Abstract
Cocaine abusers remain vulnerable to drug craving and relapse for many years after abstinence is achieved. We have recently shown that ondansetron (a 5-HT3 receptor antagonist) given 3.5 h after each daily cocaine injection reverses previously established behavioral sensitization. The purpose of the present investigation was two-fold. First, as cocaine cannot be used as therapy, we examined whether pergolide (a D1/D2 receptor agonist with reduced abuse potential) and ondansetron could reverse behavioral sensitization. Second, we investigated whether these behavioral changes were associated with parallel alterations in expression levels and/or phosphorylation changes in the NR2B and GluR1 subunits of the respective NMDA and AMPA receptors. Rats were injected for 5 consecutive days with cocaine or saline followed by 9 days of withdrawal. Starting on withdrawal day 10, animals were given vehicle, pergolide/saline, or pergolide/ondansetron for 5 consecutive days. Following a second 9-day period of withdrawal, all animals were challenged with cocaine for assessment of behavioral sensitization and tissues were collected on the following day for Western blot. Sensitization was associated with increased NR2B expression in the accumbens (NAc) shell and decreased Tyr1472 phosphorylation in the NAc core, as well as increased Ser845 phosphorylation of the GluR1 subunit in prefrontal cortex, NAc core, and shell. Pergolide/ondansetron treatment, but not pergolide alone, consistently reversed both the behavioral sensitization and the associated changes in the NMDA and AMPA receptor subunits. To the extent that sensitization plays a role in chronic cocaine abuse, a combination of these clinically available drugs may be useful in treatment of the disorder.
Similar content being viewed by others
INTRODUCTION
Cocaine dependence is a common and serious condition, and is a substantial public health problem. Total abstinence from cocaine is very difficult to accomplish in the clinic mainly because, even after abstinence is achieved, cocaine addicts remain vulnerable for years to episodes of craving and relapse triggered by stimuli previously associated with drug abuse (Gawin and Ellinwood, 1988; Wolf, 2002; Cornish and O'Brien, 1996). To date, no pharmacological treatment is available that can consistently lead to long-term cocaine abstinence. In rodents, repeated administration of cocaine results in a progressive and enduring augmentation of locomotor and stereotyped behaviors. This response is termed behavioral sensitization and it has long been considered a model of the intensification of cocaine craving in humans that characterizes addiction and promotes relapse (Wolf, 1998; Robinson and Berridge, 1993). We have previously demonstrated in rats that cocaine sensitization established by seven daily injections and a subsequent 7-day withdrawal period can be reversed by a second series of daily cocaine injections if each of these injections is followed 3.5 h later with the 5-HT3 antagonist ondansetron (Davidson et al, 2002). Furthermore, the same reversal regimen can decrease the breaking point under a progressive-ratio self-administration paradigm (Davidson et al, 2002) and reduce the cocaine intake under an oral administration paradigm (Davidson et al, 2004). Hence, blockade of 5-HT3 receptors during the acute cocaine withdrawal period (ie, a few hours after cocaine administration that includes the reduction in the acute pharmacological effects of the drug and the onset of withdrawal responses) appears to be sufficient to reverse behavioral sensitization or self-administration in rodents. However, the neurochemical changes accompanying the ondansetron-induced behavioral-reversal have not been previously determined.
Pharmacological and behavioral studies reveal an association between NMDA receptor-mediated events and behavioral sensitization to cocaine (Gainetdinov et al, 2001). In this regard, Karler et al (1989) have demonstrated that the development of sensitization in mice is prevented when MK-801, a noncompetitive NMDA receptor antagonist, is administered 30 min before each injection of cocaine. These studies suggest an association between behavioral sensitization to cocaine and NMDA receptor activation. More recently, chronic cocaine treatment has been reported to alter the expression of NMDA receptor subunits during withdrawal in a region- and time-dependent manner (Fitzgerald et al, 1996; Loftis and Janowsky, 2002, 2003). These changes may be mediated by alterations in the expression of various NMDA receptor subunits and/or post-translational changes owing to phosphorylation of the subunits (Smart 1997; Kalluri and Ticku, 1999; Westphal et al, 1999).
The NMDA receptor is a ligand-gated ion channel composed of the core NR1 subunit and the regulatory NR2 (A–D) subunits. Individual NMDA receptor subunits and splice variant combinations possess different receptor functions (Loftis and Janowsky, 2003; Hollmann and Heinemann, 1994). The NR2B subunit is one of the major NMDA regulatory subunits in the forebrain (Nakanishi, 1992; Seeburg, 1993; Omkumar et al, 1996) and midbrain (Ungless et al, 2001; Loftis and Janowsky, 2003; Hollmann and Heinemann, 1994), regions known to mediate cocaine responses. The NR2B subunit is found in the postsynaptic density fraction and its activity is regulated through phosphorylation of the Tyr1472 residue (Loftis and Janowsky, 2003; Moon et al, 1994; Nakazawa et al, 2001).
Activation of NMDA receptors also appears to enhance the sensitivity of postsynaptic neurons to glutamate stimulation through the recruitment of AMPA receptors (Clark and Cull-Candy, 2002). Systemic co-administration of AMPA receptor antagonists with cocaine has also been reported to prevent the development of behavioral sensitization as measured by locomotor activity (Li et al, 1997) or cocaine-induced stereotypy (Karler et al, 1994). Although rats that develop sensitization do not show any changes in GluR1 levels within 24 h of withdrawal, an increase in levels in the nucleus accumbens (NAc) is evident within 3 weeks of treatment (Churchill et al, 1999). Whereas this study established changes in GluR1 levels with withdrawal, it is noteworthy that the activity of the AMPA receptor can be also regulated by phosphorylation of the GluR1 subunit at the Ser831 and Ser845 residues (Lee et al, 2000; Ungless et al, 2001). The functional role of phosphorylation at these two sites has been extensively characterized, and phosphorylation at either site potentiates AMPA receptor ion channel function, albeit through separate mechanisms (Banke et al, 2000; Derkach et al, 1999). For instance, phosphorylation at Ser845 enhances AMPA currents by increasing the channel open time probability (Roche et al, 1996), whereas phosphorylation of Ser831 increases channel conductance (Derkach et al, 1999).
The purpose of the present study was several fold. First, as cocaine cannot be used in treating cocaine abusers, we tested whether or not the combination of pergolide, a D1/D2 receptor agonist, and ondansetron could reverse previously established behavioral sensitization if ondansetron is given during the acute pergolide withdrawal period (3.5 h after pergolide). Pergolide has been commonly used to treat Parkinson's disease, restless leg syndrome and other neurological disorders (Olanow et al, 2001; Staedt et al, 1998) and, to our knowledge, there have been no reports in the literature describing its abuse in these patients. This apparent lack of abuse potential may be anticipated to render this drug as a more acceptable dopamine (DA) agonist for treating cocaine abusers than cocaine itself. In addition to the behavioral outcome, we directly determined whether or not a reversal of behavioral sensitization was associated with changes in expression of the NR2B subunit and the GluR1 subunit of the respective NMDA and AMPA receptors. Finally, as phosphorylation of these subunits plays important role in the receptor functions, we examined whether altered phosphorylation state of the receptor subunits was related to behavioral sensitization and its reversal.
MATERIALS AND METHODS
Animals and Drugs
Male Sprague–Dawley rats, initially weighing 150–200 g (Charles River Laboratories, Raleigh, NC), were acclimated for 1 week in the vivarium before the experiment. Rats were housed in pairs in plastic cages in a humidity- and temperature-controlled room on a 12-h light/dark cycle (lights on 0700–1900 hours); lab chow and water were provided ad libitum. All experiments were conducted with an approved protocol from the Duke University Institutional Animal Care and Use Committee and were performed in accordance with NIH guidelines (NIH publication 865–23). Cocaine HCI (NIDA, Bethesda, MD) was dissolved (20 mg/ml) in 0.9% sterile saline. Ondansetron hydrochloride dihydrate (from the Pharmacy at Duke) stock solution was further diluted with 0.9% saline before injections (0.2 mg/ml). Pergolide (Sigma, St Louis, MO) was dissolved into 10% DMSO at 1 mg/ml and diluted 1:10 before use.
Experimental Groups
One group of rats received cocaine (7.5 mg/kg, s.c.) on day 1 and was ranked according to their behavioral responses to cocaine. Animals were evenly divided among three treatment groups (n=12–15 rats/group). All three groups received cocaine (40 mg/kg/day, s.c., at 0930hours) for 4 consecutive days and then underwent withdrawal for 9 days (Table 1). Another group of rats was randomly assigned to three groups (n=18–22 rats/group) and received 5 consecutive days of saline injections followed by 9 days of withdrawal. On day 10 of withdrawal, one cocaine and saline group each was given 0.1 mg/kg pergolide, followed by a saline injection (s.c.); these groups were termed C-P/S and S-P/S, respectively. A second cocaine (C-P/O) and saline group (S-P/O) received 0.1 mg/kg pergolide (s.c.) plus 0.2 mg/kg ondansetron (s.c.). A third cocaine (C-D/S) and saline group (S-D/S) received parallel vehicle (DMSO plus saline) injections. The interval between the two daily injections was 3.5 h for all groups and these treatments were given over 5 consecutive days followed by a second 9-day withdrawal period. On the second withdrawal day 10, all rats were acutely challenged with 7.5 mg/kg cocaine (i.p.).
Behavioral Measurements
Behavioral ratings (Ellinwood and Balster, 1974; see Table 2) and photobeam-breaks (ambulation) were monitored in the home cage (28 × 18 × 12 cm). Before behavioral assessment, rats were acclimated to the test room in their home cages for 30 min under normal lighting conditions. The rat cages were placed in Opto-Varimex photobeam monitors (8 × 8 beams; Columbus Instruments, Columbus, OH), and baseline activity was monitored over 15 min. Animals were injected with 7.5 mg/kg cocaine (i.p.) and immediately returned to their home cages where behavior was monitored over the next 60 min. For behavioral ratings, behaviors were evaluated at 5-min intervals for 20 s with 10 s elapsing between observations for separate cages.
Brain Dissection and Protein Extraction
Rats were deeply anesthetized with halothane and decapitated. Brains were rapidly removed and blocked in 1 mm coronal sections for prefrontal cortex (PFC), caudate, NAc core, NAc shell, and amygdala. Specifically, PFC samples were taken at +3.2 to +2.2 mm; caudate, NAc core and shell at +2.0 to +1.0 mm; and amygdala at −2.3 to −3.3 mm (all samples taken anterior–posterior to bregma; see Paxinos and Watson, 1986). Within the striatal section, the cortex, corpus callosum, and olfactory tubercle were removed. A diagonal cut was made above the anterior commissure, the area above this was considered caudate; in the remaining ventral section, the tissue below the commissure was sectioned with the most ventral part designated the NAc shell and the dorsal portion the NAc core. Samples were immediately frozen on dry ice, and stored at −80°C until analyses.
Tissues were homogenized in nine volumes of ice-cold homogenate buffer containing 1% sodium deoxycholate, 5 mM Tris-HCl (pH 7.4), 2 mM EDTA, 10 μg/ml aprotinin, 0.5 mM phenylmethylsulfonyl fluoride, 1 μM pepstatin A, and 0.1 mg/ml benzamidine. For phosphorylation analyses, the lysis buffer also contained phosphatase inhibitors (50 mM sodium fluoride, 50 mM sodium pyrophosphate, 20 mM 2-glycerol phosphate, 1 mM p-nitrophenyl phosphate, 2 μM microcystin LR). Samples were sonicated on ice for 10–15 s and centrifuged at 12 000g for 2–3 min at 4°C to remove large cell debris. Protein concentrations were determined by Bradford assay (1976). In preparation for Western blot, the lysate was diluted with 6 × sample buffer (48% glycerol, 6% SDS, 17.28 mM β-mercaptoethanol, 12 mM EDTA, 300 mM Tris-HCl (pH 6.8), 0.01% bromophenol blue), boiled for 5 min, and stored at −80°C until assay.
Western Blot
Samples were separated on a 7% SDS-PAGE gel and electrophoretically transferred to Immobilon-P membranes (Millipore, Bedford, MA). The membrane was rinsed with PBS and nonspecific sites were blocked by incubating the membrane with blocking buffer (PBS-0.05% Tween-20 (PBST) containing 5% nonfat dry milk) overnight at 4°C or for 3 h at room temperature. For detecting total NR2B or GluR1 levels, the membranes were incubated with primary antibody (1 : 1000 dilution) in blocking buffer for 2 h at room temperature. For detecting site-specific phosphorylation of the GluR1 subunit at the Ser845/Ser831 residues or phosphorylation of the NR2B subunit at the Tyr1472 residue, the membranes were incubated with primary antibody (Chemicon, Temecula, CA) at 1 : 1000 dilutions in the PBST buffer containing 5% BSA overnight at 4°C. The membranes were washed sequentially with PBST 4 × for 15 min and reblocked for 1 h with PBST-5% nonfat dry milk, then incubated with peroxidase-labeled secondary antibody at a 1 : 5000 dilution (Sigma, St Louis, MO) for 2 h at room temperature. The blot was washed with PBS-0.1% Tween-20 4 × for 15 min and developed with Chemiluminescent substrate (Pierce Chemical Company, Rockford, IL).
To control for loading efficiency, the blots for the GluR1, phospho-GluR1 Ser845/Ser831, NR2B and phospho-NR2B Tyr1472 subunits were stripped and reprobed with α-tubulin (Sigma). The images were scanned with Adobe photoshop (Adobe, San Jose, CA) and quantified with NIH Image J (see http://rsb.info.nih.gov). Expression of total NR2B and GluR1 and their phosphorylation levels were evaluated relative to that for α-tubulin (ie, relative density=subunit/α-tubulin levels). Background correction values were subtracted from each lane to minimize the variability across membranes.
Statistics
The behavioral and Western blot data are presented as means and standard errors of the mean. The results were analyzed with the Statistical Package for the Social Sciences, Version 14.0 (Chicago, IL). The behavioral results were analyzed by repeated-measures ANOVA (RMANOVA) and the interactive term was evaluated by Bonferroni corrected pair-wise comparisons. When the behavioral results were collapsed over the 1 h period following cocaine challenge, a univariate ANOVA was used to examine the results. For Western bots, each set of relative density results for receptor subunits was first normalized to the mean of the S-D/S group before statistical analysis. A univariate ANOVA was applied to determine statistically significant differences between treatment groups for the Western blot experiments. Post hoc multiple comparisons for these results were evaluated by Tukey test. A p<0.05 was considered statistically significant.
RESULTS
Reversal of Behavioral Sensitization by Pergolide/Ondansetron
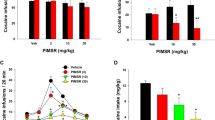
In the present study, animals were given saline or cocaine for 5 days, followed by a 9-day withdrawal period (Table 1) to establish behavioral cocaine sensitization. Animals were then administered DMSO/saline (D/S), pergolide/saline (P/S), or pergolide/ondansetron (P/O) for 5 days and another 9-day withdrawal period was imposed. Subsequently, behavioral sensitization was assessed by locomotor activity and behavioral rating scores following acute challenge with 7.5 mg/kg cocaine (i.p.) for all animals. When 15-min baseline ambulatory activity was analyzed by RMANOVA, no statistical differences among the groups were discerned (Figure 1a and b). The response to cocaine challenge was next evaluated. Comparison of the response of the three saline controls groups (eg, S-D/S, S-P/S, and S-P/O) to 7.5 mg/kg cocaine at the end of the experiment with RMANOVA found no significant main effects of the time or time by treatment interaction (Figure 1a). This result indicates that prior treatment with saline, DMSO, pergolide, or ondansetron does not differentially influence the cocaine response for the three saline-control groups. Moreover, the response to 7.5 mg/kg cocaine on day 1 of the study for the cocaine groups (eg C-D/S, C-P/S, and C-P/O groups: 367±64 ambulations) was not statistically different from that of the S-D/S group given 7.5 mg/kg cocaine (393±83 ambulations) at the end of the experiment. Hence, the S-D/S, S-P/S, and S-P/O groups were combined into a single control group and compared to the three cocaine groups: C-D/S, C-P/S, and C-P/O (Figure 1b). A RMANOVA revealed significant main effects of time (F(11,1144)=11.3, p<0.001) and a time by treatment interaction (F(33,1144)=2.07, p<0.001). Bonferroni corrected pair-wise comparisons of the interaction revealed that ambulations were higher in C-D/S animals compared to the collapsed saline control group at 10–35 and 55 min following cocaine injection (p's <0.001–0.018). Although rats treated with C-P/S exhibited fewer ambulations than C-D/S animals at 10 (p<0.014) and 20 min (p<0.036), their responses were still higher than the saline-control groups at 25–45 min (ps <0.003–0.015). Ambulations in C-P/O-treated animals did not differ from saline control rats at any time point and were lower than those of C-P/S rats at 35 and 45 min (both p<0.036). The ambulation data were collapsed over the 1 h period following cocaine challenge (Figure 1c) and a univariate ANOVA revealed significant main effects of treatment (F(3,104)=6.63, p<0.001). Bonferroni comparisons between all treatment conditions demonstrated that C-D/S rats had significantly higher ambulation levels than saline controls (p<0.001) or C-P/O rats (p<0.035) but not C-P/S rats. Ambulation levels of C-P/S rats tended to be enhanced compared to those of saline controls (p<0.071) but did not differ from those of C-P/O animals. No differences between C-P/O and saline groups were discerned. Collectively, these findings show that, while pergolide alone exerted some influence, the combined pergolide and ondansetron treatment produced the more consistent and robust reversal of behavioral sensitization to cocaine.
Effects of various treatments on previously established cocaine sensitization. (a, b) Ambulatory activities assessed during baseline and the first hour immediately following cocaine injection. Rats were administered 7.5 mg/kg cocaine (i.p.) and immediately returned to the open field for 1 h. (c) Cumulative ambulations over the 1 h of cocaine challenge. (d, e) Corresponding behavioral rating scores for stereotypy assessed during baseline and immediately after cocaine injection for 1 h. (f) Cumulative behavioral rating score over the 1 h of cocaine challenge. The arrows in panels a, b, d, and e denote when cocaine was given. See Table 1 for description of the treatment conditions (n=12–22 rats per treatment group). Control=S-D/S, S-P/S, and S-P/O groups that were collapsed into a single group because they were not statistically different from each other. *, p<0.001 & **, p<0.009 vs combined saline controls; #, p<0.035 (panel c) or p<0.001 (panel f) vs C-D/S group; +, p<0.015 vs C-P/S group, Bonferroni pair-wise comparisons.
As psychostimulant exposure can serve to restrict the behavioral repertoire of animals in the open field, such that locomotor activity is supplanted by stereotypy (Lyon and Randrup, 1972), we also rated the animal's responses to the cocaine challenge. As with baseline ambulations above, no statistical differences in basal behavioral rating scores among the groups were noted (Figure 1d and e). When the response to cocaine challenge was analyzed by RMANOVA, no significant differences among the S-D/S, S-P/S, and S-P/O treatments were observed (Figure 1d). These three groups were collapsed into a single control group and the data were reanalyzed with respect to the three cocaine groups (C-D/S, C-P/S, and C-P/O; Figure 1e). RMANOVA revealed significant main effects of time (F(11,1144)=31.9, p<0.001) and a significant time by treatment interaction (F(33,1144)=2.04, p<0.001). Bonferroni corrected pair-wise comparisons demonstrated that C-D/S rats had significantly higher behavioral scores than saline control and C-P/O rats at each time point during the entire observation period after cocaine injection (p's <0.001–0.002). C-P/S-treated rats also had higher rating scores than saline controls at 15–50 min (p's <0.001–0.034); however, these scores were less than those for C-D/S animals at 10 (p<0.018) and 20 min (p<0.023). Most importantly, scores of C-P/O rats never differed from saline controls over the entire 1 h period following cocaine challenge, and were significantly decreased relative both to C-D/S rats at all time points (p's <0.001–0.012) and to C-P/S rats at 5 (p<0.034) and 20–50 min (p's <0.001–0.012). When the behavioral ratings were collapsed over the 1 h after cocaine injection (Figure 1f) and analyzed with univariate ANOVA, a significant treatment effect was obtained (F(3,104)=11.4, p<0.001). Bonferroni comparisons demonstrated that both C-D/S and C-P/S rats had significantly higher behavioral scores than saline controls (p's <0.001 and 0.009, respectively). C-P/O rats did not differ from the controls and exhibited significantly lower ratings than those for both C-D/S (p<0.001) and C-P/S groups (p<0.015). The C-D/S and C-P/S groups did not differ from each other. Collectively, these results show that established behavioral sensitization, as assessed by both locomotor activity and behavioral ratings, is reversed when the animals are treated with pergolide followed 3.5 h later with ondansetron during the acute withdrawal period.
Pergolide/Ondansetron Effects on NR2B Subunit Expression
To determine whether the pergolide/ondansetron effects on behavioral sensitization were associated with changes in NMDA receptor function, effects of the treatments were compared on NR2B protein expression in various brain regions. Univariate ANOVA failed to reveal significant differences in levels of normalized NR2B protein in the PFC, NAc core, caudate, and amygdala among the six treatment groups (Table 3). By comparison, NR2B protein levels in the NAc shell showed significant differences among the treatment groups (F(5,44)=24.4, p<0.001). Tukey post hoc tests demonstrated that the three saline groups (S-D/S, S-P/S, and S-P/O) were not different from each other (Figure 2, left). As a result, these groups were collapsed into a single saline control group and the data were reanalyzed (Figure 2, right). Univariate ANOVA found that total NR2B levels in the NAc shell were significantly different among treatment groups (F(3,46)=38.8, p<0.001). Tukey comparisons showed that levels of the NR2B subunit were higher in the C-D/S, C-P/S, and C-P/O groups than in the saline control (all p<0.001). Importantly, concentrations of the NR2B subunit in the NAc shell were significantly lower in the animals in the C-P/O group than those in the C-D/S (p<0.019) and C-P/S (p<0.001) groups, which did not differ from each other. Taken together, these findings demonstrate that combined pergolide/ondansetron treatment can counteract the effects of chronic cocaine exposure on NR2B expression in the NAc shell.
Effects of various treatments on total NR2B levels in the NAc shell. (a) Representative Western blot of NR2B and α-tubulin expression. Each lane represents a different rat from six different groups (17.5 μg protein/lane). (b) Blots were scanned, and the relative density of total NR2B immunoreactivity to that of α-tubulin was determined for each sample. Individual density results were subsequently normalized to the S-D/S group mean. See Table 1 for descriptions of the six experimental groups (n=7–11 samples from individual rats per treatment condition). As no significant differences among the S-D/S, S-P/S, and S-P/O groups were discerned, they were collapsed into a single saline control group (Cont.) and the results analyzed against the three cocaine groups. *, p<0.001 vs saline control group; #, p<0.019 vs C-D/S group; +, p<0.001 vs C-P/S group, Tukey test.
To further relate biochemical changes to reversal of behavioral sensitization, the status of Tyr1472 phosphorylation of the NR2B subunit was examined in the PFC, NAc core, NAc shell, caudate, and amygdala (Table 4). Significant differences were only observed in the NAc core (Figure 3). As expression of phospho-NR2B protein was similar among the S-D/S, S-P/S, and S-P/O groups, the data from these treatments were collapsed into a single saline control group (Figure 3, left) and analyzed against the three cocaine treatment conditions (Figure 3, right). Univariate ANOVA revealed significant treatment effects on levels of phosphor-NR2B (F(3,33)=4.33, p<0.011), and Tukey comparisons revealed that the levels in C-D/S and C-P/S rats were reduced significantly compared to C-P/O rats (p<0.040 and 0.044). Whereas these levels were also reduced compared to that in saline controls, the differences did not reach statistical significance. Finally, the C-P/O and saline control groups were not different from each other. These findings show that pergolide/ondansetron treatment during cocaine withdrawal can normalize altered Tyr1472 phosphorylation of the NR2B subunit.
Effects of various treatments on Tyr1472-phospho-NR2B levels in the NAc core. (a) Representative Western blot of phospho-NR2B and α-tubulin expression. Each lane represents a different rat from six different groups (35 μg protein/lane). (b) Blots were scanned, and the relative density of phospho-NR2B immunoreactivity to that of α-tubulin was determined for each sample. Individual density results were subsequently normalized to the S-D/S group mean. See Table 1 for descriptions of the six experimental groups (n=6–7 samples from individual rats per treatment condition. As no significant differences among the S-D/S, S-P/S, and S-P/O groups were discerned, they were collapsed into a single saline control group (Cont) and the results analyzed against the three cocaine groups. #, p<0.040 vs C-D/S group; +, p<0.044 vs C-P/S group, Tukey test.
Pergolide/Ondansetron Effects on GluR1 Subunit Expression
To determine whether the effects of pergolide/ondansetron on reversal of behavioral sensitization are also associated with alterations in AMPA receptors, total GluR1 expression was evaluated. No significant effects among the six treatment conditions were noted in the PFC, NAc shell and core, caudate, or amygdala (Table 5). Similarly, no distinctions among the various treatment groups in the different brain regions were observed for levels of the Ser831-phospho-GluR1 subunit (data not shown). By comparison, levels of the Ser845-phospho-GluR1 subunit were increased in the PFC, NAc shell, and core (Figure 4), but not in the caudate or amygdala (Table 6). As the levels of Ser845-phospho-GluR1 were similar among the S-D/S, S-P/S, and S-P/O conditions Figure 4b, d, f, left), these data were collapsed into a single saline control group and the data were reanalyzed (Figure 4b, d, f, right). Univariate ANOVA detected significant group differences in levels of the phospho-GluR1 protein in the PFC (F(3,30)=5.31, p<0.005), NAc shell (F(3,32)=8.42, p<0.001), and core (F(3,34)=5.49, p<0.003). Tukey comparisons for PFC samples revealed that the C-P/S group exhibited significantly enhanced Ser845-phospho-GluR1 levels relative to the saline control (p<0.031) and C-P/O (p<0.030) groups, whereas the C-D/S group showed marginal enhancement compared to the saline control and C-P/O groups (p's <0.060 and 0.054, respectively). No differences were discerned between C-D/S and C-P/S rats or between saline control and C-P/O rats. In the NAc core and shell, post hoc comparisons demonstrated that Ser845-phospho-GluR1 levels were significantly enhanced in the C-D/S treatment conditions compared to the saline control (ps <0.006 and 0.001, respectively) and the C-P/O groups (p's <0.004 and 0.001). The C-D/S and C-P/S groups were not statistically different in either brain area. Together, these results show that pergolide/ondansetron treatment can consistently reverse changes in the status of Ser845 phosphorylation of the GluR1 subunit, whereas levels of the phospho-Ser831 GluR1 subunit are not modified by any of these treatment conditions.
Effects of various treatments on Ser845-phospho-GluR1 levels in the PFC and NAc core and shell. (a, c, and e) Representative Western blots of Ser845-phospho-GluR1 and α-tubulin in each brain area. Each lane represents a different rat from six different groups (PFC—28 μg protein/lane, NAc shell and core—35 μg protein/lane). (b, d, and f) Blots were scanned, and the relative density of phospho-GluR1 immunoreactivity to that of α-tubulin was determined for each sample. Individual density results for each brain area were subsequently normalized to the S-D/S group mean. See Table 1 for descriptions of the six experimental groups (n=5–7 samples from individual rats per treatment condition). As no significant differences among the S-D/S, S-P/S, and S-P/O groups were discerned for each brain area, the three groups were collapsed into a single saline control group (Cont) for statistical analyses. *, p<0.031 (panel b), p<0.006 (panel d), or p<0.001 (panel f) vs saline control group; #, p<0.004 (panel d) or p<0.001 (panel f) vs C-D/S group; +, p<0.030 vs C-P/S group.
DISCUSSION
We have previously demonstrated that behavioral sensitization to cocaine, established by 7 daily injections and a subsequent 7-day withdrawal period is reversed by combined cocaine plus ondansetron treatment if the 5-HT3 antagonist is given 3.5 h after each cocaine injection (Davidson et al, 2002). Treatment with ondansetron during this acute cocaine withdrawal period also inhibits self-administration the next day (Davidson et al, 2002, 2004). In the present study, we have substituted cocaine with a nonabused, clinically used DA agonist, pergolide (Olanow et al, 2001; Staedt et al, 1998). This direct DA agonist, given in combination with ondansetron 3.5 h later, reverses long-term behavioral sensitization to cocaine. These results suggest that daily administration of ondansetron during acute withdrawal from a DA agonist (eg, pergolide or cocaine) may provide a means of disassociating previously established relationships between long-term cocaine sensitization dynamics and the acute ‘stimulant’ effects of DA agonists. In addition to the putative lack of significant abuse potential, pergolide was selected in the present study for several reasons. First, rat studies have shown a dose similar to the one in our own experiments to reverse cocaine sensitization when given in combination with memantine (Li et al, 2000). Second, pergolide can substitute for cocaine in drug discrimination procedures (Witkin et al, 1991). Finally, the drug can activate DA-mediated behaviors (increased locomotor activity or contralateral turning) in rats after unilateral 6-hydroxydopamine lesion or reserpine pretreatment (Arnt, 1985; Clemens et al, 1993). As the behavioral effects of pergolide typically last for 3–4 h in rats (eg, Clemens et al, 1993), the 0.1 mg/kg dose (i.p.) may be expected to lead to initial DA activation with subsequent acute agonist withdrawal effects within this time interval. This prediction is consistent with our present findings.
Compared to combined pergolide/ondansetron regimen, the sensitization–reversing effects of pergolide alone were not as robust as those of the pergolide/ondansetron combination. Moreover, pergolide alone was not sufficient to consistently reverse the sensitization-associated changes in NR2B or GluR1 protein expression. Although pergolide has been reported to reduce craving in hospitalized cocaine abusers during the early withdrawal period (Malcolm et al, 1991, 1994), the inability of pergolide itself to reverse the long-term NMDA and AMPA receptor subunit changes in the present report is consistent with previous studies showing that this drug is ineffective in maintaining long-term abstinence in outpatients (Malcolm et al, 2000; Levin et al, 1999). Thus, induction of an ‘acute DA withdrawal state’ and its antagonism by delayed 5-HT3 antagonist administration may provide a potential maintenance treatment strategy for achieving long-term cocaine abstinence in chronic abusers. Previously, Li et al (2000) reported that pergolide could also reverse cocaine sensitization if the NMDA/5-HT3 antagonist memantine (Rammes et al, 2001) is given before the DA agonist. It is noted, however, that while both ondansetron and memantine are approved for the clinic (eg, anti-emetic and anti-Parkinson treatments, respectively), ondansetron produces much fewer and less serious side effects (eg, constipation) than memantine and other NMDA antagonists (eg, hallucinations and nighmares). The combination of ondansetron and pergolide (or other mixed D1/D2 agonists) may be associated with an improved therapeutic outcome in cocaine abusers owing to reduced side effects and increased therapeutic compliance. In future studies, we plan to evaluate whether the efficacy of the pergolide/ondansetron regimen can be further enhanced through changes in pergolide dose and/or by altering the time interval between the two drugs.
Increased glutamatergic neurotransmission in the NAc and ventral tegmental area have been proposed as a mechanism underlying behavioral sensitization to repeated cocaine administration (Churchill et al, 1999). Considerable evidence supports a role for NMDA receptors in mediating behavioral, electrophysiological, and neurochemical responses to cocaine (Pierce and Kalivas, 1997; Smith et al, 1993; Nestler et al, 1993). Repeated cocaine administration is reported to cause region-specific and time-dependent alterations in NR2B expression during withdrawal (Loftis and Janowsky, 2002; Yamaguchi et al, 2002; Scheggi et al, 2002; Fitzgerald et al, 1996). Our findings support the idea for changes in the NAc shell, as NR2B expression was upregulated by ∼100% in this brain region in cocaine-treated rats up to 3 weeks after cocaine withdrawal (ie C-D/S vs S-D/S groups). These results suggest that regionally specific long-term changes in NMDA receptor protein expression may contribute to difficulties encountered with the reversal of behavioral sensitization. Importantly, we have demonstrated that the combined pergolide plus ondansetron treatment can reverse not only previously established behavioral cocaine sensitization in rats but also the associated increases in the total NR2B expression in the NAc shell.
Activity of NMDA receptors can be regulated not only by changes in subunit expression but also by alterations in the phosphorylation status of the subunits. Tyrosine phosphorylation of the NR2B subunit is important both for the regulation of its channel activity and for intracellular signaling through interaction of the receptor with SH2 domain-containing molecules (Loftis and Janowsky, 2003). These events can contribute to potentiation of receptor responses and the activation of specific signal transduction pathways. Although overall protein levels of the NR2B subunit were not changed by any treatments in the NAc core, phosphorylation of the Tyr1472 residue was selectively decreased in rats sensitized to cocaine (ie C-D/S and C-P/S groups). By comparison, no significant alteration in the phosphorylation status of the subunit was detected in other brain regions under our experimental conditions.
In the present study, we did not observe any alterations in total GluR1 expression in the NAc shell and core, PFC, caudate, or amygdala following a 23-day withdrawal period. Churchill et al (1999) also found that rats withdrawn for 21 days from chronic cocaine injections exhibited no changes in the GluR1 subunit levels, although a subgroup selected a posteriori for greater than 20% increases in the cocaine sensitivity following the cocaine injections (‘sensitized’) exhibited increases in NAc GluR1 levels. We also did not observe a similar correlation between the individual cocaine sensitivity and total GluR1 expression levels in any of the cocaine-treated animals. Overall, the present experiments and those from Churchill and co-workers suggest that cocaine sensitization may not be associated with robust and consistent long-term changes in total GluR1 levels in the brain.
In contrast to total protein levels, cocaine sensitization was associated with a selective increase in Ser845 phosphorylation of GluR1 subunit in the PFC, NAc core, and NAc shell (compared to the Ser831 residue). Furthermore, reversal of behavioral sensitization was accompanied by normalization of these phophorylation changes. Other investigators have also reported changes in phosphorylation status of the GluR1 subunit during behavioral sensitization. Thus, phosphorylation of the Ser845, but not the Ser831 residue, is significantly enhanced in the PFC and NAc by cocaine treatment (Snyder et al, 2000; Wolf et al, 2003). Phosphorylation on the GluR1 Ser845 residue is mediated primarily through protein kinase A activation (Roche et al, 1996). This alteration may lead to changes at glutamate synapses in the NAc as D1 stimulation enhances phosphorylation of GluR1 subunit at a PKA site and increases surface expression of the subunit (Wolf et al, 2003; also see above). Recently, Kalivas et al (2005) have suggested that drug addiction may be associated with a shift in signaling through DA receptors such that D1 receptor signaling predominates over D2 receptor-mediated events. Thus, the enhanced phosphorylation of Ser845 on the GluR1 subunit may reflect augmented activation of protein kinase A through the enhanced D1 neurotransmission.
There may be various ways by which 5-HT3 receptor blockade during acute cocaine or pergolide withdrawal can lead to reversal of previously established cocaine sensitization. Acute cocaine withdrawal is often associated with symptoms of anhedonia, anergia, depression, and anxiety in human cocaine abusers (Gawin and Ellinwood, 1988), and the intensity of these aversive withdrawal symptoms is a strong predictor of poor treatment outcome (Mulvaney et al, 1999; Kampman et al, 2001). In animals, Koob and Le Moal (2001) have suggested that aversive ‘allosteric dysregulation’ during repeated acute cocaine withdrawal may contribute to the maintenance of long-term cocaine sensitization and abuse. To the extent that the 5-HT3 receptor system can directly or indirectly (eg, via substance P) play a major role in various aversive responses (Gavioli et al, 1999), specific 5-HT3 blockade during DA withdrawal may lead to disruption of the neural mechanisms underlying repeated cocaine abuse. Anatomically, D1 and 5-HT3 receptors in the PFC are codistributed in the inhibitory GABA interneurons (Vincent et al, 1995; Jakab and Goldman-Rakic, 1998), where 5-HT3 stimulation can rapidly activate these inhibitory neurons under adverse conditions (Jakab and Goldman-Rakic, 2000). In addition, 5HT3 terminal receptors have been shown to mediate local stimulatory actions of 5-HT on DA release in the medial PFC (Chen et al, 1992), striatum (Blandina et al, 1989), and NAc (De Deurwaerdere et al, 1998). Thus, modulations of the DA–5-HT3 interactions in the PFC, NAc core, and shell may form a basis for reversal of the sensitization-associated behavioral and NMDA/AMPA subunit changes by a pergolide/ondansetron treatment regimen.
In conclusion, the present study demonstrates that long-term behavioral sensitization to cocaine, established via chronic injections followed by a chronic withdrawal period, is associated with region-specific alterations in NR2B subunit expression and phosphorylation, as well as, changes in the phosphorylation status of the GluR1 subunit. Importantly, combined pergolide/ondansetron treatment was sufficient to reverse not only the established behavioral sensitization to cocaine but also the associated alterations in NR2B and GluR1 subunits of the respective NMDA and AMPA receptors. To the extent behavioral sensitization contributes to chronic cocaine abuse in humans, a combined pergolide/ondansetron treatment regimen may provide a means of long-term maintenance treatment for cocaine abusers.
References
Arnt J (1985). Behavioural stimulation is induced by separate dopamine D-1 and D-2 receptor sites in reserpine-pretreated but not in normal rats. Eur J Pharmacol 113: 79–88.
Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF (2000). Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci 20: 89–102.
Blandina P, Goldfarb J, Craddock-Royal B, Green JP (1989). Release of endogenous dopamine by stimulation of 5-hydroxytruptamine 3 receptors in rat stiatum. J Pharmacol Exp Ther 251: 803–809.
Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254.
Chen J, Paredes W, Van Praag HM, Lowinson JH. Gardner EL (1992). Presynaptic dopamine release is enhanced by 5-HT3 receptor activation in medial prefrontal cortex of freely moving rats. Synapse 10: 264–266.
Churchill L, Swanson CJ, Urbina M, Kalivas PW (1999). Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J Neurochem 72: 2397–2403.
Clark BA, Cull-Candy SG (2002). Activity-dependent recruitment of extrasynaptic NMDA receptor activation at an AMPA receptor-only synapse. J Neurosci 22: 4428–4436.
Clemens JA, Okimura T, Smalstig EB (1993). Dopamine agonist activities of pergolide, its metabolites and bormocriptine as measured by prolactin inhibition, compulsive turning and stereotypic behavior. Arzneimittel-Forschung 43: 281–286.
Cornish JW, O'Brien CP (1996). Crack cocaine abuse: an epidemic with many public health consequences. Annu Rev Public Health 17: 259–273.
Davidson C, Lazarus C, Lee TH, Ellinwood EH (2004). Ondansetron, given during the acute cocaine withdrawal, attenuates oral cocaine self-administration. Eur J Pharm 503: 99–102.
Davidson C, Lee TH, Xiong Z, Ellinwood EH (2002). Ondansetron given either in the acute or chronic withdrawal from repeated cocaine sensitization dosing regimens reverses the expression of sensitization and inhibits self-administration. Neuropsychopharmacology 27: 542–553.
De Deurwaerdere P, Stinus L, Spampinato U (1998). Opposite change of in vivo dopamine release in the rat nucleus accumbens and striatum that follows electrical stimulation of dorsal raphe nucleus: role of 5-HT3 receptors. J Neurosci 18: 6528–6538.
Derkach V, Barria A, Soderling TR (1999). Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA 96: 3269–3274.
Ellinwood EH, Balster RL (1974). Rating the behavioral effects of amphetamine. Eur J Pharmacol 28: 35–41.
Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ (1996). Drugs of abuse and stress increase the expression of gluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: common adaptation among cross-sensitizing agents. J Neurosci 16: 274–282.
Gainetdinov RR, Mohn AR, Bohn LM, Caron MG (2001). Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc Natl Acad Sci USA 98: 11047–11054.
Gavioli EC, Canteras NS, DeLima TC (1999). Amxiogenic-like effects induced by Substance P injected into the lateral septal nucleus. Neuro Report 10: 3399–3403.
Gawin FH, Ellinwood EH (1988). Cocaine and other stimulants. Actions, abuse, and treatment. N Engl J Med 318: 1173–1182.
Hollmann M, Heinemann S (1994). Cloned glutamate receptors. Annu Rev Neurosci 17: 31–108.
Jakab RL, Goldman-Rakic PS (1998). 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc Natl Acad Sci USA 95: 735–740.
Jakab RL, Goldman-Rakic PS (2000). Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol 417: 337–348.
Kalivas PW, Volkow N, Seamans J (2005). Unmanageable motivation in addiction: a path in prefrontal-accumbens glutamate transport. Neuron 45: 647–650.
Kalluri SG, Ticku MK (1999). Potential involvement of tyrosine kinase pathway in the antagonist induced up regulation of the NMDA receptor NR2B subunit in cortical neurons. Mol Brain Res 65: 206–210.
Kampman KM, Alterman AI, Volpicelli VR, Maany I, Muller ES, Luce DD et al (2001). Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychol Addict 15: 52–59.
Karler R, Calder LD, Bedingfield JB (1994). Cocaine behavioral sensitization and the excitatory amino acids. Psychopharmacology 115: 305–310.
Karler R, Calder LD, Chaudhry IA, Turkanis SA (1989). Blockade of ‘reverse tolerance’ to cocaine and amphetamine by MK-801. Life Sci 45: 599–606.
Koob GF, Le Moal M (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24: 97–129.
Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL (2000). Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405: 955–959.
Levin FR, McDowell D, Evans SM, Brooks D, Spano C, Nunes EV (1999). Pergolide mesylate for cocaine abuse: a controlled preliminary trial. Am J Addict 8: 120–127.
Li Y, White FJ, Wolf ME (2000). Pharmacological reversal of behavioral and cellular indices of cocaine sensitization in the rat. Psychopharmacology 151: 175–183.
Li Y, Wolf ME, White FJ (1997). Co-administration of the NMDA receptor antagonist MK-801 with the dopamine D2 receptor agonist quinpirole reverses behavioral sensitization to cocaine. Soc Neurosci Abstr 23: 261–271.
Loftis JM, Janowsky A (2002). Cocaine treatment- and withdrawal-induced alterations in the expression and serine phosphorylation of the NR1 NMDA receptor subunit. Psychopharmacology 164: 349–359.
Loftis JM, Janowsky A (2003). The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther 97: 55–85.
Lyon M, Randrup A (1972). The dose–response effect of amphetamine upon avoidance behaviour in the rat seen as a function of increasing stereotypy. Psychopharmacologia 23: 334–347.
Malcolm R, Hutto BR, Phillips JD, Ballenger JC (1991). Pergolide mesylate treatment of cocaine withdrawal. J Clin Psychiatry 52: 39–40.
Malcolm R, Kajdasz DK, Herron J, Anton RF, Brady KT (2000). A double-blind, placebo-controlled outpatient trial of pergolide for cocaine dependence. Drug Alcohol Depend 60: 161–168.
Malcolm R, Moore JW, Kajdasz DK, Ballenger JC (1994). A comparison of pergolide and bromocriptine in the initial rehabilitation of cocaine dependence. Am J Addict 3: 144–147.
Moon IS, Apperson ML, Kennedy MB (1994). The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-D-aspartate receptor subunit 2B. Proc Natl Acad Sci USA 91: 3954–3958.
Mulvaney FD, Alterman AI, Boardman CR, Kampman K (1999). Cocaine abstinence symptomatology and treatment attrition. J Sub Abuse Treat 16: 129–135.
Nakanishi S (1992). Molecular diversity of glutamate receptors and implications for brain function. Science 258: 597–603.
Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K et al (2001). Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem 276: 693–699.
Nestler EJ, Hope BT, Widnell KL (1993). Drug addiction: a model for the molecular basis of neural plasticity. Neuron 11: 995–1006.
Olanow CW, Watts RL, Koller WC (2001). An algorithm (decision tree) for the management of Parkinson's disease (2001): treatment guidelines. Neurology 56(Suppl 5): S1–S88.
Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB (1996). Identification of a phosphorylation site for calcium/calmodulin dependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem 271: 31670–31678.
Paxinos G, Watson C (1986). The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego, CA.
Pierce RC, Kalivas PW (1997). A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev 25: 192–216.
Rammes G, Rupprecht R, Ferrari U, Zieglgansberger W, Parsins CG (2001). The N-methyl-D-asparate receptor channel blockers memantine, MRZ2/579 and other amino-alkyl-cyclohexanes antagonize 5-HT3 receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neurosci Ltr 306: 81–84.
Robinson TE, Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev 18: 247–291.
Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL (1996). Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16: 1179–1188.
Scheggi S, Mangiavacchi S, Masi F, Gambarana C, Tagliamonte A, Montis MGDE (2002). Dizocilpine infusion has a different effect in the development of morphine and cocaine sensitization: behavioral and neurochemical aspects. Neuroscience 109: 267–274.
Seeburg PH (1993). The molecular biology of mammalian glutamate receptor channels. Trends Neurosci 16: 359–365.
Smart TG (1997). Regulation of excitatory and inhibitory neurotransmitter-gated ion channels by protein phosphorylation. Curr Opin Neurobiol 7: 358–367.
Smith DA, Browning M, Dunwiddie TV (1993). Cocaine inhibits hippocampal long-term potentiation. Brain Res 608: 259–265.
Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC et al (2000). Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci 20: 4480–4488.
Staedt J, Huneriager H, Ruther E, Stoppe G (1998). Pergolide: treatment of choice in restless legs syndrome (RLS) and nocturnal Myoclonus syndrome (NMS). Longterm follow up on pergolide. J Neural Transm 105: 265–268.
Ungless MA, Whistler JL, Malenka RC, Bonci A (2001). Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411: 583–587.
Vincent SL, Khan Y, Benes FM (1995). Cellular colocalization of dopamine D1 and D2 receptors in rat medial prefrontal cortex. Synapse 19: 112–120.
Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser IDC, Langeberg LK (1999). Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science 285: 93–96.
Witkin JM, Nichols DE, Terry P, Katz JL (1991). Behavioral effects of selective dopaminergic compounds in rats discriminating cocaine injections. J Pharmacol Exp Ther 257: 706–713.
Wolf ME (1998). The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol 54: 679–720.
Wolf ME (2002). Addiction: making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv 2: 146–157.
Wolf ME, Mangiavacchi S, Sun X (2003). Mechanisms by which dopamine receptors may influence synaptic plasticity. Ann NY Acad Sci 1003: 241–249.
Yamaguchi M, Suzuki T, Abe S, Hori T, Kurita H, Asada T et al (2002). Repeated cocaine administration differentially affects NMDA receptor subunit (NR1, NR2A-C) mRNAs in rat brain. Synapse 46: 157–169.
Acknowledgements
We are grateful to Dr Qiang Chen and Mrs Xueying Xiong for their excellent technical assistance. We also acknowledge the statistical assistance from Dr Ramona M Rodriguiz in the Department of Psychiatry and Behavioral Sciences at the Duke University Medical Center. This study was supported by grants from the National Institute of Drug Abuse (DA-10327 and DA-14323 to EHE and DA-06519 to THL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Lee, T., Davidson, C. et al. Reversal of Cocaine-Induced Behavioral Sensitization and Associated Phosphorylation of the NR2B and GluR1 Subunits of the NMDA and AMPA Receptors. Neuropsychopharmacol 32, 377–387 (2007). https://doi.org/10.1038/sj.npp.1301101
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301101
Keywords
This article is cited by
-
Molecular changes in the medial prefrontal cortex and nucleus accumbens are associated with blocking the behavioral sensitization to cocaine
Scientific Reports (2015)
-
Secretory leukocyte protease inhibitor is a proliferation and survival factor for pancreatic cancer cells
Clinical and Translational Oncology (2015)
-
Cocaine-Induced Changes in NMDA Receptor Signaling
Molecular Neurobiology (2014)
-
Ionic Regulation of Cell Volume Changes and Cell Death after Ischemic Stroke
Translational Stroke Research (2014)
-
Restoration of klotho expression induces apoptosis and autophagy in hepatocellular carcinoma cells
Cellular Oncology (2013)