Abstract
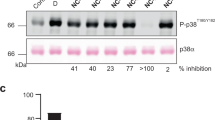
p38α mitogen-activated protein kinase (p38α) is activated by a variety of mechanisms, including autophosphorylation initiated by TGFβ-activated kinase 1 binding protein 1 (TAB1) during myocardial ischemia and other stresses. Chemical-genetic approaches and coexpression in mammalian, bacterial and cell-free systems revealed that mouse p38α autophosphorylation occurs in cis by direct interaction with TAB1(371–416). In isolated rat cardiac myocytes and perfused mouse hearts, TAT-TAB1(371–416) rapidly activates p38 and profoundly perturbs function. Crystal structures and characterization in solution revealed a bipartite docking site for TAB1 in the p38α C-terminal kinase lobe. TAB1 binding stabilizes active p38α and induces rearrangements within the activation segment by helical extension of the Thr-Gly-Tyr motif, allowing autophosphorylation in cis. Interference with p38α recognition by TAB1 abolishes its cardiac toxicity. Such intervention could potentially circumvent the drawbacks of clinical pharmacological inhibitors of p38 catalytic activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cuenda, A. & Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 1773, 1358–1375 (2007).
Mudgett, J.S. et al. Essential role for p38α mitogen-activated protein kinase in placental angiogenesis. Proc. Natl. Acad. Sci. USA 97, 10454–10459 (2000).
Beardmore, V.A. et al. Generation and characterization of p38β (MAPK11) gene-targeted mice. Mol. Cell Biol. 25, 10454–10464 (2005).
Lee, J.C. et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372, 739–746 (1994).
Gum, R.J. et al. Acquisition of sensitivity of stress-activated protein kinases to the p38 inhibitor, SB 203580, by alteration of one or more amino acids within the ATP binding pocket. J. Biol. Chem. 273, 15605–15610 (1998).
Dominguez, C., Powers, D.A. & Tamayo, N. p38 MAP kinase inhibitors: many are made, but few are chosen. Curr. Opin. Drug Discov. Devel. 8, 421–430 (2005).
Genovese, M.C. Inhibition of p38: has the fat lady sung? Arthritis Rheum. 60, 317–320 (2009).
Hammaker, D. & Firestein, G.S. “Go upstream, young man”: lessons learned from the p38 saga. Ann. Rheum. Dis. 69 (suppl. 1), i77–i82 (2010).
Martin, D.E., Felice De Nicola, G. & Marber, M.S. New therapeutic targets in cardiology: p38α mitogen-activated protein kinase for ischemic heart disease. Circulation 126, 357–368 (2012).
Tanno, M. et al. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ. Res. 93, 254–261 (2003).
Li, J., Miller, E.J., Ninomiya-Tsuji, J., Russell, R.R. III & Young, L.H. AMP-activated protein kinase activates p38 mitogen-activated protein kinase by increasing its recruitment to tab1 in the ischemic heart. Circ. Res. 97, 872–879 (2005).
Ota, A., Zhang, J., Ping, P., Han, J. & Wang, Y. Specific regulation of noncanonical p38α activation by Hsp90-Cdc37 chaperone complex in cardiomyocyte. Circ. Res. 106, 1404–1412 (2010).
Fiedler, B. et al. cGMP-dependent protein kinase type I inhibits TAB1-p38 mitogen-activated protein kinase apoptosis signaling in cardiac myocytes. J. Biol. Chem. 281, 32831–32840 (2006).
Shi, J. et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38α MAPK pathway. Proc. Natl. Acad. Sci. USA 107, 4188–4193 (2010).
Ge, B. et al. MAPKK-independent activation of p38α mediated by TAB1-dependent autophosphorylation of p38α. Science 295, 1291–1294 (2002).
Cheung, P.C., Campbell, D.G., Nebreda, A.R. & Cohen, P. Feedback control of the protein kinase TAK1 by SAPK2a/p38α. EMBO J. 22, 5793–5805 (2003).
Diskin, R., Lebendiker, M., Engelberg, D. & Livnah, O. Structures of p38α active mutants reveal conformational changes in L16 loop that induce autophosphorylation and activation. J. Mol. Biol. 365, 66–76 (2007).
Salvador, J.M. et al. Alternative p38 activation pathway mediated by T cell receptor–proximal tyrosine kinases. Nat. Immunol. 6, 390–395 (2005).
Lochhead, P.A. Protein kinase activation loop autophosphorylation in cis: overcoming a Catch-22 situation. Sci. Signal. 2, e4 (2009).
Wilson, K.P. et al. Crystal structure of p38 mitogen-activated protein kinase. J. Biol. Chem. 271, 27696–27700 (1996).
Zhang, W.X. et al. Time-resolved Forster resonance energy transfer assays for the binding of nucleotide and protein substrates to p38α protein kinase. Anal. Biochem. 343, 76–83 (2005).
Shrestha, A., Hamilton, G., O'Neill, E., Knapp, S. & Elkins, J.M. Analysis of conditions affecting auto-phosphorylation of human kinases during expression in bacteria. Protein Expr. Purif. 81, 136–143 (2012).
Jacquet, S. et al. The role of RIP2 in p38 MAPK activation in the stressed heart. J. Biol. Chem. 283, 11964–11971 (2008).
Kumphune, S. et al. A chemical genetic approach reveals that p38α MAPK activation by diphosphorylation aggravates myocardial infarction and is prevented by the direct binding of SB203580. J. Biol. Chem. 285, 2968–2975 (2010).
Sicard, P. et al. The activation of p38α, and not p38β, mitogen-activated protein kinase is required for ischemic preconditioning. J. Mol. Cell Cardiol. 48, 1324–1328 (2010).
Ge, B. et al. TAB1β (transforming growth factor-β-activated protein kinase 1-binding protein 1β), a novel splicing variant of TAB1 that interacts with p38α but not TAK1. J. Biol. Chem. 278, 2286–2293 (2003).
Zhou, H. et al. Determinants that control the specific interactions between TAB1 and p38α. Mol. Cell. Biol. 26, 3824–3834 (2006).
Rothweiler, U. et al. p38α MAP kinase dimers with swapped activation segments and a novel catalytic loop conformation. J. Mol. Biol. 411, 474–485 (2011).
Cameron, A.J., Escribano, C., Saurin, A.T., Kostelecky, B. & Parker, P.J. PKC maturation is promoted by nucleotide pocket occupation independently of intrinsic kinase activity. Nat. Struct. Mol. Biol. 16, 624–630 (2009).
Blethrow, J., Zhang, C., Shokat, K.M. & Weiss, E.L. Design and use of analog-sensitive protein kinases. Curr. Protoc. Mol. Biol. 66, 18.11 (2004).
Wang, Z. et al. The structure of mitogen-activated protein kinase p38 at 2.1-A resolution. Proc. Natl. Acad. Sci. USA 94, 2327–2332 (1997).
Kornev, A.P., Taylor, S.S. & Ten Eyck, L.F. A helix scaffold for the assembly of active protein kinases. Proc. Natl. Acad. Sci. USA 105, 14377–14382 (2008).
Bhattacharyya, R.P. et al. The Ste5 scaffold allosterically modulates signaling output of the yeast mating pathway. Science 311, 822–826 (2006).
Wang, Y. et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen activated protein kinase family. J. Biol. Chem. 273, 2161–2168 (1998).
Bukhtiyarova, M. et al. Improved expression, purification, and crystallization of p38α MAP kinase. Protein Expr. Purif. 37, 154–161 (2004).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Johnson, B.A. & Blevins, R.A. NMR View: a computer program for the visualization and analysis of NMR data. J. Biomol. NMR 4, 603–614 (1994).
Bartels, C., Xia, T.H., Billeter, M., Guntert, P. & Wuthrich, K. The program XEASY for computer-supported NMR spectral analysis of biological macromolecules. J. Biomol. NMR 6, 1–10 (1995).
Pervushin, K., Riek, R., Wider, G. & Wuthrich, K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc. Natl. Acad. Sci. USA 94, 12366–12371 (1997).
Palmer, A.G. III, Fairbrother, W.J., Cavanagh, J., Wright, P.E. & Rance, M. Improved resolution in three-dimensional constant-time triple resonance NMR spectroscopy of proteins. J. Biomol. NMR 2, 103–108 (1992).
Schleucher, J. et al. A general enhancement scheme in heteronuclear multidimensional NMR employing pulsed field gradients. J. Biomol. NMR 4, 301–306 (1994).
Foster, M.P., McElroy, C.A. & Amero, C.D. Solution NMR of large molecules and assemblies. Biochemistry 46, 331–340 (2007).
Martino, L. et al. Analysis of the interaction with the hepatitis C virus mRNA reveals an alternative mode of RNA recognition by the human La protein. Nucleic Acids Res. 40, 1381–1394 (2012).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
McCoy, A.J., Grosse-Kunstleve, R.W., Storoni, L.C. & Read, R.J. Likelihood-enhanced fast translation functions. Acta Crystallogr. D Biol. Crystallogr. 61, 458–464 (2005).
Koeberle, S.C. et al. Skepinone-L is a selective p38 mitogen-activated protein kinase inhibitor. Nat. Chem. Biol. 8, 141–143 (2012).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Bellahcene, M. et al. Activation of p38 mitogen-activated protein kinase contributes to the early cardiodepressant action of tumor necrosis factor. J. Am. Coll. Cardiol. 48, 545–555 (2006).
Acknowledgements
This work was supported by project grants from the UK Medical Research Council (MRC) (G0802033 to M.S.M., G1001138 to M.S.M. and M.R.C. and J007501 to M.S.M.) and the UK Department of Health through the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's & St Thomas' National Health Service Foundation Trust. All the ITC and part of the NMR experiments were performed at the facilities of the Centre for Biomolecular Spectroscopy, King's College London, established with a Capital Award from the Wellcome Trust to M.R.C. (085944/Z/08/Z). S.K. is grateful for support from the Structural Genomics Consortium, a registered charity (number 1097737) that receives funds from AbbVie, Boehringer Ingelheim, the Canada Foundation for Innovation, the Canadian Institutes for Health Research, Genome Canada, GlaxoSmithKline, Janssen, Lilly Canada, the Novartis Research Foundation, the Ontario Ministry of Economic Development and Innovation, Pfizer, Takeda and the Wellcome Trust (092809/Z/10/Z). A.C. is supported by the European Union FP7 grant no. 278568 “PRIMES”. We thank the scientists at the Diamond Light Source for help with data collection. We are grateful to the MRC Biomedical NMR Centre, Mill Hill, and its staff for a generous allocation of NMR time and for expert technical assistance. We also thank N. Drinkwater and B. Sutton for help at an early stage of the work. We thank Y. Wang (David Geffen School of Medicine, University of California, Los Angeles) for the pET14b vector.
Author information
Authors and Affiliations
Contributions
M.S.M. and M.R.C. conceived of the project; G.F.D.N. and R.B. performed protein cloning, expression and purification; G.F.D.N. performed ITC and NMR experiments; A.C., G.F.D.N. and S.K. performed the X-ray diffraction and structural determination; E.D.M. and S.V. performed the in vitro kinase and E. coli kinase biochemical characterizations; R.B. performed mammalian cell-culture experiments; J.C. and P.S. performed the mouse heart perfusions; and M.S.M., M.R.C., S.K., A.C. and G.F.D.N. wrote the paper. L.M. helped with ITC, R.T. with cloning and R.A.A. with NMR.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1 and 2 (PDF 2788 kb)
Rights and permissions
About this article
Cite this article
De Nicola, G., Martin, E., Chaikuad, A. et al. Mechanism and consequence of the autoactivation of p38α mitogen-activated protein kinase promoted by TAB1. Nat Struct Mol Biol 20, 1182–1190 (2013). https://doi.org/10.1038/nsmb.2668
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2668
This article is cited by
-
Structural basis of a redox-dependent conformational switch that regulates the stress kinase p38α
Nature Communications (2023)
-
Characterization of p38α autophosphorylation inhibitors that target the non-canonical activation pathway
Nature Communications (2023)
-
Fluorescence resonance energy transfer (FRET) spatiotemporal mapping of atypical P38 reveals an endosomal and cytosolic spatial bias
Scientific Reports (2023)
-
GFAT1-linked TAB1 glutamylation sustains p38 MAPK activation and promotes lung cancer cell survival under glucose starvation
Cell Discovery (2022)
-
Diversity and versatility of p38 kinase signalling in health and disease
Nature Reviews Molecular Cell Biology (2021)