311 (2004).
Shenker, A. et al. Severe endocrine and nonendocrine manifestations of the McCune–Albright syndrome associated with activating mutations of stimulatory G protein Gs . J. Pediatr. 123, 509–518 (1993).
Yu, S. et al. Variable and tissue-specific hormone resistance in heterotrimeric Gs protein α-subunit (Gsα) knockout mice is due to tissue-specific imprinting of the Gsα gene. Proc. Natl Acad. Sci. USA 95, 8715–8720 (1998).
Gao, P., Watkins, D. C. & Malbon, C. C. Constitutively active mutant GSα (G225T) and null-mutant Gαi-2 (G203T) induce primitive endoderm from stem cells. Am. J. Physiol. 268, C1460–C1466 (1995).
Aubry, L. & Firtel, R. Integration of signalling networks that regulate Dictyostelium differentiation. Annu. Rev. Cell Dev. Biol. 15, 469–517 (1999).
Schaefer, M., Shevchenko, A., Shevchenko, A. & Knoblich, J. A. A protein complex containing Inscuteable and the Gα-binding protein Pins orients asymmetric cell divisions in Drosophila. Curr. Biol. 10, 353–362 (2000). Important report that shows the essential role of heterotrimeric G proteins in regulating the proper orientation of asymmetric cell division during the development of D. melanogaster.
Voronina, E. & Wessel, G. M. Regulatory contribution of heterotrimeric G-proteins to oocyte maturation in the sea urchin. Mech. Dev. 121, 247–259 (2004).
Liu, X. et al. Activation of a frizzled-2/β-adrenergic receptor chimera promotes Wnt signalling and differentiation of mouse F9 teratocarcinoma cells via Gαo and Gαt . Proc. Natl Acad. Sci. USA 96, 14383–14388 (1999).
Ahumada, A. et al. Signalling of rat Frizzled-2 through phosphodiesterase and cyclic GMP. Science 298, 2006–2010 (2002).
Watkins, D. C., Johnson, G. L. & Malbon, C. C. Regulation of the differentiation of teratocarcinoma cells into primitive endoderm by Gαi2 . Science 258, 1373–1375 (1992).
Rudolph, U. et al. Ulcerative colitis and adenocarcinoma of the colon in Gαi2-deficient mice. Nature Genet. 10, 143–150 (1995).
Moxham, C. M. & Malbon, C. C. Insulin action impaired by deficiency of the G-protein subunit Giα2 . Nature 379, 840–844 (1996).
Neer, E. J. & Clapham, D. E. Roles of G protein subunits in transmembrane signalling. Nature 333, 129–134 (1988).
Fremion, F. et al. The heterotrimeric protein Go is required for the formation of heart epithelium in Drosophila. J. Cell Biol. 145, 1063–1076 (1999).
Kindt, K. S., Tam, T., Whiteman, S. & Schafer, W. R. Serotonin promotes Go-dependent neuronal migration in Caenorhabditis elegans. Curr. Biol. 12, 1738–1747 (2002).
Kindt, R. M. & Lander, A. D. Pertussis toxin specifically inhibits growth cone guidance by a mechanism independent of direct G protein inactivation. Neuron 15, 79–88 (1995).
Horgan, A. M., Lagrange, M. T. & Copenhaver, P. F. Developmental expression of G proteins in a migratory population of embryonic neurons. Development 120, 729–742 (1994).
Strittmatter, S. M., Valenzuela, D., Kennedy, T. E., Neer, E. J. & Fishman, M. C. Go is a major growth cone protein subject to regulation by GAP-43. Nature 344, 836–841 (1990).
Wu, H. C. & Lin, C. T. Association of heterotrimeric GTP binding regulatory protein (Go) with mitosis. Lab. Invest. 71, 175–181 (1994). Early report indicating that heterotrimeric G proteins have an essential role in cellular mitosis.
Betschinger, J. & Knoblich, J. A. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr. Biol. 14, R674–R685 (2004).
Sarma, T., Voyno-Yasenetskaya, T., Hope, T. J. & Rasenick, M. M. Heterotrimeric G-proteins associate with microtubules during differentiation in PC12 pheochromocytoma cells. FASEB J. 17, 848–859 (2003).
Wang, Y. et al. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J. Biol. Chem. 271, 4468–4476 (1996).
Wang, H. Y. & Malbon, C. C. Wnt-frizzled signalling to G-protein-coupled effectors. Cell. Mol. Life Sci. 61, 69–75 (2004).
Jiang, M. et al. Multiple neurological abnormalities in mice deficient in the G protein Go . Proc. Natl Acad. Sci. USA 95, 3269–3274 (1998).
Pang, I. H. & Sternweis, P. C. Isolation of the α subunits of GTP-binding regulatory proteins by affinity chromatography with immobilized βγ subunits. Proc. Natl Acad. Sci. USA 86, 7814–7818 (1989).
Offermanns, S. et al. Impaired motor coordination and persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking Gαq . Proc. Natl Acad. Sci. USA 94, 14089–14094 (1997).
Galvin-Parton, P. A., Chen, X., Moxham, C. M. & Malbon, C. C. Induction of Gαq-specific antisense RNA in vivo causes increased body mass and hyperadiposity. J. Biol. Chem. 272, 4335–4341 (1997).
Offermanns, S. et al. Embryonic cardiomyocyte hypoplasia and craniofacial defects in Gαq/Gα11-mutant mice. EMBO J. 17, 4304–4312 (1998).
Van Raamsdonk, C. D., Fitch, K. R., Fuchs, H., de Angelis, M. H. & Barsh, G. S. Effects of G-protein mutations on skin colour. Nature Genet. 36, 961–968 (2004).
Moghal, N., Garcia, L. R., Khan, L. A., Iwasaki, K. & Sternberg, P. W. Modulation of EGF receptor-mediated vulva development by the heterotrimeric G-protein Gαq and excitable cells in C. elegans. Development 130, 4553–4566 (2003).
Ratnaparkhi, A., Banerjee, S. & Hasan, G. Altered levels of Gq activity modulate axonal pathfinding in Drosophila. J. Neurosci. 22, 4499–4508 (2002).
Offermanns, S. In vivo functions of heterotrimeric G-proteins: studies in Gα-deficient mice. Oncogene 20, 1635–1642 (2001).
Bergmann, D. C. et al. Embryonic handedness choice in C. elegans involves the Gα protein GPA-16. Development 130, 5731–5740 (2003). Provocative demonstration that the 'handedness' of the C. elegans embryo requires the activity of a heterotrimeric G protein, GPA-16.
Wang, H., Lee, Y. & Malbon, C. C. PDE6 is an effector for the Wnt/Ca2+/cGMP-signalling pathway in development. Biochem. Soc. Trans. 32, 792–796 (2004).
Powell, K. L., Matthaei, K. I., Heydon, K. & Hendry, I. A. Gzα deficient mice: enzyme levels in the autonomic nervous system, neuronal survival and effect of genetic background. Int. J. Dev. Neurosci. 20, 39–46 (2002).
Gu, J. L., Muller, S., Mancino, V., Offermanns, S. & Simon, M. I. Interaction of Gα12 with Gα13 and Gαq signalling pathways. Proc. Natl Acad. Sci. USA 99, 9352–9357 (2002).
Meng, J. & Casey, P. J. Activation of Gz attenuates Rap1-mediated differentiation of PC12 cells. J. Biol. Chem. 277, 43417–43424 (2002).
Dutt, P., Jaffe, A. B., Merdek, K. D., Hall, A. & Toksoz, D. Gαz inhibits serum response factor-dependent transcription by inhibiting Rho signalling. Mol. Pharmacol. 66, 1508–1516 (2004).
Jho, E. H. & Malbon, C. C. Gα12 and Gα13 mediate differentiation of P19 mouse embryonal carcinoma cells in response to retinoic acid. J. Biol. Chem. 272, 24461–24467 (1997).
Lee, Y. N., Malbon, C. C. & Wang, H. Y. Gα13 signals via p115RhoGEF cascades regulating JNK1 and primitive endoderm formation. J. Biol. Chem. 279, 54896–54904 (2004).
Jho, E. H., Davis, R. J. & Malbon, C. C. c-Jun amino-terminal kinase is regulated by Gα12/Gα13 and obligate for differentiation of P19 embryonal carcinoma cells by retinoic acid. J. Biol. Chem. 272, 24468–24474 (1997).
Offermanns, S., Mancino, V., Revel, J. P. & Simon, M. I. Vascular system defects and impaired cell chemokinesis as a result of Gα13 deficiency. Science 275, 533–536 (1997).
Yau, D. M. et al. Identification and molecular characterization of the Gα12–Rho guanine nucleotide exchange factor pathway in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 100, 14748–14753 (2003).
van der Linden, A. M., Moorman, C., Cuppen, E., Korswagen, H. C. & Plasterk, R. H. Hyperactivation of the G12-mediated signalling pathway in Caenorhabditis elegans induces a developmental growth arrest via protein kinase C. Curr. Biol. 13, 516–521 (2003).
Kasai, K. et al. The G12 family of heterotrimeric G proteins and Rho GTPase mediate Sonic hedgehog signalling. Genes Cells 9, 49–58 (2004). Shows that members of the heterotrimeric G-protein G 12 family mediate the signalling of the Sonic hedgehog-Smoothened pathway.
Clapham, D. E. & Neer, E. J. New roles for G-protein βγ-dimers in transmembrane signalling. Nature 365, 403–406 (1993).
Zwaal, R. R. et al. G proteins are required for spatial orientation of early cell cleavages in C. elegans embryos. Cell 86, 619–629 (1996).
Schaefer, M., Petronczki, M., Dorner, D., Forte, M. & Knoblich, J. A. Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell 107, 183–194 (2001).
Yu, F., Cai, Y., Kaushik, R., Yang, X. & Chia, W. Distinct roles of Gαi and Gβ13F subunits of the heterotrimeric G protein complex in the mediation of Drosophila neuroblast asymmetric divisions. J. Cell Biol. 162, 623–633 (2003).
Fuse, N., Hisata, K., Katzen, A. L. & Matsuzaki, F. Heterotrimeric G proteins regulate daughter cell size asymmetry in Drosophila neuroblast divisions. Curr. Biol. 13, 947–954 (2003).
Izumi, Y., Ohta, N., Itoh-Furuya, A., Fuse, N. & Matsuzaki, F. Differential functions of G protein and Baz–aPKC signalling pathways in Drosophila neuroblast asymmetric division. J. Cell Biol. 164, 729–738 (2004).
Gotta, M. & Ahringer, J. Distinct roles for Gα and Gβγ in regulating spindle position and orientation in Caenorhabditis elegans embryos. Nature Cell Biol. 3, 297–300 (2001).
Schwindinger, W. F. et al. Mice with deficiency of G protein γ3 are lean and have seizures. Mol. Cell Biol. 24, 7758–7768 (2004).
Schwindinger, W. F. et al. Loss of G protein γ7 alters behaviour and reduces striatal αolf level and cAMP production. J. Biol. Chem. 278, 6575–6579 (2003).
D'Angelo, D. D. et al. Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc. Natl Acad. Sci. USA 94, 8121–8126 (1997).
Malbon, C. C., Wang, H. & Moon, R. T. Wnt signalling and heterotrimeric G-proteins: strange bedfellows or a classic romance? Biochem. Biophys. Res. Commun. 287, 589–593 (2001).
Wang, H. Y. & Malbon, C. C. Wnt signalling, Ca2+, and cyclic GMP: visualizing Frizzled functions. Science 300, 1529–1530 (2003).
Cadigan, K. M. Wnt signalling — 20 years and counting. Trends Genet. 18, 340–342 (2002).
Aigaki, T., Seong, K. H. & Matsuo, T. Longevity determination genes in Drosophila melanogaster. Mech Ageing Dev. 123, 1531–1541 (2002).
Lum, L. & Beachy, P. A. The Hedgehog response network: sensors, switches, and routers. Science 304, 1755–1759 (2004).
Cvejic, S., Zhu, Z., Felice, S. J., Berman, Y. & Huang, X. Y. The endogenous ligand Stunted of the GPCR Methuselah extends lifespan in Drosophila. Nature Cell Biol. 6, 540–546 (2004).
Chen, J. K., Taipale, J., Young, K. E., Maiti, T. & Beachy, P. A. Small molecule modulation of Smoothened activity. Proc. Natl Acad. Sci. USA 99, 14071–14076 (2002).
Chen, W. et al. Activity-dependent internalization of smoothened mediated by β-arrestin 2 and GRK2. Science 306, 2257–2260 (2004).
Xiang, Y. et al. Nerve growth cone guidance mediated by G protein-coupled receptors. Nature Neurosci. 5, 843–848 (2002).
Piao, X. et al. G protein-coupled receptor-dependent development of human frontal cortex. Science 303, 2033–2036 (2004).
Ivey, K. et al. Gαq and Gα11 proteins mediate endothelin-1 signalling in neural crest-derived pharyngeal arch mesenchyme. Dev. Biol. 255, 230–237 (2003).
Battu, G., Hoier, E. F. & Hajnal, A. The C. elegans G-protein-coupled receptor SRA-13 inhibits RAS/MAPK signalling during olfaction and vulval development. Development 130, 2567–2577 (2003).
Malbon, C. C. Frizzleds: new members of the superfamily of G-protein-coupled receptors. Front. Biosci. 9, 1048–1058 (2004).
Hollinger, S. & Hepler, J. R. Cellular regulation of RGS proteins: modulators and integrators of G protein signalling. Pharmacol. Rev. 54, 527–559 (2002).
Granderath, S. et al. loco encodes an RGS protein required for Drosophila glial differentiation. Development 126, 1781–1791 (1999).
Wu, C., Zeng, Q., Blumer, K. J. & Muslin, A. J. RGS proteins inhibit Xwnt-8 signalling in Xenopus embryonic development. Development 127, 2773–2784 (2000). First demonstration that regulators of G-protein signalling proteins function in Wnt signalling and embryonic development in the X. laevis model.
Grillet, N., Dubreuil, V., Dufour, H. D. & Brunet, J. F. Dynamic expression of RGS4 in the developing nervous system and regulation by the neural type-specific transcription factor Phox2b. J. Neurosci. 23, 10613–10621 (2003).
Yamada, L. et al. Morpholino-based gene knockdown screen of novel genes with developmental function in Ciona intestinalis. Development 130, 6485–6495 (2003).
Sun, B. & Firtel, R. A. A regulator of G protein signalling-containing kinase is important for chemotaxis and multicellular development in Dictyostelium. Mol. Biol. Cell 14, 1727–1743 (2003).
Lanier, S. M. AGS proteins, GPR motifs and the signals processed by heterotrimeric G proteins. Biol. Cell 96, 369–372 (2004).
Blumer, J. B. & Lanier, S. M. Accessory proteins for G protein-signalling systems: activators of G protein signalling and other nonreceptor proteins influencing the activation state of G proteins. Recept. Channels 9, 195–204 (2003).
Yu, F., Morin, X., Cai, Y., Yang, X. & Chia, W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell 100, 399–409 (2000).
Gotta, M., Dong, Y., Peterson, Y. K., Lanier, S. M. & Ahringer, J. Asymmetrically distributed C. elegans homologues of AGS3/PINS control spindle position in the early embryo. Curr. Biol. 13, 1029–1037 (2003).
Du, Q. & Macara, I. G. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell 119, 503–516 (2004).
Wong, W. & Scott, J. D. AKAP signalling complexes: focal points in space and time. Nature Rev. Mol. Cell Biol. 5, 959–970 (2004).
Malbon, C. C., Tao, J. & Wang, H. Y. AKAPs (A-kinase anchoring proteins) and molecules that compose their G-protein-coupled receptor signalling complexes. Biochem. J. 379, 1–9 (2004).
Tao, J., Wang, H. Y. & Malbon, C. C. Protein kinase A regulates AKAP250 (gravin) scaffold binding to the β2-adrenergic receptor. EMBO J. 22, 6419–6429 (2003).
Malbon, C. C., Tao, J., Shumay, E. & Wang, H. Y. AKAP (A-kinase anchoring protein) domains: beads of structure–function on the necklace of G-protein signalling. Biochem. Soc. Trans. 32, 861–864 (2004).
DeCostanzo, A. J., Huang, X. P., Wang, H. Y. & Malbon, C. C. The Frizzled-1/β2-adrenergic receptor chimera: pharmacological properties of a unique G protein-linked receptor. Naunyn Schmiedebergs Arch. Pharmacol. 365, 341–348 (2002).
Brzostowski, J. A., Johnson, C. & Kimmel, A. R. Gα-mediated inhibition of developmental signal response. Curr. Biol. 12, 1199–1208 (2002).
Couwenbergs, C., Spilker, A. C. & Gotta, M. Control of embryonic spindle positioning and Gα activity by C. elegans RIC-8. Curr. Biol. 14, 1871–1876 (2004).
Hess, H. A., Roper, J. C., Grill, S. W. & Koelle, M. R. RGS-7 completes a receptor-independent heterotrimeric G protein cycle to asymmetrically regulate mitotic spindle positioning in C. elegans. Cell 119, 209–218 (2004).
Martin-McCaffrey, L. et al. RGS14 is a mitotic spindle protein essential from the first division of the mammalian zygote. Dev. Cell 7, 763–769 (2004).
Afshar, K. et al. RIC-8 is required for GPR-1/2-dependent Gα function during asymmetric division of C. elegans embryos. Cell 119, 219–230 (2004).
Mehlmann, L. M., Jones, T. L. & Jaffe, L. A. Meiotic arrest in the mouse follicle maintained by a Gs protein in the oocyte. Science 297, 1343–1345 (2002).
Pace, A. M., Wong, Y. H. & Bourne, H. R. A mutant α subunit of Gi2 induces neoplastic transformation of Rat-1 cells. Proc. Natl Acad. Sci. USA 88, 7031–7035 (1991).
Liu, T., Liu, X., Wang, H., Moon, R. T. & Malbon, C. C. Activation of rat frizzled-1 promotes Wnt signalling and differentiation of mouse F9 teratocarcinoma cells via pathways that require Gαq and Gαo function. J. Biol. Chem. 274, 33539–33544 (1999).
Chen, J. F., Guo, J. H., Moxham, C. M., Wang, H. Y. & Malbon, C. C. Conditional, tissue-specific expression of Q205L Gαi2 in vivo mimics insulin action. J. Mol. Med. 75, 283–289 (1997).
Kanungo, J., Potapova, I., Malbon, C. C. & Wang, H. MEKK4 mediates differentiation in response to retinoic acid via activation of c-Jun N-terminal kinase in rat embryonal carcinoma P19 cells. J. Biol. Chem. 275, 24032–24039 (2000).
Kuhl, M., Sheldahl, L. C., Malbon, C. C. & Moon, R. T. Ca2+/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologues and promotes ventral cell fates in Xenopus. J. Biol. Chem. 275, 12701–12711 (2000).
Acknowledgements
I thank the members of the Malbon laboratory, and especially Dr. H.-Y. Wang, Department of Physiology and Biophysics, State University of New York at Stony Brook, for the critical reading of the manuscript, M. Feigin for assistance in compiling the tables, E. Shumay for the fluorescence images, and R. Brockner for help with the references and with the compilation of background material. This work was generously supported by a National Institutes of Health grant to C.C.M.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Flybase
OMIM
Albrights hereditary osteodystrophy
Swiss-Prot
Wormbase
FURTHER INFORMATION
Glossary
- G-PROTEIN EFFECTOR
-
A protein that is directly downstream of G proteins in the signalling pathway. This protein responds to the activation of either a heterotrimeric G-protein α subunit or the Gβγ-subunit complex with a specific change in its activity. G-protein effectors include adenylyl cyclase, phospholipase C and various ion channels.
- FATTY ACYLATION
-
Addition of a fatty acid moiety to N-terminal glycine by N-myristoylation and/or palmitoylation of an internal cysteine residue of a protein.
- ISOPRENYLATION
-
Enzyme-mediated post-translational covalent attachment of a hydrophobic isoprenyl moiety to proteins.
- INNER LEAFLET
-
A lipid layer that faces the inside of the cell.
- G-PROTEIN-COUPLED RECEPTOR
-
(GPCR). A seven-helix membrane-spanning cell-surface receptor that signals through heterotrimeric GTP-binding and -hydrolysing G proteins to stimulate or inhibit the activity of a downstream enzyme.
- GROWTH CONE
-
Motile tip of the axon or dendrite of a growing nerve cell that spreads out into a large cone-shaped appendage.
- GASTRULATION
-
Series of morphogenetic movements observed during the early development of most animals that leads to the formation of a multilayered embryo with an outer cell layer (ectoderm), an inner cell layer (endoderm), and an intermediate cell layer (mesoderm).
- KNOCK-DOWN
-
Suppression of the expression of a gene product, typically achieved by the use of antisense oligodeoxynucleotides, antisense morpholinos and RNAi that specifically target the RNA product of the gene.
- PERTUSSIS TOXIN
-
A protein toxin composed of A and B protomers that is produced by Bordetella pertussis. It inactivates Gαi proteins by specifically catalysing ADP ribosylation of the α subunit.
- TERATOCARCINOMA CELLS
-
Embryonal carcinoma cells derived from a malignant germ-cell tumour arising from the ovary or testis.
- KNOCKOUT
-
A mouse product of genetic manipulation that yields the inactivation of a specific gene (that is, complete deficiency of the gene product) by gene interruption.
- SYNAPTIC BOUTON
-
An enlargement of the presynaptic nerve terminal that contains the vesicles and apparatus for the release of the neurotransmitter.
- PRIMITIVE ENDODERM
-
The extraembryonic tissue that gives rise to the visceral and parietal endoderm.
- ASYMMETRIC CELL DIVISION
-
Cell division in which the derivative daughter cells are different from each other because cytoplasmic determinants have been distributed unequally.
- WNT PROTEINS
-
A family of highly conserved secreted signalling molecules that regulate cell–cell interactions during embryogenesis.
- RETINOIC ACID
-
A morphogen and regulator of differentiation during embryogenesis. It is a product of vitamin A, which the body synthesizes from carotenes.
- PLANAR CELL POLARITY
-
The pattern of organization of cells in the plane of an epithelium.
- MITOTIC SPINDLE
-
A bipolar array of microtubules that forms during mitosis, to which chromosomes attach and by which chromosomes are segregated to daughter cells.
- HANDEDNESS
-
Asymmetry of internal organs (such as the heart, lung and liver) with respect to left and right sides in organisms with bilateral body symmetry for structures such as eyes, ears and limbs.
- HYDROPATHY PLOT
-
A measure of the hydrophobicity of a protein region that provides an indication of the likelihood that it will reside in a membrane.
- CYCLIC-NUCLEOTIDE-GATED CHANNEL
-
(CNGC). Conserved protein family with six predicted transmembrane helices that can form cation-conducting channels and that is activated by the binding of cyclic nucleotides such as cAMP and cGMP.
- CELL DIFFERENTIATION
-
A normal process in development in which cells become structurally and functionally different from one another and develop a mature phenotype — for example, fat, muscle and liver cells.
- GUANINE NUCLEOTIDE-EXCHANGE FACTOR
-
A protein that activates a specific small GTPase by catalysing the exchange of bound GDP for GTP.
- MORPHOGEN
-
A substance that induces pattern formation, differentiation and morphogenesis. The concentration of a morphogen often forms a gradient to which cells respond differently.
- GTPase-ACTIVATING PROTEIN
-
(GAP). Proteins that inactivate small GTP-binding proteins, such as Ras-family members, by increasing their rate of GTP hydrolysis.
Rights and permissions
About this article
Cite this article
Malbon, C. G proteins in development. Nat Rev Mol Cell Biol 6, 689–701 (2005). https://doi.org/10.1038/nrm1716
Issue Date:
DOI: https://doi.org/10.1038/nrm1716
This article is cited by
-
Comparative transcriptomic analysis of races 1, 2, 5 and 6 of Fusarium oxysporum f.sp. pisi in a susceptible pea host identifies differential pathogenicity profiles
BMC Genomics (2021)
-
Nerve growth factor interacts with CHRM4 and promotes neuroendocrine differentiation of prostate cancer and castration resistance
Communications Biology (2021)
-
Synthesis and muscarinic acetylcholine receptor (mAChR) antagonist activity of substituted piperazine-triazoles
Monatshefte für Chemie - Chemical Monthly (2021)
-
Design and synthesis of muscarinic acetylcholine receptor (mAChR) antagonist: pharmacophore-based screening and structure-based optimization
Monatshefte für Chemie - Chemical Monthly (2019)
-
M3 muscarinic acetylcholine receptors regulate epithelial–mesenchymal transition, perineural invasion, and migration/metastasis in cholangiocarcinoma through the AKT pathway
Cancer Cell International (2018)