Abstract
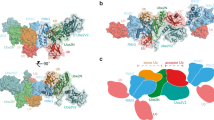
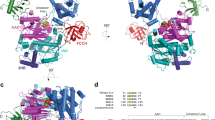
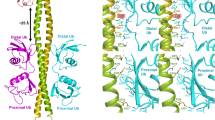
The protein kinase TAK1 is activated by binding to Lys63 (K63)-linked ubiquitin chains through its subunit TAB2. Here we analyze crystal structures of the TAB2 NZF domain bound to Lys63-linked di- and triubiquitin, revealing that TAB2 binds adjacent ubiquitin moieties via two distinct binding sites. The conformational constraints imposed by TAB2 on a Lys63 dimer cannot be adopted by linear chains, explaining why TAK1 cannot be activated by linear ubiquitination events.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Skaug, B., Jiang, X. & Chen, Z.J. Annu. Rev. Biochem. 78, 769–796 (2009).
Iwai, K. & Tokunaga, F. EMBO Rep. 10, 706–713 (2009).
Chen, Z.J. & Sun, L.J. Mol. Cell 33, 275–286 (2009).
Xia, Z.P. et al. Nature 461, 114–119 (2009).
Kanayama, A. et al. Mol. Cell 15, 535–548 (2004).
Besse, A. et al. J. Biol. Chem. 282, 3918–3928 (2007).
Komander, D. et al. EMBO Rep. 10, 466–473 (2009).
Alam, S.L. et al. EMBO J. 23, 1411–1421 (2004).
Sims, J.J., Haririnia, A., Dickinson, B.C., Fushman, D. & Cohen, R.E. Nat. Struct. Mol. Biol. 16, 883–889 (2009).
Komander, D. Biochem. Soc. Trans. 37, 937–953 (2009).
Dikic, I., Wakatsuki, S. & Walters, K.J. Nat. Rev. Mol. Cell Biol. 10, 659–671 (2009).
Rahighi, S. et al. Cell 136, 1098–1109 (2009).
Yoshikawa, A. et al. FEBS Lett. 583, 3317–3322 (2009).
Lo, Y.C. et al. Mol. Cell 33, 602–615 (2009).
Varadan, R., Assfalg, M., Raasi, S., Pickart, C. & Fushman, D. Mol. Cell 18, 687–698 (2005).
Windheim, M., Stafford, M., Peggie, M. & Cohen, P. Mol. Cell. Biol. 28, 1783–1791 (2008).
Acknowledgements
We would like to thank P. Cohen (Medical Research Council Protein Phosphorylation Unit), H. Scheel (Miltenyi Biotec), D. Veprintsev, A. Pobbati, R. Williams, O. Perisic, F. Gorrec, Y. Ye (Medical Research Council Laboratory of Molecular Biology) for help and reagents. Y.K., M.A. and A.B. are Medical Research Council Career Development Fellows.
Author information
Authors and Affiliations
Contributions
Y.K. designed and performed all experiments and analyzed the data, M.A. and A.B. contributed to experiments, K.H. provided NZF classification, and D.K. designed experiments, analyzed the data and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Methods, Supplementary Figures 1–8 and Supplementary Tables 1 and 2 (PDF 1673 kb)
Rights and permissions
About this article
Cite this article
Kulathu, Y., Akutsu, M., Bremm, A. et al. Two-sided ubiquitin binding explains specificity of the TAB2 NZF domain. Nat Struct Mol Biol 16, 1328–1330 (2009). https://doi.org/10.1038/nsmb.1731
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.1731
This article is cited by
-
A modular toolbox to generate complex polymeric ubiquitin architectures using orthogonal sortase enzymes
Nature Communications (2021)
-
The SUMOylation of TAB2 mediated by TRIM60 inhibits MAPK/NF-κB activation and the innate immune response
Cellular & Molecular Immunology (2021)
-
TNK1 is a ubiquitin-binding and 14-3-3-regulated kinase that can be targeted to block tumor growth
Nature Communications (2021)
-
Structural insights into ubiquitin recognition and Ufd1 interaction of Npl4
Nature Communications (2019)
-
A network-centric approach to drugging TNF-induced NF-κB signaling
Nature Communications (2019)