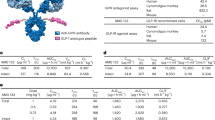
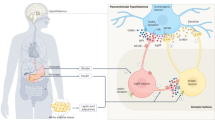
Abstract
Genetic defects underlying the melanocortin-4 receptor (MC4R) signaling pathway lead to severe obesity. Three severely obese LEPR-deficient individuals were administered the MC4R agonist setmelanotide, resulting in substantial and durable reductions in hyperphagia and body weight over an observation period of 45–61 weeks. Compared to formerly developed and tested MC4R agonists, setmelanotide has the unique capability of activating nuclear factor of activated T cell (NFAT) signaling and restoring function of this signaling pathway for selected MC4R variants. Our data demonstrate the potency of setmelanotide in treatment of individuals with diverse MC4R-related pathway deficiencies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Farooqi, I. S. & O’Rahilly, S. J. Endocrinol. 223, T63–T70 (2014).
Krishna, R. et al. Clin. Pharmacol. Ther. 86, 659–666 (2009).
Farooqi, I. S. et al. N. Engl. J. Med. 341, 879–884 (1999).
Clément, K. et al. Nature 392, 398–401 (1998).
Jackson, V. M., Price, D. A. & Carpino, P. A. Expert Opin. Investig. Drugs 23, 1055–1066 (2014).
Kühnen, P. et al. N. Engl. J. Med. 375, 240–246 (2016).
Farooqi, I. S. et al. N. Engl. J. Med. 356, 237–247 (2007).
Huvenne, H. et al. J. Clin. Endocrinol. Metab. 100, E757–E766 (2015).
Bischof, J. M., Van Der Ploeg, L. H., Colmers, W. F. & Wevrick, R. Br. J. Pharmacol. 173, 2614–2621 (2016).
Collet, T. H. et al. Mol. Metab. 6, 1321–1329 (2017).
Gantz, I. et al. J. Biol. Chem. 268, 15174–15179 (1993).
Ghamari-Langroudi, M. et al. Nature 520, 94–98 (2015).
Yang, L. K. & Tao, Y. X. Biochim. Biophys. Acta 1863, 2486–2495 (2017).
Li, Y. Q. et al. J. Clin. Invest. 126, 40–49 (2016).
Ollmann, M. M. et al. Science 278, 135–138 (1997).
Büch, T. R., Heling, D., Damm, E., Gudermann, T. & Breit, A. J. Biol. Chem. 284, 26411–26420 (2009).
Stutzmann, F. et al. Diabetes 57, 2511–2518 (2008).
Dinter, J. et al. J. Mol. Endocrinol. 54, 205–216 (2015).
Tarnow, P., Schoneberg, T., Krude, H., Gruters, A. & Biebermann, H. J. Biol. Chem. 278, 48666–48673 (2003).
Rasmussen, S. G. et al. Nature 477, 549–555 (2011).
Hanson, M. A. et al. Science 335, 851–855 (2012).
Koehbach, J. et al. Proc. Natl Acad. Sci. USA 110, 21183–21188 (2013).
Ericson, M. D. et al. Biochim. Biophys. Acta 1863, 2414–2435 (2017).
Acknowledgements
We thank D. Schnabel for his support and A. Stielow, S. Zvorc, S. Jyrch, and C. Cetindag (Charité Universitätsmedizin Berlin, Germany) and H. Guermoudi, V. Lemoine and F. Marchelli (Pitié-Sapêtrière hospital, Paris, France) for excellent study assistance. This work was supported by grants from the German Research Foundation (DFG) (KU 2673/2-1; SPP1629: BI839/5-2, KR1701/5-1; KFO218 and HI 865/2-1), Bundesministerium für Bildung und Forschung (BMBF) (NGFN- Plus, 01GS0820), the Helmholtz Association (ICEMED) (WB19) and the Institute of Cardiometabolism and Nutrition (K.C.) as well as Assistance Publique Hôpitaux de Paris (clinical research contracts to K.C. and C.P.) and Institut Benjamin Delessert. P.K. was supported by the Charité /Berlin Institute of Health (BIH) Clinical Scientist Program. H.K. and K.M. were supported by the Berlin Institute of Health (BIH). P.S. was supported by the DFG (SFB740-B6, SFB1078-B6), and G.K. and P.S. were supported by the DFG Cluster of Excellence ‘Unifying Concepts in Catalysis’ (Research Field E). I.S.F. was supported by the Wellcome Trust.
Author information
Authors and Affiliations
Contributions
P.K., H.K., K.G and F.F. designed the clinical study and developed the protocol; P.K., B.W. and L.P. performed the clinical investigation of the patients; P.K. supervised and managed data generation and analysis; K.C. and C.P. recruited subject 1 and 3 and performed routine follow-up; I.S.F. recruited subject 2; U.B-P. and I.J. performed regular dermatological examination; K.W. was involved as a cardiologist; K.M. and J.S. contributed to the clinical investigation and metabolic phenotyping (including data analysis) of the individuals; S.W. supported clinical investigation of the patients; H.B., A.M. and L.V.d.P. performed in vitro experiments; P.K., H.B., A.M., J.M, I.S.F. and L.V.d.P. critically discussed the results of in vitro experiments; A.G., O.B. and S.S. contributed to the discussion of the data; G.K., N.H. and P.S. performed the MC4 receptor modeling and docking studies, analyzed the structural data and described related parts; P.K., L.V.d.P., H.B., H.K., K.G., I.S.F., K.C., F.F., G.K., N.H. and P.S. contributed to the analysis and interpretation of the data and reviewed all drafts of the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.V.d.P., F.F. and K.G. are employees and stock holders of Rhythm Pharmaceuticals. S. Sharma is a consultant for Rhythm Pharmaceuticals.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Figures 1–10 and Supplementary Tables 1–5
Source data
Supplementary Data
Source Data for Figure 2
Rights and permissions
About this article
Cite this article
Clément, K., Biebermann, H., Farooqi, I.S. et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat Med 24, 551–555 (2018). https://doi.org/10.1038/s41591-018-0015-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-018-0015-9
This article is cited by
-
Hypothalamic POMC neuron-specific knockout of MC4R affects insulin sensitivity by regulating Kir2.1
Molecular Medicine (2024)
-
Approccio all’obesità ipotalamica: presente e futuro
L'Endocrinologo (2024)
-
Rare genetic forms of obesity in childhood and adolescence: A narrative review of the main treatment options with a focus on innovative pharmacological therapies
European Journal of Pediatrics (2024)
-
Relevance and consequence of chronic inflammation for obesity development
Molecular and Cellular Pediatrics (2023)
-
Targeting the central melanocortin system for the treatment of metabolic disorders
Nature Reviews Endocrinology (2023)