Key Points
-
The principal action of GABA (γ-aminobutyric acid) in the adult CNS is to increase membrane permeability to chloride and bicarbonate ions. The increase in membrane conductance and hyperpolarization that is associated with the activation of postsynaptic GABA type A (GABAA) receptors following brief exposure to a high concentration of GABA released from presynaptic vesicles underlies what is known as 'phasic' inhibition.
-
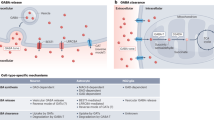
In recent years, it has become evident that GABA receptor activation can also take place in a much less spatially and temporally restricted manner. GABA that escapes from the synaptic cleft can activate receptors on presynaptic terminals or at neighbouring synapses on the same or adjacent neurons, and low concentrations of GABA in the extracellular space can result in the persistent 'tonic' activation of GABAA receptors.
-
One important function of phasic inhibition is the generation of rhythmic activities in neuronal networks. A notable example is the action of cortical and hippocampal basket cells that innervate the perisomatic regions of pyramidal cells. These interneurons have an essential role in generating and maintaining theta and gamma frequency network oscillations.
-
Tonic activation of GABAA receptors causes a persistent increase in the cell's input conductance, thereby affecting the magnitude and duration of the voltage response to an injected current. For a given excitatory input, the size and duration of the excitatory postsynaptic potential (EPSP) will be reduced, and the temporal and spatial window over which signal integration can occur will be narrowed, thereby reducing the probability that an action potential will be generated.
-
The different modes of GABAA receptor activation seem to be determined by the subcellular location and biophysical properties of receptor subtypes. Receptors that contain a γ2 subunit in association with α1, α2 or α3 subunits are the predominant subtypes that mediate phasic synaptic inhibition. Receptors that contain α4, α5 or α6 subunits are predominantly or exclusively extrasynaptic, implying that they are more likely to mediate tonic inhibition.
-
The macroscopic and microscopic properties of GABAA receptors depend on their subunit composition. The most important biophysical differences between receptors that mediate phasic inhibition and those that have been implicated in tonic inhibition are their affinities for GABA and the speed and extent of their desensitization.
-
The pattern of phasic inhibition is determined by the number, variety and activity of presynaptic GABA-releasing neurons, but the relationship between neuronal activity and tonic inhibition is less clear. In the hippocampus and cerebellum, changes in presynaptic activity or release can modify the magnitude of the tonic conductance. GABA transporters have an important influence on ambient GABA release, so it is possible that tonic inhibition could also be modulated by changes in uptake.
-
Many processes modulate GABAA receptor number or function and are likely to be relevant to both phasic and tonic inhibition. Reversible post-translational modifications, such as phosphorylation and palmitoylation, affect both the properties and subcellular location of the receptors. In addition, clustering of GABAA receptors changes both their kinetic behaviour and single-channel conductance.
-
Just as the biophysical properties of GABAA receptors are determined by their subunit composition, so are their pharmacological properties. Differences in subunit composition between synaptic and extra- or perisynaptic receptors are reflected in differential modulation of phasic and tonic inhibition by benzodiazepine site ligands and other clinically relevant drugs.
Abstract
The proper functioning of the adult mammalian brain relies on the orchestrated regulation of neural activity by a diverse population of GABA (γ-aminobutyric acid)-releasing neurons. Until recently, our appreciation of GABA-mediated inhibition focused predominantly on the GABAA (GABA type A) receptors located at synaptic contacts, which are activated in a transient or 'phasic' manner by GABA that is released from synaptic vesicles. However, there is growing evidence that low concentrations of ambient GABA can persistently activate certain subtypes of GABAA receptor, which are often remote from synapses, to generate a 'tonic' conductance. In this review, we consider the distinct roles of synaptic and extrasynaptic GABA receptor subtypes in the control of neuronal excitability.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mody, I., De Koninck, Y., Otis, T. S. & Soltesz, I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 17, 517–525 (1994).
Edwards, F. A., Konnerth, A. & Sakmann, B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J. Physiol. (Lond.) 430, 213–249 (1990).
Nusser, Z., Cull-Candy, S. & Farrant, M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron 19, 697–709 (1997).
Brickley, S. G., Cull-Candy, S. G. & Farrant, M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J. Neurosci. 19, 2960–2973 (1999).
Overstreet, L. S., Westbrook, G. L. & Jones, M. V. in Transmembrane Transporters (ed. Quick, M. W.) 259–275 (Wiley–Liss Inc., Hoboken, New Jersey, 2002).
Mozrzymas, J. W., Zarmowska, E. D., Pytel, M. & Mercik, K. Modulation of GABAA receptors by hydrogen ions reveals synaptic GABA transient and a crucial role of the desensitization process. J. Neurosci. 23, 7981–7992 (2003). The authors investigated the properties of mIPSCs at various pH values, and, from responses to exogenous GABA, separately determined that protons affect the binding and desensitization of GABA A receptors. By modelling mIPSCs as responses to exponentially decaying GABA concentration transients, they were able to reproduce the pH effects by assuming a concentration transient of GABA in the synaptic cleft that peaked at ∼3 mM, with a clearance time constant of ∼100 μs.
Mozrzymas, J. W. Dynamism of GABAA receptor activation shapes the 'personality' of inhibitory synapses. Neuropharmacology 47, 945–960 (2004).
Baumann, S. W., Baur, R. & Sigel, E. Individual properties of the two functional agonist sites in GABAA receptors. J. Neurosci. 23, 11158–11166 (2003).
Jones, M. V., Sahara, Y., Dzubay, J. A. & Westbrook, G. L. Defining affinity with the GABAA receptor. J. Neurosci. 18, 8590–8604 (1998).
Frerking, M. & Wilson, M. Saturation of postsynaptic receptors at central synapses? Curr. Opin. Neurobiol. 6, 395–403 (1996).
Perrais, D. & Ropert, N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. J. Neurosci. 19, 578–588 (1999).
Hajos, N., Nusser, Z., Rancz, E. A., Freund, T. F. & Mody, I. Cell type- and synapse-specific variability in synaptic GABAA receptor occupancy. Eur. J. Neurosci. 12, 810–818 (2000).
Weiss, D. S. & Magleby, K. L. Gating scheme for single GABA-activated Cl− channels determined from stability plots, dwell-time distributions, and adjacent-interval durations. J. Neurosci. 9, 1314–1324 (1989).
Twyman, R. E., Rogers, C. J. & Macdonald, R. L. Intraburst kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J. Physiol. (Lond.) 423, 193–220 (1990).
Jones, M. V. & Westbrook, G. L. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron 15, 181–191 (1995). Using rapid GABA application to receptors in excised membrane patches, the authors revealed how desensitization could shape IPSCs. They showed that entry to desensitized states held the channel in bound conformations that allowed channel re-opening after GABA removal, thereby prolonging the response to a brief synaptic GABA concentration transient.
Jayaraman, V., Thiran, S. & Hess, G. P. How fast does the γ-aminobutyric acid receptor channel open? Kinetic investigations in the microsecond time region using a laser-pulse photolysis technique. Biochemistry 38, 11372–11378 (1999).
Haas, K. F. & Macdonald, R. L. GABAA receptor subunit γ2 and δ subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J. Physiol. (Lond.) 514, 27–45 (1999). A detailed biophysical analysis of responses from recombinant α 1 β 3 γ 2 and α 1 β 3 δ receptors, which shows that the δ subunit confers unique kinetic properties on GABA A receptors.
Burkat, P. M., Yang, J. & Gingrich, K. J. Dominant gating governing transient GABAA receptor activity: a first latency and Po analysis. J. Neurosci. 21, 7026–7036 (2001).
Bier, M., Kits, K. S. & Borst, J. G. Relation between rise times and amplitudes of GABAergic postsynaptic currents. J. Neurophysiol. 75, 1008–1012 (1996).
Maconochie, D. J., Zempel, J. M. & Steinbach, J. H. How quickly can GABAA receptors open? Neuron 12, 61–71 (1994).
McClellan, A. M. & Twyman, R. E. Receptor system response kinetics reveal functional subtypes of native murine and recombinant human GABAA receptors. J. Physiol. (Lond.) 515, 711–727 (1999).
Chang, Y. & Weiss, D. S. Channel opening locks agonist onto the GABAC receptor. Nature Neurosci. 2, 219–225 (1999).
Bianchi, M. T. & Macdonald, R. L. Agonist trapping by GABAA receptor channels. J. Neurosci. 21, 9083–9091 (2001).
Okada, M., Onodera, K., Van Renterghem, C., Sieghart, W. & Takahashi, T. Functional correlation of GABAA receptor α subunits expression with the properties of IPSCs in the developing thalamus. J. Neurosci. 20, 2202–2208 (2000).
Vicini, S. et al. GABAA receptor α1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J. Neurosci. 21, 3009–3016 (2001).
Nusser, Z., Sieghart, W. & Mody, I. Differential regulation of synaptic GABAA receptors by cAMP-dependent protein kinase in mouse cerebellar and olfactory bulb neurones. J. Physiol. (Lond.) 521, 421–435 (1999).
Bacci, A., Rudolph, U., Huguenard, J. R. & Prince, D. A. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J. Neurosci. 23, 9664–9674 (2003).
Ramadan, E. et al. GABAA receptor β3 subunit deletion decreases α2/3 subunits and IPSC duration. J. Neurophysiol. 89, 128–134 (2003).
Barbour, B. & Hausser, M. Intersynaptic diffusion of neurotransmitter. Trends Neurosci. 20, 377–384 (1997).
Kullmann, D. M. Spillover and synaptic cross talk mediated by glutamate and GABA in the mammalian brain. Prog. Brain Res. 125, 339–351 (2000).
Telgkamp, P., Padgett, D. E., Ledoux, V. A., Woolley, C. S. & Raman, I. M. Maintenance of high-frequency transmission at Purkinje to cerebellar nuclear synapses by spillover from boutons with multiple release sites. Neuron 41, 113–126 (2004). An elegant study that combines electrophysiology, electron microscopy reconstructions of Purkinje cell synaptic connections and simulations to show how multiple active zones in one bouton enable spillover-mediated transmission, which allows high-frequency inhibition at corticonuclear synapses.
Mody, I. Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem. Res. 26, 907–913 (2001).
Kullmann, D. M. et al. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog. Biophys. Mol. Biol. 87, 33–46 (2005).
Valeyev, A. Y., Cruciani, R. A., Lange, G. D., Smallwood, V. S. & Barker, J. L. Cl− channels are randomly activated by continuous GABA secretion in cultured embryonic rat hippocampal neurons. Neurosci. Lett. 155, 199–203 (1993).
LoTurco, J. J., Owens, D. F., Heath, M. J., Davis, M. B. & Kriegstein, A. R. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron 15, 1287–1298 (1995).
Owens, D. F., Liu, X. & Kriegstein, A. R. Changing properties of GABAA receptor-mediated signaling during early neocortical development. J. Neurophysiol. 82, 570–583 (1999).
Demarque, M. et al. Paracrine intercellular communication by a Ca2+- and SNARE- independent release of GABA and glutamate prior to synapse formation. Neuron 36, 1051–1061 (2002).
Otis, T. S., Staley, K. J. & Mody, I. Perpetual inhibitory activity in mammalian brain slices generated by spontaneous GABA release. Brain Res. 545, 142–150 (1991).
Salin, P. A. & Prince, D. A. Spontaneous GABAA receptor-mediated inhibitory currents in adult rat somatosensory cortex. J. Neurophysiol. 75, 1573–1588 (1996).
Hausser, M. & Clark, B. A. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron 19, 665–678 (1997).
Kaneda, M., Farrant, M. & Cull-Candy, S. G. Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J. Physiol. (Lond.) 485, 419–435 (1995).
Brickley, S., Cull-Candy, S. & Farrant, M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J. Physiol. (Lond.) 497, 753–759 (1996). The authors investigated the postnatal development and inhibitory effects of the tonic GABA A receptor-mediated conductance in cerebellar granule cells. This was first identified in reference 41 and was shown to be distinct from the superimposition of phasic synaptic events. The conductance was found to increase with age, in line with known changes in subunit expression, and to reduce action potential generation in response to current injection.
Tia, S., Wang, J. F., Kotchabhakdi, N. & Vicini, S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABAA receptor α6 subunit. J. Neurosci. 16, 3630–3640 (1996).
Wall, M. J. & Usowicz, M. M. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur. J. Neurosci. 9, 533–548 (1997).
Nusser, Z. & Mody, I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J. Neurophysiol. 87, 2624–2628 (2002).
Porcello, D. M., Huntsman, M. M., Mihalek, R. M., Homanics, G. E. & Huguenard, J. R. Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking δ subunit. J. Neurophysiol. 89, 1378–1386 (2003).
Yamada, J., Yamamoto, S., Ueno, S., Furukawa, T. & Fukuda, A. GABAA receptor-mediated tonic inhibition in rat somatosensory cortex. FENS Forum Abstr. 2, A083.027 (2004).
Bai, D. L. et al. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol. Pharmacol. 59, 814–824 (2001).
Semyanov, A., Walker, M. C. & Kullmann, D. M. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nature Neurosci. 6, 484–490 (2003). Showed that when GABA uptake is intact, guinea pig hippocampal interneurons, but not pyramidal cells, exhibit a tonic GABA A receptor-mediated conductance. Reducing the tonic conductance in interneurons with a low concentration of picrotoxin increased their excitability and the inhibitory input to pyramidal cells.
Sigel, E., Baur, R., Malherbe, P. & Mohler, H. The rat β1-subunit of the GABAA receptor forms a picrotoxin-sensitive anion channel open in the absence of GABA. FEBS Lett. 257, 377–379 (1989).
Maksay, G., Thompson, S. A. & Wafford, K. A. The pharmacology of spontaneously open α1β3ε GABAA receptor-ionophores. Neuropharmacology 44, 994–1002 (2003).
Lindquist, C. E., Dalziel, J. E., Cromer, B. A. & Birnir, B. Penicillin blocks human α1β1 and α1β1γ2S GABAA channels that open spontaneously. Eur. J. Pharmacol. 496, 23–32 (2004).
Birnir, B., Everitt, A. B., Lim, M. S. & Gage, P. W. Spontaneously opening GABAA channels in CA1 pyramidal neurones of rat hippocampus. J. Membr. Biol. 174, 21–29 (2000).
Attwell, D., Barbour, B. & Szatkowski, M. Nonvesicular release of neurotransmitter. Neuron 11, 401–407 (1993).
Lerma, J., Herranz, A. S., Herreras, O., Abraira, V. & Martin del Rio, R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 384, 145–155 (1986).
Tossman, U., Jonsson, G. & Ungerstedt, U. Regional distribution and extracellular levels of amino acids in rat central nervous system. Acta Physiol. Scand. 127, 533–545 (1986).
Kennedy, R. T., Thompson, J. E. & Vickroy, T. W. In vivo monitoring of amino acids by direct sampling of brain extracellular fluid at ultralow flow rates and capillary electrophoresis. J. Neurosci. Methods 114, 39–49 (2002).
Xi, Z. X. et al. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J. Neurosci. 23, 3498–3505 (2003).
Brickley, S. G., Cull-Candy, S. G. & Farrant, M. Vesicular release of GABA contributes to both phasic and tonic inhibition of granule cells in the cerebellum of mature mice. J. Physiol. 547.P, C30 (2003).
Carta, M., Mameli, M. & Valenzuela, C. F. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J. Neurosci. 24, 3746–3751 (2004).
Rossi, D. J., Hamann, M. & Attwell, D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J. Physiol. (Lond.) 548, 97–110 (2003).
Jensen, K., Chiu, C. S., Sokolova, I., Lester, H. A. & Mody, I. GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J. Neurophysiol. 90, 2690–2701 (2003).
Buzsaki, G. & Chrobak, J. J. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr. Opin. Neurobiol. 5, 504–510 (1995).
Singer, W. The changing face of inhibition. Curr. Biol. 6, 395–397 (1996).
Somogyi, P. P. & Klausberger, T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J. Physiol. (Lond.) 562, 9–26 (2005).
Freund, T. F. Interneuron diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci. 26, 489–495 (2003).
Whittington, M. A. & Traub, R. D. Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 26, 676–682 (2003).
Jonas, P., Bischofberger, J., Fricker, D. & Miles, R. Interneuron diversity series: fast in, fast out — temporal and spatial signal processing in hippocampal interneurons. Trends Neurosci. 27, 30–40 (2004).
Cobb, S. R., Buhl, E. H., Halasy, K., Paulsen, O. & Somogyi, P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378, 75–78 (1995). Showed that a single hippocampal GABA-releasing basket cell can time the output and synchronize the activity of a large population of postsynaptic pyramidal cells. This study reveals that phasic inhibition does not necessarily have a purely inhibitory role in the CNS.
Galarreta, M. & Hestrin, S. Electrical synapses between GABA-releasing interneurons. Nature Rev. Neurosci. 2, 425–433 (2001).
Wang, X. -J. & Buzsaki, G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J. Neurosci. 16, 6402–6413 (1996).
Traub, R. D. et al. Gamma-frequency oscillations: a neuronal population phenomenon, regulated by synaptic and intrinsic cellular processes, and inducing synaptic plasticity. Prog. Neurobiol. 55, 563–575 (1998).
Huntsman, M. M., Porcello, D. M., Homanics, G. E., DeLorey, T. M. & Huguenard, J. R. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science 283, 541–543 (1999).
Laurent, G. Olfactory network dynamics and the coding of multidimensional signals. Nature Rev. Neurosci. 3, 884–895 (2002).
Miles, R., Toth, K., Gulyas, A. I., Hajos, N. & Freund, T. F. Differences between somatic and dendritic inhibition in the hippocampus. Neuron 16, 815–823 (1996).
Spruston, N., Schiller, Y., Stuart, G. & Sakmann, B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science 268, 297–300 (1995).
Pouille, F. & Scanziani, M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293, 1159–1163 (2001). Shows, in hippocampal pyramidal cells, how the disynaptic feed-forward inhibition that follows monosynaptic excitation substantially restricts the window in which temporal summation can occur. Regional differences in the strength of inhibition allow the dendrites to integrate input over a broad time window while enforcing precise coincidence detection at the soma.
Gulledge, A. T. & Stuart, G. J. Excitatory actions of GABA in the cortex. Neuron 37, 299–309 (2003).
Williams, S. R. & Stuart, G. J. Voltage- and site-dependent control of the somatic impact of dendritic IPSPs. J. Neurosci. 23, 7358–7367 (2003).
Freund, T. F. & Buzsaki, G. Interneurons of the hippocampus. Hippocampus 6, 347–470 (1996).
Hamann, M., Rossi, D. J. & Attwell, D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron 33, 625–633 (2002). Shows that in the adult cerebellar cortex, mossy fibre input to Purkinje cells is controlled by furosemide-sensitive GABA A receptors on granule cells, which are activated tonically both by ambient GABA and following spillover of synaptically released GABA.
Chadderton, P., Margrie, T. W. & Hausser, M. Integration of quanta in cerebellar granule cells during sensory processing. Nature 428, 856–860 (2004). The authors carried out the first in vivo patch-clamp recordings from cerebellar granule cells, and revealed the presence of a tonic GABA A receptor-mediated conductance in the intact brain.
Mitchell, S. J. & Silver, R. A. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38, 433–445 (2003).
Chance, F. S., Abbott, L. F. & Reyes, A. D. Gain modulation from background synaptic input. Neuron 35, 773–782 (2002). References 83 and 84 provide elegant demonstrations of how tonic inhibition, when combined with phasic excitation, can alter the way in which action potentials are generated in response to changing levels of excitatory input, effectively altering neuronal gain.
Semyanov, A., Walker, M. C., Kullmann, D. M. & Silver, R. A. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 27, 262–269 (2004).
Marr, D. A theory of cerebellar cortex. J. Physiol. (Lond.) 202, 437–470 (1969).
Tyrrell, T. & Willshaw, D. Cerebellar cortex: its simulation and the relevance of Marr's theory. Phil. Trans. R. Soc. Lond. B 336, 239–257 (1992).
Maex, R. & Schutter, E. D. Synchronization of Golgi and granule cell firing in a detailed network model of the cerebellar granule cell layer. J. Neurophysiol. 80, 2521–2537 (1998).
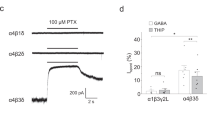
Stell, B. M., Brickley, S. G., Tang, C. Y., Farrant, M. & Mody, I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc. Natl Acad. Sci. USA 100, 14439–14444 (2003). Showed that, in granule cells of the dentate gyrus and cerebellum, the neurosteroid THDOC, at a low concentration that is known to occur in vivo , specifically enhanced the tonic inhibitory conductance that was mediated by extrasynaptic δ-subunit-containing GABA A receptors.
Caraiscos, V. B. et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc. Natl Acad. Sci. USA 101, 3662–3667 (2004). By recording from hippocampal slices previously incubated in the GABA transaminase blocker vigabatrin, the authors showed that the tonic GABA A receptor-mediated conductance in CA1 pyramidal neurons was reduced in cells from mice that lacked the α5 subunit of the GABA A receptor.
Stell, B. M. & Mody, I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J. Neurosci. 22, RC223 (2002).
Wisden, W. et al. Ectopic expression of the GABAA receptor α6 subunit in hippocampal pyramidal neurons produces extrasynaptic receptors and an increased tonic inhibition. Neuropharmacology 43, 530–549 (2002).
Bieda, M. C. & MacIver, M. B. A major role for tonic GABAA conductances in anaesthetic supression of intrinsic neuronal excitability. J. Neurophysiol. (in the press).
Richards, J. G., Schoch, P., Haring, P., Takacs, B. & Mohler, H. Resolving GABAA/benzodiazepine receptors: cellular and subcellular localization in the CNS with monoclonal antibodies. J. Neurosci. 7, 1866–1886 (1987).
Somogyi, P., Takagi, H., Richards, J. G. & Mohler, H. Subcellular localization of benzodiazepine/GABAA receptors in the cerebellum of rat, cat, and monkey using monoclonal antibodies. J. Neurosci. 9, 2197–2209 (1989). The first high-resolution demonstration of the presence of non-synaptic GABA A receptors on the surface of CNS neurons.
Waldvogel, H. J. et al. GABA, GABA receptors and benzodiazepine receptors in the human spinal cord: an autoradiographic and immunohistochemical study at the light and electron microscopic levels. Neuroscience 39, 361–385 (1990).
Soltesz, I. et al. Synaptic and nonsynaptic localization of benzodiazepine/GABAA receptor/Cl− channel complex using monoclonal antibodies in the dorsal lateral geniculate nucleus of the cat. Eur. J. Neurosci. 2, 414–429 (1990).
Nusser, Z., Roberts, J. D. B., Baude, A., Richards, J. G. & Somogyi, P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J. Neurosci. 15, 2948–2960 (1995). Showed that in cerebellar granule cells, the total number of extrasynaptic GABA A receptors exceeds that in GABA-releasing synapses.
Nusser, Z., Sieghart, W. & Somogyi, P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 18, 1693–1703 (1998). A demonstration, using immunogold electron microscopy, of the exclusively extrasynaptic presence of the δ subunit in cerebellar granule cells.
Craig, A. M., Blackstone, C. D., Huganir, R. L. & Banker, G. Selective clustering of glutamate and γ-aminobutyric acid receptors opposite terminals releasing the corresponding neurotransmitters. Proc. Natl Acad. Sci. USA 91, 12373–12377 (1994).
Somogyi, P., Fritschy, J. M., Benke, D., Roberts, J. D. & Sieghart, W. The γ2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the α1 and β2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology 35, 1425–1444 (1996).
Fritschy, J. M., Johnson, D. K., Mohler, H. & Rudolph, U. Independent assembly and subcellular targeting of GABAA-receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo. Neurosci. Lett. 249, 99–102 (1998).
Brunig, I., Scotti, E., Sidler, C. & Fritschy, J. M. Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J. Comp. Neurol. 443, 43–55 (2002).
Wei, W., Zhang, N., Peng, Z., Houser, C. R. & Mody, I. Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J. Neurosci. 23, 10650–10661 (2003).
Barnard, E. A. et al. International union of pharmacology. XV. Subtypes of γ-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 50, 291–313 (1998).
Luscher, B. & Keller, C. A. Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol. Ther. 102, 195–221 (2004).
Moss, S. J. & Smart, T. G. Constructing inhibitory synapses. Nature Rev. Neurosci. 2, 240–250 (2001).
Fritschy, J. M. & Brunig, I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol. Ther. 98, 299–323 (2003).
Essrich, C., Lorez, M., Benson, J. A., Fritschy, J. M. & Luscher, B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nature Neurosci. 1, 563–571 (1998). In this elegant study, the authors showed that cortical and hippocampal neurons from mice that lacked the γ2 subunit failed to accumulate GABA A receptors at developing synaptic sites. The loss of GABA A receptor clusters was accompanied by a loss of gephyrin and a loss of normal synaptic function.
Schweizer, C. et al. The γ2 subunit of GABAA receptors is required for maintenance of receptors at mature synapses. Mol. Cell. Neurosci. 24, 442–450 (2003).
Farrant, M. et al. Loss of IPSCs following selective ablation of the GABAA receptor γ2 subunit in cerebellar granule cells. Soc. Neurosci. Abstr. 170.3 (2004).
Crestani, F. et al. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc. Natl Acad. Sci. USA 99, 8980–8985 (2002).
Collinson, N. et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J. Neurosci. 22, 5572–5580 (2002).
Rossi, D. J. & Hamann, M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity α6 subunit GABAA receptors and glomerular geometry. Neuron 20, 783–795 (1998).
Mody, I. & Pearce, R. A. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 27, 569–575 (2004).
Brickley, S. G., Revilla, V., Cull-Candy, S. G., Wisden, W. & Farrant, M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409, 88–92 (2001). Deletion of the α6 subunit of the GABA A receptor abolished the GABA A receptor-mediated tonic conductance in cerebellar granule cells. Together with reference 89, this study showed that the conductance is mediated by α 6 βδ receptors. Although tonic GABA A receptor activation was lost, normal granule cell excitability was maintained by the upregulation of a voltage-independent potassium conductance.
Peng, Z. et al. GABAA receptor changes in δ subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J. Comp. Neurol. 446, 179–197 (2002).
Colquhoun, D. Binding gating affinity and efficacy: the interpretation of structure–activity relationships for agonists and of the effects of mutating receptors. Br. J. Pharmacol. 125, 924–947 (1998).
Knoflach, F. et al. Pharmacological modulation of the diazepam-insensitive recombinant γ-aminobutyric acid A receptors α4β2γ2 and α6β2γ2. Mol. Pharmacol. 50, 1253–1261 (1996).
Fisher, J. L. & Macdonald, R. L. Single channel properties of recombinant GABAA receptors containing γ2 or δ subtypes expressed with α1 and β3 subtypes in mouse L929 cells. J. Physiol. (Lond.) 505, 283–297 (1997).
Bohme, I., Rabe, H. & Luddens, H. Four amino acids in the α subunits determine the γ-aminobutyric acid sensitivities of GABAA receptor subtypes. J. Biol. Chem. 279, 35193–35200 (2004).
Feng, H. J. & Macdonald, R. L. Multiple actions of propofol on αβγ and αβδ GABAA receptors. Mol. Pharmacol. 66, 1517–1524 (2004).
Minier, F. & Sigel, E. Positioning of the α-subunit isoforms confers a functional signature to γ-aminobutyric acid type A receptors. Proc. Natl Acad. Sci. USA 101, 7769–7774 (2004).
Brown, N., Kerby, J., Bonnert, T. P., Whiting, P. J. & Wafford, K. A. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br. J. Pharmacol. 136, 965–974 (2002).
Verdoorn, T. A., Draguhn, A., Ymer, S., Seeburg, P. H. & Sakmann, B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron 4, 919–928 (1990).
Puia, G. et al. Neurosteroids act on recombinant human GABAA receptors. Neuron 4, 759–765 (1990).
Angelotti, T. P. & Macdonald, R. L. Assembly of GABAA receptor subunits: α1β1 and α1β1γ2S subunits produce unique ion channels with dissimilar single-channel properties. J. Neurosci. 13, 1429–1440 (1993).
Akk, G., Bracamontes, J. & Steinbach, J. H. Activation of GABAA receptors containing the α4 subunit by GABA and pentobarbital. J. Physiol. 556, 387–399 (2004).
Adkins, C. E. et al. α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J. Biol. Chem. 276, 38934–38939 (2001).
Bianchi, M. T. & Macdonald, R. L. Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J. Neurosci. 23, 10934–10943 (2003).
Gingrich, K. J., Roberts, W. A. & Kass, R. S. Dependence of the GABAA receptor gating kinetics on the α-subunit isoform: implications for structure–function relations and synaptic transmission. J. Physiol. 489, 529–543 (1995).
Lavoie, A. M., Tingey, J. J., Harrison, N. L., Pritchett, D. B. & Twyman, R. E. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on α-subunit isoform. Biophys. J. 73, 2518–2526 (1997).
Boileau, A. J., Li, T., Benkwitz, C., Czajkowski, C. & Pearce, R. A. Effects of γ2S subunit incorporation on GABAA receptor macroscopic kinetics. Neuropharmacology 44, 1003–1012 (2003).
Benkwitz, C., Banks, M. I. & Pearce, R. A. Influence of GABAA receptor γ2 splice variants on receptor kinetics and isoflurane modulation. Anesthesiology 101, 924–936 (2004).
Bianchi, M. T., Haas, K. F. & Macdonald, R. L. α1 and α6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAA receptors containing the δ subunit. Neuropharmacology 43, 492–502 (2002).
Jones, M. V. & Westbrook, G. L. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 19, 96–101 (1996).
Bianchi, M. T. & Macdonald, R. L. Slow phases of GABAA receptor desensitization: structural determinants and possible relevance for synaptic function. J. Physiol. (Lond.) 544, 3–18 (2002).
Mellor, J. R. & Randall, A. D. Synaptically released neurotransmitter fails to desensitize postsynaptic GABAA receptors in cerebellar cultures. J. Neurophysiol. 85, 1847–1857 (2001).
Behrends, J. C., Lambert, J. D. C. & Jensen, K. Repetitive activation of postsynaptic GABAA receptors by rapid, focal agonist application onto intact rat striatal neurones in vitro. Pflugers Arch. 443, 707–712 (2002).
Overstreet, L. S., Jones, M. V. & Westbrook, G. L. Slow desensitization regulates the availability of synaptic GABAA receptors. J. Neurosci. 20, 7914–7921 (2000).
Mozrzymas, J. W., Barberis, A., Mercik, K. & Zarnowska, E. D. Binding sites, singly bound states, and conformation coupling shape GABA-evoked currents. J. Neurophysiol. 89, 871–883 (2003).
Saxena, N. & Macdonald, R. Properties of putative cerebellar γ-aminobutyric acid A receptor isoforms. Mol. Pharmacol. 49, 567–579 (1996).
Tia, S., Wang, J. F., Kotchabhakdi, N. & Vicini, S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharmacology 35, 1375–1382 (1996).
Bianchi, M. T. & Macdonald, R. L. Mutation of the 9′ leucine in the GABAA receptor γ2L subunit produces an apparent decrease in desensitization by stabilizing open states without altering desensitized states. Neuropharmacology 41, 737–744 (2001).
Petrini, E. M., Marchionni, I., Zacchi, P., Sieghart, W. & Cherubini, E. Clustering of extrasynaptic GABAA receptors modulates tonic inhibition in cultured hippocampal neurons. J. Biol. Chem. 279, 45833–45843 (2004).
Frerking, M., Petersen, C. C. & Nicoll, R. A. Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus. Proc. Natl Acad. Sci. USA 96, 12917–12922 (1999).
Kullmann, D. M. & Semyanov, A. Glutamatergic modulation of GABAergic signaling among hippocampal interneurons: novel mechanisms regulating hippocampal excitability. Epilepsia 43, 174–178 (2002).
Leao, R. M., Mellor, J. R. & Randall, A. D. Tonic benzodiazepine-sensitive GABAergic inhibition in cultured rodent cerebellar granule cells. Neuropharmacology 39, 990–1003 (2000).
Richerson, G. B. & Wu, Y. M. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J. Neurophysiol. 90, 1363–1374 (2003).
Wu, Y., Wang, W. & Richerson, G. B. Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J. Neurophysiol. 89, 2021–2034 (2003).
Overstreet, L. S. & Westbrook, G. L. Paradoxical reduction of synaptic inhibition by vigabatrin. J. Neurophysiol. 86, 596–603 (2001).
Allen, N. J., Rossi, D. J. & Attwell, D. Sequential release of GABA by exocytosis and reversed uptake leads to neuronal swelling in simulated ischemia of hippocampal slices. J. Neurosci. 24, 3837–3849 (2004).
Corey, J. L., Davidson, N., Lester, H. A., Brecha, N. & Quick, M. W. Protein kinase C modulates the activity of a cloned γ-aminobutyric acid transporter expressed in Xenopus oocytes via regulated subcellular redistribution of the transporter. J. Biol. Chem. 269, 14759–14767 (1994).
Quick, M. W., Hu, J., Wang, D. & Zhang, H. Y. Regulation of a γ-aminobutyric acid transporter by reciprocal tyrosine and serine phosphorylation. J. Biol. Chem. 279, 15961–15967 (2004).
Hansra, N., Arya, S. & Quick, M. W. Intracellular domains of a rat brain GABA transporter that govern transport. J. Neurosci. 24, 4082–4087 (2004).
Kittler, J. T. & Moss, S. J. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr. Opin. Neurobiol. 13, 341–347 (2003).
Keller, C. A. et al. The γ2 subunit of GABAA receptors is a substrate for palmitoylation by GODZ. J. Neurosci. 24, 5881–5891 (2004).
Jones, M. V. & Westbrook, G. L. Shaping of IPSCs by endogenous calcineurin activity. J. Neurosci. 17, 7626–7633 (1997).
Hinkle, D. J. & Macdonald, R. L. β subunit phosphorylation selectively increases fast desensitization and prolongs deactivation of α1β1γ2L and α1β3γ2L GABAA receptor currents. J. Neurosci. 23, 11698–11710 (2003).
Wang, Q. et al. Control of synaptic strength, a novel function of Akt. Neuron 38, 915–928 (2003).
Rathenberg, J., Kittler, J. T. & Moss, S. J. Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol. Cell. Neurosci. 26, 251–257 (2004).
Triller, A. & Choquet, D. Synaptic structure and diffusion dynamics of synaptic receptors. Biol. Cell 95, 465–476 (2003).
Chen, L., Wang, H., Vicini, S. & Olsen, R. W. The γ-aminobutyric acid type A (GABAA) receptor-associated protein (GABARAP) promotes GABAA receptor clustering and modulates the channel kinetics. Proc. Natl Acad. Sci. USA 97, 11557–11562 (2000).
Everitt, A. B. et al. Conductance of recombinant GABAA channels is increased in cells co-expressing GABAA receptor-associated protein. J. Biol. Chem. 279, 21701–21706 (2004).
Petrini, E. M., Zacchi, P., Barberis, A., Mozrzymas, J. W. & Cherubini, E. Declusterization of GABAA receptors affects the kinetic properties of GABAergic currents in cultured hippocampal neurons. J. Biol. Chem. 278, 16271–16279 (2003).
Peng, Z., Huang, C. S., Stell, B. M., Mody, I. & Houser, C. R. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J. Neurosci. 24, 8629–8639 (2004).
Follesa, P., Biggio, F., Caria, S., Gorini, G. & Biggio, G. Modulation of GABAA receptor gene expression by allopregnanolone and ethanol. Eur. J. Pharmacol. 500, 413–425 (2004).
Yeung, J. Y. T. et al. Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol. Pharmacol. 63, 2–8 (2003).
Korpi, E. R., Kuner, T., Seeburg, P. H. & Luddens, H. Selective antagonist for the cerebellar granule cell-specific γ-aminobutyric acid type A receptor. Mol. Pharmacol. 47, 283–289 (1995).
Wall, M. J. Furosemide reveals heterogeneous GABAA receptor expression at adult rat Golgi cell to granule cell synapses. Neuropharmacology 43, 737–749 (2002).
Hevers, W. & Luddens, H. Pharmacological heterogeneity of γ-aminobutyric acid receptors during development suggests distinct classes of rat cerebellar granule cells in situ. Neuropharmacology 42, 34–47 (2002).
Belelli, D., Casula, A., Ling, A. & Lambert, J. J. The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology 43, 651–661 (2002).
Wohlfarth, K. M., Bianchi, M. T. & Macdonald, R. L. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J. Neurosci. 22, 1541–1549 (2002).
Sundstrom-Poromaa, I. et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nature Neurosci. 5, 721–722 (2002).
Wallner, M., Hanchar, H. J. & Olsen, R. W. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc. Natl Acad. Sci. USA 100, 15218–15223 (2003). References 174 and 175 provide an elegant demonstration that concentrations of ethanol that can be reached with moderate, social alcohol consumption enhance responses from δ but not γ2 subunit-containing GABA A receptors.
Wei, W., Faria, L. C. & Mody, I. Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J. Neurosci. 24, 8379–8382 (2004).
Hanchar, H. J., Dodson, P. D., Olsen, R. W., Otis, T. S. & Wallner, M. Alcohol induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nature Neurosci. (in the press). Shows that alcohol impairs motor coordination by enhancing tonic inhibition, which is mediated by extrasynaptic α 6 β 3 δ GABA A receptors in cerebellar granule cells. Moreover, in ANT rats, a naturally occurring single nucleotide polymorphism (R100Q) in the α6 subunit results in a GABA A receptor that is significantly more sensitive to the potentiating effects of alcohol.
Caraiscos, V. B. et al. Selective enhancement of tonic GABAergic inhibition in murine hippocampal neurons by low concentrations of the volatile anesthetic isoflurane. J. Neurosci. 24, 8454–8458 (2004).
Bormann, J., Hamill, O. P. & Sakmann, B. Mechanism of anion permeation through channels gated by glycine and γ-aminobutyric acid in mouse cultured spinal neurones. J. Physiol. (Lond.) 385, 243–286 (1987).
Kaila, K. Ionic basis of GABAA receptor channel function in the nervous system. Prog. Neurobiol. 42, 489–537 (1994).
Payne, J. A., Rivera, C., Voipio, J. & Kaila, K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 26, 199–206 (2003).
Chavas, J., Forero, M. E., Collin, T., Llano, I. & Marty, A. Osmotic tension as a possible link between GABAA receptor activation and intracellular calcium elevation. Neuron 44, 701–713 (2004).
Llinas, R. & Muhlethaler, M. Electrophysiology of guinea-pig cerebellar nuclear cells in the in vitro brain stem–cerebellar preparation. J. Physiol. (Lond.) 404, 241–258 (1988).
Rivera, C., Voipio, J. & Kaila, K. Two developmental switches in GABAergic signalling: the K+–Cl− cotransporter KCC2, and carbonic anhydrase CAVII. J. Physiol. (Lond.) 562, 27–36 (2005).
Chavas, J. & Marty, A. Coexistence of excitatory and inhibitory GABA synapses in the cerebellar interneuron network. J. Neurosci. 23, 2019–2031 (2003).
Stein, V. & Nicoll, R. A. GABA generates excitement. Neuron 37, 375–378 (2003).
Gao, X. B., Chen, G. & van den Pol, A. N. GABA-dependent firing of glutamate-evoked action potentials at AMPA/kainate receptors in developing hypothalamic neurons. J. Neurophysiol. 79, 716–726 (1998).
Staley, K. J. & Mody, I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J. Neurophysiol. 68, 197–212 (1992).
Ben-Ari, Y. Excitatory actions of GABA during development: the nature of the nurture. Nature Rev. Neurosci. 3, 728–739 (2002).
Owens, D. F. & Kriegstein, A. R. Is there more to GABA than synaptic inhibition? Nature Rev. Neurosci. 3, 715–727 (2002).
Nguyen, L. et al. Autocrine/paracrine activation of the GABAA receptor inhibits the proliferation of neurogenic polysialylated neural cell adhesion molecule-positive (PSA-NCAM+) precursor cells from postnatal striatum. J. Neurosci. 23, 3278–3294 (2003).
Lester, H. A., Dibas, M. I., Dahan, D. S., Leite, J. F. & Dougherty, D. A. Cys-loop receptors: new twists and turns. Trends Neurosci. 27, 329–336 (2004).
Simon, J., Wakimoto, H., Fujita, N., Lalande, M. & Barnard, E. A. Analysis of the set of GABAA receptor genes in the human genome. J. Biol. Chem. 279, 41422–41435 (2004).
Wisden, W., Laurie, D. J., Monyer, H. & Seeburg, P. H. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 12, 1040–1062 (1992).
Fritschy, J. -M. & Mohler, H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 359, 154–194 (1995).
Pirker, S., Schwarzer, C., Wieselthaler, A., Sieghart, W. & Sperk, G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101, 815–850 (2000).
Kittler, J. T., McAinsh, K. & Moss, S. J. Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol. Neurobiol. 26, 251–268 (2002).
Sieghart, W. & Sperk, G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr. Top. Med. Chem. 2, 795–816 (2002).
McKernan, R. M. & Whiting, P. J. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 19, 139–143 (1996).
Whiting, P. J. GABAA receptor subtypes in the brain: a paradigm for CNS drug discovery? Drug Discov. Today 8, 445–450 (2003).
Tretter, V., Ehya, N., Fuchs, K. & Sieghart, W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J. Neurosci. 17, 2728–2737 (1997).
Farrar, S. J., Whiting, P. J., Bonnert, T. P. & McKernan, R. M. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J. Biol. Chem. 274, 10100–10104 (1999).
Baumann, S. W., Baur, R. & Sigel, E. Forced subunit assembly in α1β2γ2 GABAA receptors. Insight into the absolute arrangement. J. Biol. Chem. 277, 46020–46025 (2002).
Neelands, T. R., Fisher, J. L., Bianchi, M. & Macdonald, R. L. Spontaneous and γ-aminobutyric acid (GABA)-activated GABAA receptor channels formed by ε-subunit-containing isoforms. Mol. Pharmacol. 55, 168–178 (1999).
Neelands, T. R. & Macdonald, R. L. Incorporation of the π subunit into functional γ-aminobutyric acid A receptors. Mol. Pharmacol. 56, 598–610 (1999).
Bonnert, T. P. et al. θ, a novel γ-aminobutyric acid type A receptor subunit. Proc. Natl Acad. Sci. USA 96, 9891–9896 (1999).
Bormann, J. The 'ABC' of GABA receptors. Trends Pharmacol. Sci. 21, 16–19 (2000).
Johnston, G. A. Medicinal chemistry and molecular pharmacology of GABA(C) receptors. Curr. Top. Med. Chem. 2, 903–913 (2002).
Qian, H. & Ripps, H. Response kinetics and pharmacological properties of heteromeric receptors formed by coassembly of GABA ρ- and γ2-subunits. Proc. R. Soc. Lond. B 266, 2419–2425 (1999).
Milligan, C. J., Buckley, N. J., Garret, M., Deuchars, J. & Deuchars, S. A. Evidence for inhibition mediated by coassembly of GABAA and GABAC receptor subunits in native central neurons. J. Neurosci. 24, 7241–7250 (2004).
Hevers, W. & Luddens, H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol. Neurobiol. 18, 35–86 (1998).
Compagnone, N. A. & Mellon, S. H. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front. Neuroendocrinol. 21, 1–56 (2000).
Acknowledgements
We thank T. Smart (University College London) for comments on the manuscript, M. Wallner (University of California, Los Angeles) and E. Petrini (Trieste) for sharing data prior to publication, and S. Brickley (Imperial College London) for many helpful discussions during a long collaberation with M.F. M.F.'s research is supported by the Wellcome Trust. Z.N. is the recipient of a Wellcome Trust International Senior Research Fellowship, an International Scholarship from Howard Hughes Medical Institute and a Postdoctoral Fellowship from the Boehringer Ingelheim Fond.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
FURTHER INFORMATION
Glossary
- ALTERNATIVE SPLICING
-
During splicing, introns are excised from RNA after transcription and the cut ends are rejoined to form a continuous message. Alternative splicing allows the production of different messages from the same DNA molecule.
- PARACRINE SIGNALLING
-
A signalling process that involves the secretion from a cell of molecules that act on other cells expressing appropriate receptors in the immediate neighbourhood, rather than acting on the same cell (autocrine signalling) or on remote cells (endocrine signalling).
- GLOMERULUS
-
Axon terminals end in various configurations within the neuropil. The most common is en passant or de passage, in which axons make simple synapses as they pass dendrites or cell bodies. By contrast, some axons end in — or produce strings of — enlargements that are often packed with synaptic vesicles. These glomerular-type endings might synapse with large numbers of dendrites. In the cerebellum, each large excitatory mossy fibre terminal contacts dendrites from many granule cells and, together with inhibitory Golgi cell axon terminals, forms a glomerular structure that is wrapped with glia.
- THETA FREQUENCY NETWORK OSCILLATION
-
Rhythmic neural activity with a frequency of 4–8 Hz.
- GAMMA FREQUENCY NETWORK OSCILLATIONS
-
Rhythmic neural activity with a frequency of 25–70 Hz.
- COINCIDENCE DETECTION
-
A situation in which two different subthreshold excitatory inputs are sufficiently closely timed that they summate to trigger the generation of an action potential.
- TETRODOTOXIN
-
A potent marine neurotoxin that blocks voltage-gated sodium channels. Tetrodotoxin was originally isolated from the tetraodon pufferfish.
- PALMITOYLATION
-
The covalent attachment of a palmitate (16-carbon, saturated fatty acid) to a cysteine residue through a thioester bond.
- ALLOSTERIC
-
A term originally used to describe enzymes that have two or more receptor sites, one of which (the active site) binds the principal substrate, whereas the other(s) bind(s) effector molecules that can influence the enzyme's biological activity. More generally, it is used to describe the indirect coupling of distinct sites within a protein, mediated by conformational changes.
- NOOTROPIC
-
Refers to agents that enhance memory or other cognitive functions.
- ALCOHOL NON-TOLERANT RATS
-
(ANT rats). A rat line that has been selectively bred to be highly sensitive to motor impairment after ethanol intake.
Rights and permissions
About this article
Cite this article
Farrant, M., Nusser, Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 6, 215–229 (2005). https://doi.org/10.1038/nrn1625
Issue Date:
DOI: https://doi.org/10.1038/nrn1625
This article is cited by
-
Modulators of GABAA receptor-mediated inhibition in the treatment of neuropsychiatric disorders: past, present, and future
Neuropsychopharmacology (2024)
-
Neurosteroids: mechanistic considerations and clinical prospects
Neuropsychopharmacology (2024)
-
Schizophrenia: from neurochemistry to circuits, symptoms and treatments
Nature Reviews Neurology (2024)
-
The Role of Extrasynaptic GABA Receptors in Postpartum Depression
Molecular Neurobiology (2024)
-
Neurosteroids and their potential as a safer class of general anesthetics
Journal of Anesthesia (2024)