Abstract
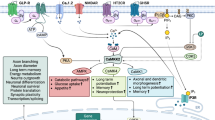
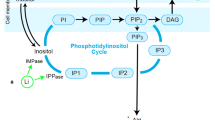
Inositol, a simple six-carbon sugar, forms the basis of a number of important intracellular signaling molecules. Over the last 35 years, a series of biochemical and cell biological experiments have shown that lithium (Li+) reduces the cellular concentration of myo-inositol and as a consequence attenuates signaling within the cell. Based on these observations, inositol-depletion was proposed as a therapeutic mechanism in the treatment of bipolar mood disorder. Recent results have added significant new dimensions to the original hypothesis. However, despite a number of clinical studies, this hypothesis still remains to be either proven or refuted. In this review of our current knowledge, I will consider where the inositol-depletion hypothesis stands today and how it may be further investigated in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Berridge MJ, Downes CP, Hanley MR . Neural and developmental actions of lithium: a unifying hypothesis. Cell 1989; 59: 411–419.
Harwood AJ, Agam G . Search for a common mechanism of mood stabilizers. Biochem Pharmacol 2003; 66: 179–189.
Allison JH, Stewart MA . Reduced brain inositol in lithium-treated rats. Nat New Biol 1971; 233: 267–268.
Allison JH, Blisner ME, Holland WH, Hipps PP, Sherman WR . Increased brain myo-inositol 1-phosphate in lithium-treated rats. Biochem Biophys Res Commun 1976; 71: 664–670.
Naccarato WF, Ray RE, Wells WW . Biosynthesis of myo-inositol in rat mammary gland. Isolation and properties of the enzymes. Arch Biochem Biophys 1974; 164: 194–201.
Hallcher LM, Sherman WR . The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem 1980; 255: 10896–10901.
Atack JR, Broughton HB, Pollack SJ . Structure and mechanism of inositol monophosphatase. FEBS Lett 1995; 361: 1–7.
Mikoshiba K . Inositol 1,4,5-trisphosphate receptor. Trends Pharmacol Sci 1993; 14: 86–89.
Shears SB . How versatile are inositol phosphate kinases? Biochem J 2004; 377: 265–280.
Fisher SK, Novak JE, Agranoff BW . Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem 2002; 82: 736–754.
Inhorn RC, Majerus PW . Inositol polyphosphate 1-phosphatase from calf brain. Purification and inhibition by Li+, Ca2+, and Mn2+. J Biol Chem 1987; 262: 15946–15952.
Emilien G, Maloteaux JM, Seghers A, Charles G . Lithium compared to valproic acid and carbamazepine in the treatment of mania: a statistical meta-analysis. Eur Neuropsychopharmacol 1996; 6: 245–252.
O'Donnell T, Rotzinger S, Nakashima TT, Hanstock CC, Ulrich M, Silverstone PH . Chronic lithium and sodium valproate both decrease the concentration of myo-inositol and increase the concentration of inositol monophosphates in rat brain. Brain Res 2000; 880: 84–91.
Vaden DL, Ding D, Peterson B, Greenberg ML . Lithium and valproate decrease inositol mass and increase expression of the yeast INO1 and INO2 genes for inositol biosynthesis. J Biol Chem 2001; 276: 15466–15471.
Williams RS, Cheng L, Mudge AW, Harwood AJ . A common mechanism of action for three mood-stabilizing drugs. Nature 2002; 417: 292–295.
Shamir A, Shaltiel G, Greenberg ML, Belmaker RH, Agam G . The effect of lithium on expression of genes for inositol biosynthetic enzymes in mouse hippocampus; a comparison with the yeast model. Brain Res Mol Brain Res 2003; 115: 104–110.
Williams RS, Eames M, Ryves WJ, Viggars J, Harwood AJ . Loss of a prolyl oligopeptidase confers resistance to lithium by elevation of inositol (1,4,5) trisphosphate. EMBO J 1999; 18: 2734–2745.
Shaltiel G, Shamir A, Shapiro J, Ding D, Dalton E, Bialer M et al. Valproate decreases inositol biosynthesis. Biol Psychiatry 2004 (in press).
Schulz I, Gerhartz B, Neubauer A, Holloschi A, Heiser U, Hafner M et al. Modulation of inositol 1,4,5-triphosphate concentration by prolyl endopeptidase inhibition. Eur J Biochem 2002; 269: 5813–5820.
Eickholt BJ, Williams RSB, Harwood AJ . Mood stabilizers and the cell biology of neuronal growth cones. Clin Neurosci Res 2004 (in press).
Emilien G, Maloteaux JM, Seghers A, Charles G . Lithium therapy in the treatment of manic-depressive illness. Present status and future perspectives. A critical review. Arch Int Pharmacodyn Ther 1995; 330: 251–278.
Shaltiel G, Dalton E, Belmaker RH, Harwood AJ, Agam G . Specificity of mood stabilizer action on neuronal growth cones. (ms submitted). 2004.
van Calker D, Belmaker RH . The high affinity inositol transport system—implications for the pathophysiology and treatment of bipolar disorder. Bipolar Disord 2000; 2: 102–107.
Inoue K, Shimada S, Minami Y, Morimura H, Miyai A, Yamauchi A et al. Cellular localization of Na+/MYO-inositol co-transporter mRNA in the rat brain. Neuroreport 1996; 7: 1195–1198.
Lubrich B, Spleiss O, Gebicke-Haerter PJ, van Calker D . Differential expression, activity and regulation of the sodium/myo-inositol cotransporter in astrocyte cultures from different regions of the rat brain. Neuropharmacology 2000; 39: 680–690.
Uldry M, Ibberson M, Horisberger JD, Chatton JY, Riederer BM, Thorens B . Identification of a mammalian H(+)-myo-inositol symporter expressed predominantly in the brain. EMBO J 2001; 20: 4467–4477.
Uldry M, Steiner P, Zurich MG, Beguin P, Hirling H, Dolci W et al. Regulated exocytosis of an H(+)/myo-inositol symporter at synapses and growth cones. EMBO J 2004; 23: 531–540.
Wolfson M, Bersudsky Y, Zinger E, Simkin M, Belmaker RH, Hertz L . Chronic treatment of human astrocytoma cells with lithium, carbamazepine or valproic acid decreases inositol uptake at high inositol concentrations but increases it at low inositol concentrations. Brain Res 2000; 855: 158–161.
Wolfson M, Hertz E, Belmaker RH, Hertz L . Chronic treatment with lithium and pretreatment with excess inositol reduce inositol pool size in astrocytes by different mechanisms. Brain Res 1998; 787: 34–40.
Berry GT, Wu S, Buccafusca R, Ren J, Gonzales LW, Ballard PL et al. Loss of murine Na+/myo-inositol cotransporter leads to brain myo-inositol depletion and central apnea. J Biol Chem 2003; 278: 18297–18302.
Berry GT, Buccafusca R, Greer JJ, Eccleston E . Phosphoinositide deficiency due to inositol depletion is not a mechanism of lithium action in brain. Mol Genet Metab 2004; 82: 87–92.
Berry GT, Wang ZJ, Dreha SF, Finucane BM, Zimmerman RA . In vivo brain myo-inositol levels in children with Down syndrome. J Pediatr 1999; 135: 94–97.
Atack JR, Broughton HB, Pollack SJ . Inositol monophosphatase—a putative target for Li+ in the treatment of bipolar disorder. Trends Neurosci 1995; 18: 343–349.
Lopez-Coronado JM, Belles JM, Lesage F, Serrano R, Rodriguez PL . A novel mammalian lithium-sensitive enzyme with a dual enzymatic activity, 3′-phosphoadenosine 5′-phosphate phosphatase and inositol-polyphosphate 1-phosphatase. J Biol Chem 1999; 274: 16034–16039.
Miyamoto R, Sugiura R, Kamitani S, Yada T, Lu Y, Sio SO et al. Tol1, a fission yeast phosphomonoesterase, is an in vivo target of lithium, and its deletion leads to sulfite auxotrophy. J Bacteriol 2000; 182: 3619–3625.
Ray Jr WJ, Post CB, Puvathingal JM . Comparison of rate constants for (PO3−) transfer by the Mg(II), Cd(II), and Li(I) forms of phosphoglucomutase. Biochemistry 1989; 28: 559–569.
Masuda CA, Xavier MA, Mattos KA, Galina A, Montero-Lomeli M . Phosphoglucomutase is an in vivo lithium target in yeast. J Biol Chem 2001; 276: 37794–37801.
Pang H, Koda Y, Soejima M, Kimura H . Identification of human phosphoglucomutase 3 (PGM3) as N-acetylglucosamine-phosphate mutase (AGM1). Ann Hum Genet 2002; 66: 139–144.
Klein PS, Melton DA . A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA 1996; 93: 8455–8459.
Woodgett JR . Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J 1990; 9: 2431–2438.
Ryves WJ, Harwood AJ . Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem Biophys Res Commun 2001; 280: 720–725.
Ryves WJ, Dajani R, Pearl L, Harwood AJ . Glycogen synthase kinase-3 inhibition by lithium and beryllium suggests the presence of two magnesium binding sites. Biochem Biophys Res Commun 2002; 290: 967–972.
Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J . Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J 2001; 20: 27–39.
King TD, Bijur GN, Jope RS . Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithium. Brain Res 2001; 919: 106–114.
Bijur GN, De Sarno P, Jope RS . Glycogen synthase kinase-3beta facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J Biol Chem 2000; 275: 7583–7590.
Takashima A, Noguchi K, Sato K, Hoshino T, Imahori K . Tau protein kinase I is essential for amyloid beta-protein-induced neurotoxicity. Proc Natl Acad Sci USA 1993; 90: 7789–7793.
Takashima A, Noguchi K, Michel G, Mercken M, Hoshi M, Ishiguro K et al. Exposure of rat hippocampal neurons to amyloid beta peptide (25–35) induces the inactivation of phosphatidyl inositol-3 kinase and the activation of tau protein kinase I/glycogen synthase kinase-3 beta. Neurosci Lett 1996; 203: 33–36.
Harwood AJ . Signal transduction: Life, the universe and development. Curr Biol 2000; 10: R116–R119.
Pap M, Cooper GM . Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem 1998; 273: 19929–19932.
Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW et al. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci USA 2000; 97: 11074–11079.
Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ et al. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol 2000; 7: 793–803.
Eickholt BJ, Walsh FS, Doherty P . An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. J Cell Biol 2002; 157: 211–217.
Nusse R (2004); http://www.stanford.edu/~rnusse/wntwindow.html.
Lucas FR, Goold RG, Gordon-Weeks PR, Salinas PC . Inhibition of GSK-3beta leading to the loss of phosphorylated MAP-1B is an early event in axonal remodelling induced by WNT-7a or lithium. J Cell Sci 1998; 111: 1351–1361.
Ciani L, Krylova O, Smalley MJ, Dale TC, Salinas PC . A divergent canonical WNT-signaling pathway regulates microtubule dynamics: dishevelled signals locally to stabilize microtubules. J Cell Biol 2004; 164: 243–253.
Garrity PA . Developmental biology: how neurons avoid derailment. Nature 2003; 422: 570–571.
He X . Wnt signaling went derailed again: a new track via the LIN-18 receptor? Cell 2004; 118: 668–670.
Lu W, Yamamoto V, Ortega B, Baltimore D . Mammalian ryk is a wnt coreceptor required for stimulation of neurite outgrowth. Cell 2004; 119: 97–108.
O'Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S et al. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci 2004; 24: 6791–6798.
Gould TD, Einat H, Bhat R, Manji HK . AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol 2004; 1–4.
Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H . Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry 2004; 55: 781–784.
Chen G, Huang LD, Jiang YM, Manji HK . The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem 1999; 72: 1327–1330.
Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS . Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 2001; 276: 36734–36741.
Hall AC, Brennan A, Goold RG, Cleverley K, Lucas FR, Gordon-Weeks PR et al. Valproate regulates GSK-3-mediated axonal remodeling and synapsin I clustering in developing neurons. Mol Cell Neurosci 2002; 20: 257–270.
De Sarno P, Li X, Jope RS . Regulation of Akt and glycogen synthase kinase-3beta phosphorylation by sodium valproate and lithium. Neuropharmacology 2002; 43: 1158–1164.
Allison JH, Boshans RL, Hallcher LM, Packman PM, Sherman WR . The effects of lithium on myo-inositol levels in layers of frontal cerebral cortex, in cerebellum, and in corpus callosum of the rat. J Neurochem 1980; 34: 456–458.
Sherman WR, Leavitt AL, Honchar MP, Hallcher LM, Phillips BE . Evidence that lithium alters phosphoinositide metabolism: chronic administration elevates primarily D-myo-inositol-1-phosphate in cerebral cortex of the rat. J Neurochem 1981; 36: 1947–1951.
Lee CH, Dixon JF, Reichman M, Moummi C, Los G, Hokin LE . Li+ increases accumulation of inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate in cholinergically stimulated brain cortex slices in guinea pig, mouse and rat. The increases require inositol supplementation in mouse and rat but not in guinea pig. Biochem J 1992; 282(Part 2): 377–385.
Dixon JF, Hokin LE . Lithium stimulates accumulation of second-messenger inositol 1,4,5-trisphosphate and other inositol phosphates in mouse pancreatic minilobules without inositol supplementation. Biochem J 1994; 304(Part 1): 251–258.
Hokin LE, Dixon JF, Los GV . A novel action of lithium: stimulation of glutamate release and inositol 1,4,5 trisphosphate accumulation via activation of the N-methyl D-aspartate receptor in monkey and mouse cerebral cortex slices. Adv Enzyme Regul 1996; 36: 229–244.
Sullivan NR, Burke T, Siafaka-Kapadai A, Javors M, Hensler JG . Effect of valproic acid on serotonin-2A receptor signaling in C6 glioma cells. J Neurochem 2004; 90: 1269–1275.
Kofman O, Belmaker RH . Intracerebroventricular myo-inositol antagonizes lithium-induced suppression of rearing behaviour in rats. Brain Res 1990; 534: 345–347.
Kofman O, Belmaker RH, Grisaru N, Alpert C, Fuchs I, Katz V et al. Myo-inositol attenuates two specific behavioral effects of acute lithium in rats. Psychopharmacol Bull 1991; 27: 185–190.
Kato T, Inubushi T, Kato N . Magnetic resonance spectroscopy in affective disorders. J Neuropsychiatry Clin Neurosci 1998; 10: 133–147.
Kato T, Shioiri T, Takahashi S, Inubushi T . Measurement of brain phosphoinositide metabolism in bipolar patients using in vivo 31P-MRS. J Affect Disord 1991; 22: 185–190.
Shimon H, Agam G, Belmaker RH, Hyde TM, Kleinman JE . Reduced frontal cortex inositol levels in postmortem brain of suicide victims and patients with bipolar disorder. Am J Psychiatry 1997; 154: 1148–1150.
Moore CM, Breeze JL, Gruber SA, Babb SM, Frederick BB, Villafuerte RA et al. Choline, myo-inositol and mood in bipolar disorder: a proton magnetic resonance spectroscopic imaging study of the anterior cingulate cortex. Bipolar Disord 2000; 2: 207–216.
Davanzo P, Thomas MA, Yue K, Oshiro T, Belin T, Strober M et al. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology 2001; 24: 359–369.
Shapiro J, Belmaker RH, Biegon A, Seker A, Agam G . Scyllo-inositol in post-mortem brain of bipolar, unipolar and schizophrenic patients. J Neural Transm 2000; 107: 603–607.
Silverstone PH, Wu RH, O'Donnell T, Ulrich M, Asghar SJ, Hanstock CC . Chronic treatment with both lithium and sodium valproate may normalize phosphoinositol cycle activity in bipolar patients. Hum Psychopharmacol 2002; 17: 321–327.
Friedman SD, Dager SR, Parow A, Hirashima F, Demopulos C, Stoll AL et al. Lithium and valproic acid treatment effects on brain chemistry in bipolar disorder. Biol Psychiatry 2004; 56: 340–348.
Belmaker RH, Shapiro J, Vainer E, Nemanov L, Ebstein RP, Agam G . Reduced inositol content in lymphocyte-derived cell lines from bipolar patients. Bipolar Disord 2002; 4: 67–69.
Banks RE, Aiton JF, Cramb G, Naylor GJ . Incorporation of inositol into the phosphoinositides of lymphoblastoid cell lines established from bipolar manic-depressive patients. J Affect Disord 1990; 19: 1–8.
Soares JC, Mallinger AG, Dippold CS, Forster Wells K, Frank E, Kupfer DJ . Effects of lithium on platelet membrane phosphoinositides in bipolar disorder patients: a pilot study. Psychopharmacology (Berlin) 2000; 149: 12–16.
Morain P, Lestage P, De Nanteuil G, Jochemsen R, Robin JL, Guez D et al. S 17092: a prolyl endopeptidase inhibitor as a potential therapeutic drug for memory impairment. Preclinical and clinical studies. CNS Drug Rev 2002; 8: 31–52.
Shinoda M, Miyazaki A, Toide K . Effect of a novel prolyl endopeptidase inhibitor, JTP-4819, on spatial memory and on cholinergic and peptidergic neurons in rats with ibotenate-induced lesions of the nucleus basalis magnocellularis. Behav Brain Res 1999; 99: 17–25.
Toide K, Iwamoto Y, Fujiwara T, Abe H . JTP-4819: a novel prolyl endopeptidase inhibitor with potential as a cognitive enhancer. J Pharmacol Exp Ther 1995; 274: 1370–1378.
Shishido Y, Furushiro M, Tanabe S, Nishiyama S, Hashimoto S, Ohno M et al. ZTTA, a postproline cleaving enzyme inhibitor, improves cerebral ischemia-induced deficits in a three-panel runway task in rats. Pharmacol Biochem Behav 1996; 55: 333–338.
Maes M, Goossens F, Scharpe S, Meltzer HY, D'Hondt P, Cosyns P . Lower serum prolyl endopeptidase enzyme activity in major depression: further evidence that peptidases play a role in the pathophysiology of depression. Biol Psychiatry 1994; 35: 545–552.
Maes M, Goossens F, Scharpe S, Calabrese J, Desnyder R, Meltzer HY . Alterations in plasma prolyl endopeptidase activity in depression, mania, and schizophrenia: effects of antidepressants, mood stabilizers, and antipsychotic drugs. Psychiatry Res 1995; 58: 217–225.
Breen G, Harwood AJ, Gregory K, Sinclair M, Collier D, St Clair D et al. Two peptidase activities decrease in treated bipolar disorder not schizophrenic patients. Bipolar Disord 2004; 6: 156–161.
Majerus PW . Inositol phosphate biochemistry. Annu Rev Biochem 1992; 61: 225–250.
Gould E, Gross CG . Neurogenesis in adult mammals: some progress and problems. J Neurosci 2002; 22: 619–623.
Renshaw PF, Wicklund S . In vivo measurement of lithium in humans by nuclear magnetic resonance spectroscopy. Biol Psychiatry 1988; 23: 465–475.
Andreopoulos S, Wasserman M, Woo K, Li PP, Warsh JJ . Chronic lithium treatment of B lymphoblasts from bipolar disorder patients reduces transient receptor potential channel 3 levels. Pharmacogenomics J 2004; 4: 365–373.
Wasserman MJ, Corson TW, Sibony D, Cooke RG, Parikh SV, Pennefather PS et al. Chronic lithium treatment attenuates intracellular calcium mobilization. Neuropsychopharmacology 2004; 29: 759–769.
Mellor H, Parker PJ . The extended protein kinase C superfamily. Biochem J 1998; 332(Part 2): 281–292.
Nishizuka Y . Membrane phospholipid degradation and protein kinase C for cell signaling. Neurosci Res 1992; 15: 3–5.
Wang HY, Johnson GP, Friedman E . Lithium treatment inhibits protein kinase C translocation in rat brain cortex. Psychopharmacology (Berlin) 2001; 158: 80–86.
Wang H, Friedman E . Increased association of brain protein kinase C with the receptor for activated C kinase-1 (RACK1) in bipolar affective disorder. Biol Psychiatry 2001; 50: 364–370.
Lenox RH, Watson DG, Patel J, Ellis J . Chronic lithium administration alters a prominent PKC substrate in rat hippocampus. Brain Res 1992; 570: 333–340.
Manji HK, Lenox RH . Ziskind-Somerfeld Research Award. Protein kinase C signaling in the brain: molecular transduction of mood stabilization in the treatment of manic-depressive illness. Biol Psychiatry 1999; 46: 1328–1351.
Watson DG, Lenox RH . Chronic lithium-induced down-regulation of MARCKS in immortalized hippocampal cells: potentiation by muscarinic receptor activation. J Neurochem 1996; 67: 767–777.
Pacheco MA, Jope RS . Modulation of carbachol-stimulated AP-1 DNA binding activity by therapeutic agents for bipolar disorder in human neuroblastoma SH-SY5Y cells. Brain Res Mol Brain Res 1999; 72: 138–146.
Boyle WJ, Smeal T, Defize LH, Angel P, Woodgett JR, Karin M et al. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell 1991; 64: 573–584.
Manji HK, Moore GJ, Chen G . Bipolar disorder: leads from the molecular and cellular mechanisms of action of mood stabilizers. Br J Psychiatry Suppl 2001; 41: s107–s119.
York JD, Odom AR, Murphy R, Ives EB, Wente SR . A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science 1999; 285: 96–100.
Shen X, Xiao H, Ranallo R, Wu WH, Wu C . Modulation of ATP-dependent chromatin-remodeling complexes by inositol polyphosphates. Science 2003; 299: 112–114.
Steger DJ, Haswell ES, Miller AL, Wente SR, O'Shea EK . Regulation of chromatin remodeling by inositol polyphosphates. Science 2003; 299: 114–116.
Rando OJ, Chi TH, Crabtree GR . Second messenger control of chromatin remodeling. Nat Struct Biol 2003; 10: 81–83.
Mora A, Komander D, van Aalten DM, Alessi DR . PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 2004; 15: 161–170.
Levchenko A, Iglesias PA . Models of eukaryotic gradient sensing: application to chemotaxis of amoebae and neutrophils. Biophys J 2002; 82: 50–63.
van Horck FP, Lavazais E, Eickholt BJ, Moolenaar WH, Divecha N . Essential role of type I(alpha) phosphatidylinositol 4-phosphate 5-kinase in neurite remodeling. Curr Biol 2002; 12: 241–245.
Yamazaki M, Miyazaki H, Watanabe H, Sasaki T, Maehama T, Frohman MA et al. Phosphatidylinositol 4-phosphate 5-kinase is essential for ROCK-mediated neurite remodeling. J Biol Chem 2002; 277: 17226–17230.
Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD . A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2001; 2: 287–293.
Sanai N, Tramontin AD, Quinones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 2004; 427: 740–744.
van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH . Functional neurogenesis in the adult hippocampus. Nature 2002; 415: 1030–1034.
Williams R, Ryves JW, Dalton EC, Eickholt B, Shaltiel G, Agam G et al. A molecular cell biology of lithium. Biochem Soc Trans 2004; 32: 799–802.
Harrison PJ . The neuropathology of primary mood disorder. Brain 2002; 125: 1428–1449.
Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA . Convergent evidence for impaired AKT1-GSK3β signaling in schizophrenia. Nat Genet 2004; 36: 131–137.
Acknowledgements
AJH is supported by a Wellcome Senior Biomedical Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harwood, A. Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. Mol Psychiatry 10, 117–126 (2005). https://doi.org/10.1038/sj.mp.4001618
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001618
Keywords
This article is cited by
-
SPIRAL MRI for in vivo lithium-7 imaging: a feasibility study in mice after oral lithium treatment
Scientific Reports (2024)
-
Antimicrobial, antioxidant, and sun protection potential of the isolated compounds from Spermacoce princeae (K. Schum)
BMC Complementary Medicine and Therapies (2023)
-
Metabolomic profiling relates tianeptine effectiveness with hippocampal GABA, myo-inositol, cholesterol, and fatty acid metabolism restoration in socially isolated rats
Psychopharmacology (2022)
-
Lithium as a Neuroprotective Agent for Bipolar Disorder: An Overview
Cellular and Molecular Neurobiology (2022)
-
Entangled radicals may explain lithium effects on hyperactivity
Scientific Reports (2021)