-
PDF
- Split View
-
Views
-
Cite
Cite
Fei Luo, Zhonglan Zou, Xinlu Liu, Min Ling, Qingling Wang, Qi Wang, Lu Lu, Le Shi, Yonglian Liu, Qizhan Liu, Aihua Zhang, Enhanced glycolysis, regulated by HIF-1α via MCT-4, promotes inflammation in arsenite-induced carcinogenesis, Carcinogenesis, Volume 38, Issue 6, June 2017, Pages 615–626, https://doi.org/10.1093/carcin/bgx034
Close - Share Icon Share
Abstract
Arsenite is well established as a human carcinogen, but the molecular mechanisms leading to arsenite-induced carcinogenesis are complex and elusive. Accelerated glycolysis, a common process in tumor cells called the Warburg effect, is associated with various biological phenomena. However, the role of glycolysis induced by arsenite is unknown. We have found that, with chronic exposure to arsenite, L-02 cells undergo a metabolic shift to glycolysis. In liver cells exposed to arsenite, hypoxia inducible factor-1α (HIF-1α) and monocarboxylate transporter-4 (MCT-4) are over-expressed. MCT-4, directly mediated by HIF-1α, maintains a high level of glycolysis, and the enhanced glycolysis promotes pro-inflammatory properties, which are involved in arsenite carcinogenesis. In addition, serum lactate and cytokines are higher in arsenite-exposed human populations, and there is a positive correlation between them. Moreover, there is a positive relationship between lactate and cytokines with arsenic in hair. In sum, these findings indicate that MCT-4, mediated by HIF-1α, enhances the glycolysis induced by arsenite. Lactate, the end product of glycolysis, is released into the extracellular environment. The acidic microenvironment promotes production of pro-inflammatory cytokines, which contribute to arsenite-induced liver carcinogenesis. These results provide a link between the induction of glycolysis and inflammation in liver cells exposed to arsenite, and thus establish a previously unknown mechanism for arsenite-induced hepatotoxicity.
Introduction
Arsenic, a common metalloid toxicant contaminating groundwater, is a global concern (1). Inorganic arsenic, which is toxic acutely and chronically, is classified as a carcinogen by the International Agency for Research on Cancer (2). Chronic exposure to arsenicals has been associated with diabetes mellitus, black-foot disease, skin lesions, and liver and kidney cancer (1). Arsenite induces neoplastic transformation of cultured human cells, and such models have been used to investigate the mechanisms of arsenite-induced carcinogenesis (3). However, the mechanisms by which arsenite induces disease remain poorly understood.
For production of ATP, most cancer cells display upregulation of glycolysis. This phenomenon, discovered 80 years ago, is termed the ‘Warburg effect’ (4). A change in glucose metabolism has been implicated as a contributor to malignant progression (5). However, why this glycolysis occurs, and whether it is a cause or a consequence, has remained a matter of debate. In our previous study, we found that acute exposure of cells to arsenite induces glycolysis (6), suggesting that this process is associated with arsenite carcinogenesis. The potential for environmental exposures to contribute to carcinogenesis via altered energy metabolism has been reviewed, but there are as yet few examples of such a mechanism (7).
Chronic inflammation is believed to promote tumorigenesis, and the link between inflammation and cancer is supported by clinical studies (8). The pro-inflammatory cytokines, interleukin (IL)-6 and 8, and tumor necrosis factor alpha (TNF-α) are also involved in the microenvironment of tumorigenesis (9). In macrophages, metabolic intermediates have been suggested to regulate IL-1β production. For example, succinate drives production of IL-1β, and 2-deoxyglucose (2-DG), which blocks glycolysis, inhibits lipopolysaccharide and induces transcription of IL-1β and TNF-α (10). In our investigations of the molecular mechanism of arsenite carcinogenesis, we found that inflammation causally contributes to arsenite-induced malignant transformation of cells (11). However, the molecular link between glycolysis and inflammation in arsenite carcinogenesis has not been extensively evaluated.
Chronic hypobaric hypoxia up-regulates MCT-4 in rat skeletal muscle (12) and in some tumor cells (13), at least in part through a transcriptional mechanism. In COS cells, the hypoxia mimic, cobalt, also upregulates MCT-4 expression, another indicator of the role of the transcription factor, hypoxia inducible factor-1α (HIF-1α) (14). Furthermore, enhancement of MCT-4 expression under such conditions is dependent on HIF-1α, and the promoter region of the MCT-4 gene contains hypoxia response elements (HREs) to which HIF-1α can bind and activate transcription (15). In aggressive bladder cancers, monocarboxylate transporter-4 (MCT-4) is increased, consistent with the enhanced rates of glycolysis of these cancers and thus their need to export large amounts of lactic acid (16). Glycolysis is involved in the inflammation of immune cells (17), and enhanced glycolysis leads to increased formation of intracellular lactate, which is exported to the extracellular environment by MCT-4 (18). In macrophages, upregulation of MCT-4 presents a positive feedback mechanism to maintain a high glycolytic rate that is essential for a fully activated inflammatory response (18).
MCT-4 is responsible for exporting lactic acid into the extracellular microenvironment, and an acidic microenvironment promotes cytokine production (18). Chronic inflammation promotes arsenite carcinogenesis (11), providing a link from glycolysis to malignant transformation of cells through induction of inflammation. In this report, we establish that MCT-4, mediated by HIF-1α, is involved in arsenite-induced glycolysis. The results contribute to an understanding of arsenite-induced liver oncogenesis.
Materials and methods
Cell culture and reagents
Human liver L-02 cells are an immortalized, non-tumor cell line derived from normal liver tissue. Expressing a distinct ultrastructure compared to hepatic carcinoma cells, they are considered to be a model for nonmalignant liver (19). L-02 cells were obtained from the Shanghai Cell Bank of Chinese Academy of Sciences on December 14, 2013. THLE-3 cells, a line human liver cells, were obtained from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China) on September 24, 2016. Cells were maintained in 5% CO2 at 37°C in RPMI-1640 medium, supplemented with 10% fetal bovine serum (FBS, Life Technologies/Gibco, Grand Island, NY), 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies/Gibco, Gaithersburg, MD). For chronic exposure, 1 × 106 L-02 cells were seeded into 10 cm (diameter) dishes for 24 h and maintained in 0 or 2 μM sodium arsenite (NaAsO2, Sigma, St. Louis, MO; purity, 99.0%) for 48–72 h per passage. This process was continued for about 15 weeks (30 passages). 2-DG was purchased from Sigma. Human recombinant IL-6 and IL-8 were purchased from R&D Systems (Minneapolis, MN). All other reagents were of analytical grade or the highest grade available.
Quantitative real-time PCR
Total cellular RNA was isolated by use of TRIzol (Invitrogen) according to the manufacturer’s recommendations. For detection of mRNA, 2 μg of total RNA and MMLV reverse transcriptase (Promega Corp., Madison, WI) were used in reverse transcription following the manufacturer’s protocol. GAPDH was used as a control. Quantitative real-time PCR was performed with an Applied Biosystems 7300HT machine and MaximaTM SYBR Green/ROX qPCR Master Mix (Fermentas). Fold changes in expression of each gene were calculated by a comparative threshold cycle (Ct) method using the formula 2−(ΔΔCt) (20). The PCR reaction was evaluated by melting curve analysis and by checking the PCR products on 2% wt/vol agarose gels. The primers, listed in Supplementary Table 1, available at Carcinogenesis Online, were synthesized by Invitrogen.
Western blots
Protein concentrations were measured with the BCA Protein Assay according to the manufacturer’s manual (Beyotime Institute of Biotechnology, Shanghai, China). Equal amounts (80 μg) of protein were separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and were transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA). Membranes were incubated overnight at 4°C with a 1:1000 dilution of anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH, Sigma) and antibodies for HIF-1α, MCT-4, inducible nitric oxide synthase (iNOS) (Abcam), p-p65 (Ser 536) or p65 (Cell Signaling Technology, Beverly, MA). After additional incubation with a 1:1000 dilution of an anti-immunoglobin horseradish peroxidase-linked antibody for 1 h, the immune complexes were detected by enhanced chemiluminescence (Cell Signaling Technology). For densitometric analyses, protein bands on the blots were measured by the use of Eagle Eye II software.
Metabolic assays
Lactate in cell culture media and sera was measured with a lactate assay kit (BioVision, Milpitas, CA). Glucose levels were determined by use of a glucose assay kit (BioVision), and glucose consumption was calculated as the difference of glucose concentrations between the original media and media from cell cultures.
Transmission electron microscopy
Arsenite-treated L-02 cells were suspended in 50 μl of phosphate-buffered saline and fixed with 4% paraformaldehyde and 4% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, at the incubation temperature and kept at 4°C until transmission electron microscopy analysis. The samples were placed on carbon-coated copper grids and immersed in 2% phosphotungstic acid solution (pH 7.0) for 30 s. The preparations were examined with a transmission electron microscope (JEM-1200EX; JEOL Ltd., Tokyo, Japan) at an acceleration voltage of 80 kV. All visible mitochondria in arsenite-treated L-02 cells were assessed for damage semi-quantitatively based on previously published criteria (21) as follows: Score 1 = no major damage, clear inner and outer membranes with dense matrix and densely aligned cristae. Score 2 = cristae membranes are disrupted with occasional vacuolation, although the matrix largely remains dense. Inner and outer mitochondrial membranes may have separated somewhat. Score 3 = the mitochondrial matrix is largely reduced and few cristae remain. Score 4 = cristae are mostly absent. The matrix is either absent or electron-dense, and outer membrane rupture is seen in some. Score 5 = triple and quadruple membrane rings indicative of autophagy or mitophagy are present. In these evaluations, relative sizes and densities of mitochondria were not considered.
RNA interference
Transfections of L-02 and THLE-3 cells were performed with the N-TER™ and AccuTarget TMN nanoparticle siRNA Transfection System (Sigma) following the manufacturer’s protocol. Briefly, 5 × 105 cells were seeded into each well of six-well plates, 18–24 h prior to transfection. The siRNA nanoparticle formation solution was prepared by adding target gene siRNA dilutions to N-TER peptide dilutions. The preparations were incubated at room temperature for 30 min. Nanoparticle formation solution transfection medium (2 ml) containing target gene siRNA was transferred to each well of the culture plates, and, after 24 h, cells were treated and harvested for analysis. Control siRNA and MCT-4 siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Enzyme-linked immunosorbent assay (ELISA) assays
To determine the amounts of inflammatory cytokines produced by cells and the amounts in human sera, ELISA tests were performed according to the manufacturer’s instructions. Cells were plated at 1 × 106 per 100 cm2 dish. At confluency, culture supernatants were harvested, centrifuged and placed at −70°C. Mouse and human sera were preserved at −70°C. Human and mouse-specific IL-6, IL-8 and TNF-α ELISAs from Beijing 4A Biotech Co., Ltd (Beijing) were used to determine the amounts of inflammatory cytokines. All assays were performed in duplicate and repeated three times. The lower limits of detection of IL-6, IL-8 and TNF-α were 2, 7 and 4 pg/ml, respectively.
Anchorage-independent growth
Soft-agar dishes were prepared with under-layers of 0.70% agarose in RPMI-1640 medium supplemented with 10% FBS. To test their capacity for soft-agar growth, cells were plated in triplicate at a density of 1 × 104 in 2 ml of 0.35% agarose. Cultures were fed every 3 days. After 14 days, the colonies were observed under a microscope, and those with diameters >80 μm were counted. These represent colonies with >30 cells.
Transwell assays
Arsenite-transformed L-02 cells were exposed to 2 mM 2-DG or incubated with human recombinant IL-6/8 (10 ng/ml) for 24 h or were transfected with 20 nM of control siRNA or 10 nM of MCT-4 siRNA for 24 h, then incubated with human recombinant IL-6/8 (10 ng/ml) for 24 h. Then, 5 × 104/100 μl cells, in serum-free medium, were plated onto the upper chambers. RPMI-1640 medium containing 10% FBS was added to the lower chamber as a chemoattractant. After incubation for 24 h at 37°C, non-migrating cells were removed with cotton swabs. Cells that migrated to the bottom of the membrane were fixed with 4% paraformaldehyde, stained with crystal violet staining solution for 30 min, and washed twice with phosphate-buffered saline. Stained cells were visualized under a microscope (high-power field), and the numbers of cells counted in five random fields were averaged. To assess the capacity of arsenite-transformed HBE cells for invasion, 5 × 104/100 µl transfected cells were added to upper chambers that had been coated with 35 μl of Matrigel (BD Biosciences, Franklin Lakes, NJ). MEM medium containing 10% FBS was added to the lower chamber. Cells were incubated for 24 h at 37°C, and then non-invading cells were removed with cotton swabs. Invading cells were fixed, stained and counted.
Luciferase activity assay
The pGL3-MCT-4-Luc construct was purchased from Genechem (Shanghai, China). The plasmid phRL-tk (used as an internal control for transfection efficiency and for cytotoxicity of test chemicals) containing the Renilla luciferase gene was purchased from Promega (Madison, WI). The cells proliferated to 60–80% confluence after 24 h of culture. Then, the cells were co-transfected with 2 μg of DNA of the reporter constructs and HIF-1α siRNA using the Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The amounts of luciferase and Renilla luciferase were measured with the Dual-Luciferase Reporter Assay System Kit (Promega) following the manufacturer’s instructions. The values of luciferase activity for each lysate were normalized to the Renilla luciferase activity. The relative transcriptional activity was converted into fold induction above the vehicle control value.
Subjects
The populations exposed to arsenic were identified according to Standard of diagnosis for endemic arsenism of China (22). All subjects resided in Xinren County and gave informed consent. Our team examined the target population in 2014, with a total of 120 villagers who had been exposed to arsenic for more than 15 years and who agreed to participate in the study. Arsenicosis was categorized based on the degree of symptoms: non-patient (internal control, n = 30), mild (n = 30), intermediate (n = 30) and severe (n = 30). Symptoms were classified according to the Chinese National Arsenicosis Diagnosis Standard protocol (23). Two control groups were included. First, non-patients (n = 30), individuals exposed to arsenic but showing no symptoms of arsenicosis, were designated as the internal control group. The external control group included 30 villagers who did not use coal containing high levels of arsenic. Inductively coupled mass spectrometry (IC-MS) was used to measure levels of arsenic in the hair of all subjects (22).
Statistical analyses
Derived values are presented as the means ± SD. Comparison of mean data among multiple groups was analyzed by one-way analysis of variance (ANOVA), and a multiple range least significant difference was used for inter-group comparisons. P values < 0.05 were considered statistically significant. All statistical analyses were performed with SPSS 16.0.
Results
Chronic arsenite exposure induces glycolysis and causes mitochondrial damage in L-02 cells
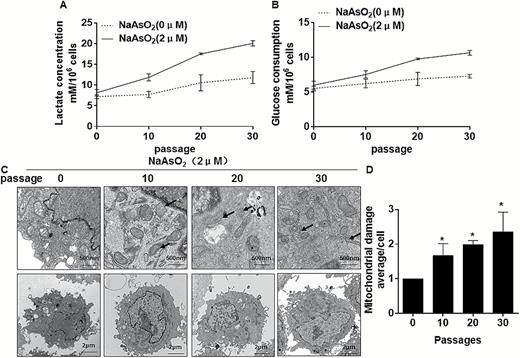
General properties of primary cancers are their high glycolytic rate and high lactate production, which results from increased glucose consumption (24). Our previous study has established that acute exposure of cells to arsenite induces glycolysis (6). As described in our previous article, the proliferation of L-02 cells exposed to arsenite at concentrations of 0, 1, 2, 5, 10 or 20 μM was assessed by CCK-8. Relative proliferation was highest in cells incubated with 2 μM arsenite at 24 h (25). Thus, we chose 2 μM arsenite for chronic exposures. To determine if chronic exposure to arsenite alters the metabolic phenotype in the same manner, L-02 cells were exposed to 0 or 2 μM arsenite for 0, 10, 20 or 30 passages. After 30 passages, they acquired the capacity for anchorage-independent growth (25). As determined by measurement of cell culture media, arsenite increased lactate production in a time-dependent manner (Figure 1A). Moreover, on exposure to arsenite for 10, 20 and 30 passages, cells consumed more glucose in a time-dependent manner (Figure 1B). Arsenite also increased expression of glycolysis-related genes (hexokinase 2 [HK-2], enolase 1 [Eno-1] and glucose transporter type 4 [Glut-4]) (Supplementary Figure s1A, available at Carcinogenesis Online). To determine if arsenite induces a metabolic shift from oxidative phosphorylation to aerobic glycolysis, transmission electron microscopy was used to observe mitochondrial morphology. In normal L-02 cells, mitochondria had regular, oval-shaped morphology, in contrast to a mixture of irregular (black arrows) and regular-shaped mitochondria in cells exposed to arsenite for 10, 20 or 30 passages (Figure 1C). Damaged mitochondria were semi-quantified. With longer times of exposure to arsenite, there were more damaged mitochondria (Figure 1D), suggesting alterations in mitochondrial function. Moreover, the expressions of genes related to the tricarboxylic acid cycle (aconitase 1 [ACO-1], isocitrate dehydrogenase 1 [IDH-1], and isocitrate dehydrogenase 2 [IDH-2]) were down-regulated with longer times of arsenite exposure (Supplementary Figure s1B, available at Carcinogenesis Online). Thus, with chronic exposure to arsenite, L-02 cells underwent a metabolic shift to enhanced glycolysis.
Chronic exposure to arsenite causes elevated glycolysis and mitochondrial defects in L-02 cells. Densities of bands were quantified by Eagle Eye II software. GAPDH levels, measured in parallel, served as controls. L-02 cells were exposed to 0 or 2 μM of arsenite for 0, 10, 20 or 30 passages. (A) Levels of lactate in the culture media were measured and normalized to cell numbers. (B) Glucose consumption was determined in cells as in A; the numbers are means ± SD (n = 3) from three independent experiments. (C) Representative transmission electron microscopy images of mitochondria in the indicated groups. Abnormal mitochondrial morphology is indicated by black arrows. (D) Average scores for semi-quantitative mitochondrial damage from arsenite-treated L-02 cells showing that damage increases in the period of 0–30 passages. *P < 0.05 different from control cells.
Chronic exposure to arsenite induces an inflammatory phenotype in L-02 cells
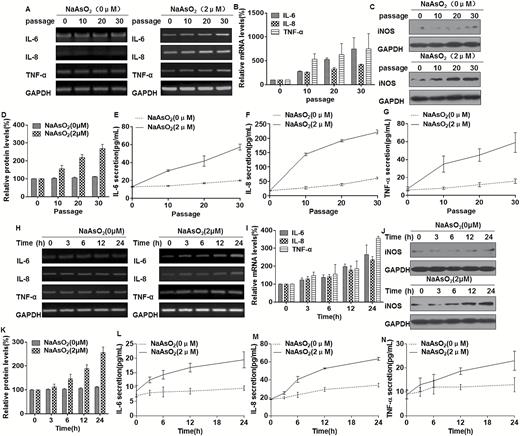
In our previous study, we found that arsenite-induced inflammatory properties, which was involved in transformation of human bronchial epithelial cells (11). The capacity of arsenite to induce secretion of pro-inflammatory cytokines by L-02 cells was investigated. The focus was on IL-6, IL-8 and TNF-α, which are chemotactic cytokines that are over-produced in the human liver in development of an inflammatory phenotype (26). With increased time of exposure to arsenite (10–30 passages), there was a generalized up-regulation of cytokine production (Figure 2A, B, E, F and G). Further, with 10 or more passages in the presence of arsenite, the levels of iNOS were high (Figure 2C and D). In addition, the levels of pro-inflammatory cytokines after arsenite (0 or 2 μM) exposure over periods ranging from 0 to 24 h were compared. The results showed that pro-inflammatory cytokines were over-expressed and released, reaching a peak at 24 h (Figure 2H, I, L, M and N). iNOS was over-expressed in L-02 cells exposed to arsenite for 24 h (Figure 2J and K). These results indicate that, for L-02 cells, arsenite affects pro-inflammatory cytokines.
Chronic exposure to arsenite induces an inflammatory phenotype in L-02 cells Densities of bands were quantified by Eagle Eye II software. GAPDH levels, measured in parallel, served as controls. L-02 cells were exposed to 0 or 2 μM of arsenite for 0, 10, 20 or 30 passages. (A) The mRNA levels of IL-6, IL-8 and TNF-α were determined by RT-PCR, (B) Quantitative RT-PCR was used to measure the transcript levels of IL-6, IL-8 and TNF-α (means ± SD, n = 3). (C) The levels of iNOS were determined by Western blot analyses and (D) the relative protein levels (means ± SD, n = 3) of iNOS were determined. (E–G) The levels of IL-6, IL-8 and TNF-α present in the media (means ± SD, n = 3) were measured by ELISA. L-02 cells were exposed to 0 or 2 μM arsenite for 0, 3, 6, 12 or 24 h. (H) The mRNA levels of IL-6, IL-8 and TNF-α were determined by RT-PCR. (I) Quantitative RT-PCR was used to measure the transcript levels of IL-6, IL-8 and TNF-α (means ± SD, n = 3). (J) The levels of iNOS were determined by Western blot analyses and (K) the relative protein levels (means ± SD, n = 3) of iNOS were determined. (L–N) The levels of IL-6, IL-8 and TNF-α present in the medium (means ± SD, n = 3) were measured by ELISA.
In liver cells, arsenite-induced up-regulation of MCT-4 is dependent on HIF-1α
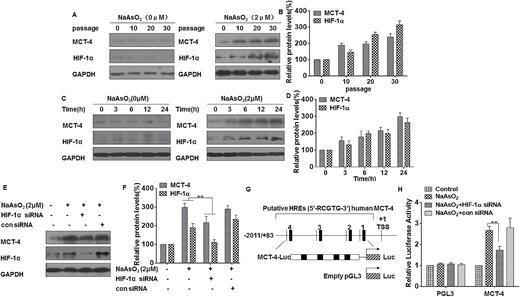
We confirmed that, in L-02 cells, arsenite induces glycolysis (Figure 1). Because MCT-4 is the predominant MCT found in glycolytic cells, it might be predicted that its expression would be increased by arsenite exposure to allow export of increased quantities of lactic acid. As determined in the present research, MCT-4 and HIF-1α are increased in arsenite-treated L-02 cells, and their expressions are up-regulated with increased numbers of passages (Figure 3A and B). Moreover, the expressions of MCT-4 and HIF-1α increased after arsenite exposure over periods ranging from 0 to 24 h (Figure 3C and D). Further, we hypothesized that MCT-4 is regulated by HIF-1α. To establish this, knockdown of HIF-1α attenuated arsenite-induced up-regulation of MCT-4 (Figure 3E and F). This effect was also evident in THLE-3 cells (Supplementary Figure s2A and s2B, available at Carcinogenesis Online), confirming that HIF-1α is responsible for arsenite-induced expression of MCT-4 in liver cells exposed to arsenite. In addition, inspection of the cloned human MCT-4 promoter revealed the presence of four putative HREs with the characteristic motif, 5'-RCGTG-3' (Figure 3G). Since these sequences could be responsible for mediating the response to HIF-1α, we created the full-length promoter constructs shown in Figure 3G. Luciferase expression of the full-length promoter reporter was decreased by inhibiting expression of HIF-1α in L-02 cells and THLE-3 cells (Figure 3H and Supplementary Figure s2C, available at Carcinogenesis Online). These results demonstrate that, in liver cells, arsenite induces MCT-4 over-expression, which is transcriptionally regulated by HIF-1α.
In L-02 cells, arsenite-induced up-regulation of MCT-4 is dependent on HIF-1α. Densities of bands were quantified by Eagle Eye II software. GAPDH levels, measured in parallel, served as controls. L-02 cells were exposed to 0 or 2 μM of arsenite for 0, 10, 20 or 30 passages. (A) Western blots were performed, and (B) relative protein levels (means ± SD, n = 3) of HIF-1α and MCT-4 were determined. L-02 cells were exposed to 0 or 2 μM arsenite for 0, 3, 6, 12 or 24 h. (C) Western blots were performed and (D) relative protein levels (means ± SD, n = 3) of HIF-1α and MCT-4 were determined. L-02 cells were exposed to 20 nM of control siRNA or to 10 nM HIF-1α siRNA for 24 h, and then incubated with 0 or 2 μM arsenite for 24 h. (E) Western blots were performed, and (F) relative protein levels (means ± SD, n = 3) of HIF-1α and MCT-4 were determined. **P < 0.01, different from arsenite-treated cells in the absence of HIF-1α siRNA. (G) Schematic illustration of the consensus HIF-1α HREs in the MCT-4 gene promoter. (H) Luciferase activities of MCT-4 were measured and normalized to Renilla luciferase activity (means ± SD, n = 3); **P < 0.01 different from arsenite-treated cells in the absence of HIF-1α siRNA.
Glycolysis is involved in the arsenite-induced increase of inflammatory cytokines in liver cells
Hypoxia alters cellular bioenergetics by inducing mitochondrial dysfunction and promoting a switch to glycolysis, which supports an inflammatory response, abnormal angiogenesis, cellular invasion and pannus formation (17). To determine if glycolysis enhances the inflammation induced by arsenite, 2-DG, an inhibitor of glycolysis, was used to determine the change of arsenite-enhanced inflammation. The increases of lactate production and glucose consumption induced by arsenite were reduced when L-02 and THLE-3 cells were exposed to 2-DG (Supplementary Figures s3A, s3B, s4A and s4B, available at Carcinogenesis Online). Further, inhibition of glycolysis diminished the increases of IL-6, IL-8, TNF-α and iNOS induced by arsenite (Supplementary Figures s3C-s3G and s4C-s4F, available at Carcinogenesis Online). These data confirm that, in liver cells, glycolysis is involved in the arsenite-induced increases of these pro-inflammatory cytokines.
MCT-4 is involved in the inflammatory properties of liver cells exposed to arsenite
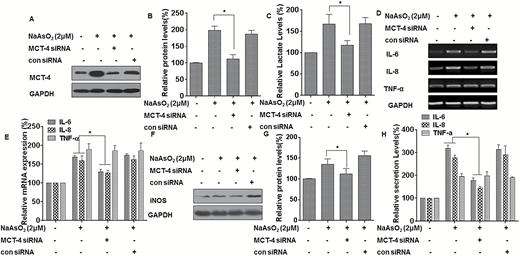
MCT-4 is upregulated in activated macrophages, and, in these cells, increased expression of MCT-4, required for lactate export, is necessary to sustain enhanced glycolysis and the inflammatory response (18). MCT-4 is a metabolic target for reversal of the Warburg effect (27). Based on the observations that MCT-4 is induced by arsenite and is responsible for transporting intracellular lactate to the extracellular environment, we reasoned that MCT-4 is involved in the inflammatory response of liver cells to arsenite. To test this hypothesis, we knocked down MCT-4 in L-02 and THLE-3 cells by use of an siRNA and assessed the expression of pro-inflammatory cytokines after arsenite treatment. As shown in Figure 4A–C and Supplementary Figures s5A–s5C, available at Carcinogenesis Online, expression of MCT-4 was inhibited by the siRNA, and MCT-4 knockdown blocked release of lactate in L-02 cells and THLE-3 cells. MCT4 knockdown attenuated arsenite-induced expression of IL-6, IL-8 and iNOS (Figure 4D–G and Supplementary Figures s5D–s5F, available at Carcinogenesis Online), but failed to affect arsenite-induced increases of TNF-α. Moreover, knockdown of MCT-4 reduced arsenite-induced secretion of IL-6 and IL-8 but failed to affect arsenite-induced release of TNF-α (Figure 4H and Supplementary Figure s5G, available at Carcinogenesis Online). These data indicate that, in liver cells, MCT-4 maintains a high level of glycolysis, which is involved in the arsenite-induced increases of IL-6 and IL-8, but not TNF-α.
MCT-4 is involved in the inflammatory properties of L-02 cells exposed to arsenite. Densities of bands were quantified by Eagle Eye II software. GAPDH levels, measured in parallel, served as controls. L-02 cells were exposed to 20 nM of control siRNA or to 10 nM MCT-4 siRNA for 24 h, and then incubated with 0 or 2 μM arsenite for 24 h. (A) Western blots were performed, and (B) relative protein levels (means ± SD, n = 3) of MCT-4 were determined. (C) Levels of lactate in the culture medium were measured and normalized to cell numbers. (D)The mRNA levels of IL-6, IL-8 and TNF-α were determined by RT-PCR. (E) Quantitative RT-PCR was used to measure the transcript levels of IL-6, IL-8 and TNF-α (means ± SD, n = 3). (F) The levels of iNOS were determined by Western blot analyses and (G) the relative protein levels (means ± SD, n = 3) of iNOS were measured. (H) The levels of IL-6, IL-8, and TNF-α present in the media (means ± SD, n = 3) were measured by ELISA. *P < 0.05, different from arsenite-treated cells in the absence of MCT-4 siRNA.
Glycolysis and enhanced inflammatory properties maintain malignant progression of arsenite-transformed L-02 cells
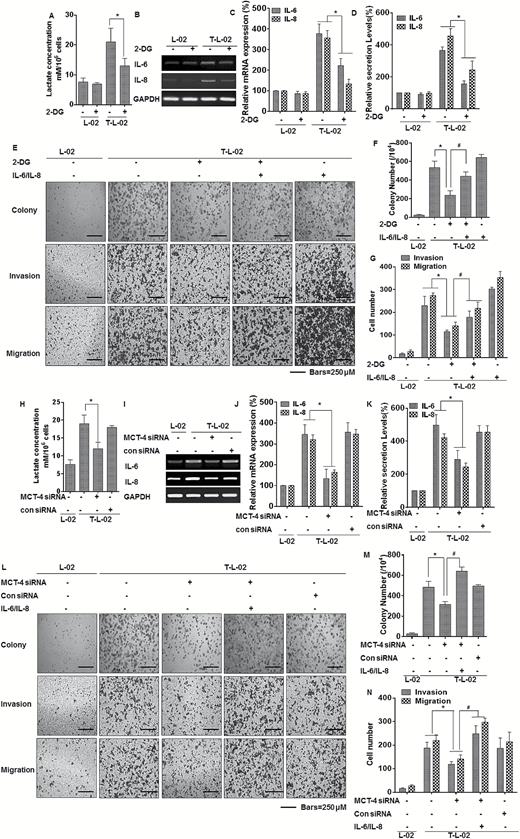
Since glycolysis is involved in the arsenite-induced increases of IL-6 and IL-8, and since cytokines released during inflammation enhance carcinogenesis (28), the role of glycolysis in IL-6 and IL-8 expression in maintenance of arsenite-induced transformation and malignant progression was evaluated. In arsenite-transformed L-02 cells, 2-DG reduced the increase of lactate (Figure 5A), and the expression and secretion of IL-6 and IL-8 were diminished by treating the arsenite-transformed L-02 cells with 2-DG (Figure 5B–D). To evaluate the effects of IL-6 and IL-8 mediated by glycolysis on maintenance of arsenite-induced transformation, the role of IL-6 and IL-8 on the capacity for colony formation, invasion, and migration were examined by adding recombinant IL-6 and IL-8 proteins to the arsenite-transformed L-02 cells with glycolysis reduced by 2-DG. For arsenite-transformed L-02 cells, inhibition of glycolysis reduced anchorage-independent growth and the invasion and migration capacities of cells, but the decreased anchorage-independent growth and the invasion and migration capacities were reversed by adding recombinant IL-6 and IL-8 proteins (Figure 5E–G).
Glycolysis, by enhancing inflammation, promotes malignant progression of arsenite-transformed L-02 cells. C-L-02, passage control L-02 cells; T-L-02, arsenite-transformed L-02 cells; Lac, lactate. L-02 cells and arsenite-transformed L-02 cells were treated with 2-DG for 3 h. (A) Levels of lactate in the culture media were measured and normalized to cell numbers. (B) The mRNA levels of IL-6 and IL-8 were determined by RT-PCR. (C) Quantitative RT-PCR was used to measure the transcript levels of IL-6 and IL-8 (means ± SD, n = 3). (D) The levels of IL-6 and IL-8 present in the media (means ± SD, n = 3) were measured by ELISA. *P < 0.05, different from arsenite-transformed cells. Arsenite-transformed L-02 cells were exposed to 2 mM 2-DG or incubated with human recombinant IL-6/8 (10 ng/ml) three times a week for 2 weeks. (E) Colony formation was assessed in soft agar, and representative images of cell migration and cell invasion and (F) their colony numbers and (G) migrating/invading cells (means ± SD, n = 3) were quantified, bars = 250 μm. *P < 0.05, different from arsenite-transformed cells in the absence of 2-DG; #P < 0.05, different from arsenite- transformed cells in the presence of 2-DG. Arsenite-transformed L-02 cells were exposed to 20 nM of control siRNA or to 10 nM MCT-4 siRNA for 24 h, and then incubated with 0 or 2 μM arsenite for 24 h. (H) Levels of lactate in the culture medium were measured and normalized to cell numbers. (I) The mRNA levels of IL-6 and IL-8 were determined by RT-PCR. (J) Quantitative RT-PCR was used to measure the transcript levels of IL-6 and IL-8 (means ± SD, n = 3). (K) The levels of IL-6 and IL-8 present in the medium (means ± SD, n = 3) were measured by ELISA. *P < 0.05, different from arsenite-transformed cells in the absence of MCT-4 siRNA. Arsenite-transformed L-02 cells were transfected with 20 nM of control siRNA or with 10 nM of MCT-4 siRNA for 24 h, then incubated with human recombinant IL-6/8 (10 ng/ml) three times a week for 2 weeks. (L) Colony formation, assessed in soft agar, and representative images of cell migration and cell invasion. (M) Their colony numbers and (N) migrating/invading cells (means ± SD, n = 3) were quantified, bars = 250 μm. *P < 0.05, different from arsenite-transformed cells in the absence of MCT-4 siRNA; #P < 0.05, different from arsenite-transformed cells in the absence of IL-6/IL-8.
In cell culture models of breast and lung cancer, high expression of MCT4 is associated with increased cellular motility and invasive potential (29,30). Based on the fact that, in L-02 cells, MCT-4 maintains high levels of glycolysis and is necessary for arsenite-induced inflammatory properties, the role of MCT-4 in IL-6 and IL-8 expression and in maintenance of arsenite-induced transformation and malignant progression was determined. In arsenite-transformed L-02 cells, MCT-4 knockdown reduced the increase of lactate (Figure 5H) and diminished the expression and secretion of IL-6 and IL-8 (Figure 5I–K). Moreover, the role of IL-6 and IL-8 on the capacities for colony formation and invasion and migration were examined by adding recombinant IL-6 and IL-8 proteins to arsenite-transformed L-02 cells with MCT-4 knockdown. For arsenite-transformed L-02 cells, silencing of MCT-4 reduced anchorage-independent growth and their invasion and migration capacities, but these effects were reversed by adding recombinant IL-6 and IL-8 proteins (Figure 5L–N). Thus, MCT-4 enhances glycolysis, acting via IL-6 and IL-8, and is involved in maintenance of arsenite-induced transformation and malignant progression.
With the increases of arsenicosis, the levels of lactate and inflammatory cytokine are gradually increased in sera of people exposed to arsenic
A common feature of the tumor microenvironment is extracellular acidosis, and a low extracellular pH promotes tumor growth and cancer progression (31). Lactate also stimulates inflammation and angiogenesis and could therefore contribute to the metastatic process (32,33). We investigated the inflammatory condition and its relationship with the levels of glycolysis. Blood samples were examined to measure the extent of exposure and to assess liver damage in those exposed to arsenite (Supplementary Table 2, available at Carcinogenesis Online). Arsenicosis symptoms were categorized based on the degree of symptoms: non-patient (internal control, n = 30), mild (n = 30), intermediate (n = 30) and severe (n = 30). Two control groups (an internal control and an external control) were included. These groups were examined clinically (general clinical symptoms, signs, biochemical indexes of liver function and liver by ultrasound examination), excluding their previous history of occupational exposure, alcohol consumption, hypertension, genetic, drug exposure of liver damage, viral hepatitis and long-term contact with X rays. Relative to values for the external and internal control groups, arsenic concentrations in hair were higher (P < 0.01 or P < 0.05, Supplementary Table 2, available at Carcinogenesis Online). The levels of total protein and the albumin/globulin ratios were decreased in the intermediate and severe groups, compared with the external control groups (P < 0.01; Supplementary Table 2, available at Carcinogenesis Online). Further, albumin levels were lower in the exposed group relative to the control group (P < 0.01 or P < 0.05; Supplementary Table 2, available at Carcinogenesis Online). Moreover, the albumin levels were lower in the intermediate and severe groups relative to the mild group (P < 0.05; Supplementary Table 2, available at Carcinogenesis Online). However, the levels of glutamic–pyruvic transaminase (ALT) and glutamic–oxalacetic transaminase (AST) showed no significant difference. Continued elevation of AST levels, higher than those for ALT, are often associated with severe liver damage and are a sign of increased chronicity (34). In the present research, although ALT and AST were not elevated by exposure to arsenic, AST levels were higher than ALT levels, which implies that, in the exposed group, the livers were damaged to some extent. In addition, the γ-glutamyl transpeptidase levels in the intermediate and severe groups were higher than those for the control group and the mild group (P < 0.01 or P < 0.05; Supplementary Table 2, available at Carcinogenesis Online). Further, the levels of total bile acids were higher in the mild, intermediate and severe groups, compared with the control (P < 0.01 or P < 0.05; Supplementary Table 2, available at Carcinogenesis Online). These data indicate that, for humans, arsenic exposure is associated with liver damage.
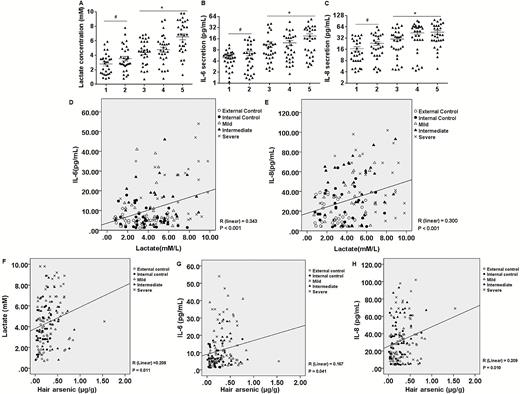
Further, blood samples were examined to measure the levels of lactate and the pro-inflammatory IL-6 and IL-8 cytokines. In sera, the concentrations of lactate and the levels of IL-6 and IL-8 were higher in the mild, intermediate and severe groups, compared with the internal and external control groups. Further, the concentrations of lactate and the levels of IL-6 and IL-8 were higher in the external control group than in the internal group. The severe group had the highest levels of lactate, IL-6 and IL-8 (Figure 6A–C). TNF-α levels in the exposed group were slightly higher than in the external control group and the internal control group (Supplementary Figure s6A, available at Carcinogenesis Online). These findings indicate that with the increases of arsenicosis, the levels of lactate and inflammatory cytokines are gradually increased. The levels of IL-6, IL-8 and TNF-α in human sera positively correlated with the levels of lactate (Figure 6D and 6E and Supplementary Figure s6B, available at Carcinogenesis Online). In addition, there was a positive correlation between the levels of arsenic in hair with lactate, IL-6, and IL-8 in the sera of the external control, internal control, mild, intermediate or severe populations (Figure 6F–H). However, there was no positive relationship between the levels of arsenic in hair and TNF-α (Supplementary Figure s6C, available at Carcinogenesis Online). Thus, with the increases of arsenicosis, the levels of lactate and inflammatory cytokines are gradually increased. These factors may serve as markers for arsenic poisoning.
There is a positive correlation between lactate concentrations and pro-inflammatory cytokines in sera of people exposed to arsenic and between these factors and arsenic concentrations in hair. 1, External control; 2, Internal control; 3, Mild; 4, Intermediate; 5, Severe. The levels of serum lactate (A), serum IL-6 (B) and serum IL-8 (C) were measured (n = 30 per group, values are means ± SD). *P < 0.05, different from the external and internal control groups. (D) There is a positive correlation between the levels of lactate and IL-6 in the sera of the external control, internal control, mild, intermediate, or severe populations (n = 30, R = 0.343, P < 0.001). (E) There is a positive correlation between the levels of lactate and IL-8 in the sera of the external control, internal control, mild, intermediate or severe populations (n = 30, R = 0.300, P < 0.001). (F) There is a positive correlation between the levels of arsenic in hair and lactate in the sera of the following populations: external control, internal control, mild, intermediate, or severe (n = 30, R = 0.203, P = 0.011). (G) There is a positive correlation between the arsenic levels in hair and IL-6 in the sera of the five populations (n = 30, R = 0.167, P = 0.041). (H) There is a positive correlation between the levels of arsenic in hair and IL-8 in the sera of the five populations (n = 30, R = 0.209, P = 0.010).
Discussion
Arsenic is a widely distributed environmental contaminant affecting millions of people worldwide (35). In humans, environmental and occupational exposures to arsenic are associated with various chronic health effects, including increased risk of skin, lung, liver, kidney, prostate and urinary bladder cancers (36). For humans, the liver is a target of arsenite, and arsenite exposure is associated with the development of hepatocellular carcinomas and other lesions (37). However, despite decades of research, the precise mechanisms by which arsenic induces disease remain poorly understood.
In cells, arsenite causes various alterations in normal signaling pathways. Production of reactive oxygen species (ROS) (38), DNA damage (39), altered DNA methylation (40), and enzyme inhibition (41) are all thought to be involved. Moreover, arsenite causes depletion of ATP by inhibiting lipoic acid or by competing with phosphate in human erythrocytes (42), reverses glioblastoma resistance to mTOR-targeted therapies (43), increases oxidative stress due to inhibition of GSH reductase and thioredoxin reductase (44), alters DNA methylation of the promoter of the tumor suppressor gene p53 (45), and inhibits DNA repair through regulation of DNA ligase activity (46). These effects suggest that arsenic-associated outcomes are complex and that interpretation of relevant data should be cautious.
Most solid tumors program their energetic metabolism toward glycolysis, independently from oxygen levels, a phenomenon known as the Warburg effect or aerobic glycolysis (47). Although interest in the metabolic reprogramming of cancer cells is increasing (9), few studies have focused on the role of this metabolic profile in arsenite carcinogenesis. Our previous study indicated that, in L-02 cells, the lncRNA, MALAT1, acting through HIF-1α stabilization, enhances arsenite-induced glycolysis after short-term exposure (6). Further research is needed to determine if this metabolic profile has effects on arsenite-induced liver carcinogenesis. As demonstrated in the present research, with increased time of exposure to arsenite, the levels of glycolysis were elevated and mitochondrial defects developed, confirming that, with chronic exposure, arsenite induces a metabolic shift from oxidative phosphorylation to aerobic glycolysis. Thus, these results demonstrate a metabolic shift to glycolysis, which may be involved in the neoplastic transformation induced in L-02 cells by arsenite.
Inflammation is associated with gastric, colon, esophageal and lung cancers, and with hepatocellular carcinomas (28), and chronic inflammation is involved in human carcinogenesis (28,48). Arsenite-induced alterations in expression of pro-inflammatory genes have been evaluated in other cellular systems. For example, in the previous study, we suggested that HIF-2α-mediated inflammation promotes arsenite-induced transformation of human bronchial epithelial cells (11). When cells are exposed to arsenite, pro-inflammatory and anti-inflammatory cytokines, including IL-1α and β, IL-2, IL-4, IL-6 and IL-8, are released into the culture medium (49). In intact animals, the serum levels of TNF-α are increased in those exposed to arsenite (50). iNOS, expressed in response to harmful stimuli such as oxidative stress or infection, is responsible for inflammation (51). In the present effort, a main focus was on showing alterations in expression of inflammation-related genes after exposure of cells to low levels of arsenite. The results showed that arsenite differentially affects pro-inflammatory cytokines with increased time of exposure. Thus, in L-02 and THLE-3 liver cells, arsenite induces inflammatory properties.
MCT-4, a low-affinity, high-capacity lactate transporter that is present in cells exhibiting elevated glycolytic activity, is involved in lactate release from glycolytic cells (52). Furthermore, enhancement of MCT-4 expression is dependent on the transcription factor HIF-1a, and the promoter region of the MCT4 gene contains HREs, to which HIF-1α can bind and activate transcription (15). Our previous study indicated that HIF-1α enhances arsenite-induced glycolysis in L-02 cells after short-term exposure (6). In the present report, we demonstrate that, in L-02 and THLE-3 cells, MCT-4 and HIF-1α are up-regulated with increased time of exposure. We hypothesize that, in these cells, arsenite induces an increase of HIF-1α and that HIF-1α, acting through MCT-4, promotes lactate release and maintains a high level of glycolytic activity. Further, HIF-1α is responsible for the expression of MCT-4, suggesting that, in L-02 and THLE-3 cells exposed to arsenite, MCT-4 is mediated by HIF-1α.
Long-term exposure to arsenite induces the generation of ROS, including superoxide and hydroperoxides; and high cellular levels of ROS mediate activation of ERK1/2 (53). We previously provided evidence that the arsenite-induced activation of ERK is caused by high levels of superoxide and/or hydroperoxides (38). ROS production activates HIF-1α via the ERK pathway (54). In addition, chronic exposure to arsenic causes angiogenesis in human bronchial epithelial cells via the ROS/miR-199a-5p/HIF-1α/COX-2 pathway (55). Thus, exposure to arsenite could induce the generation of ROS, and the high levels of ROS induce HIF-1α overexpression, which increases pro-inflammatory cytokines. Thus, in arsenite-exposed cells, induction of HIF-1α and MCT-4 and the metabolic changes observed are ROS-dependent.
The Warburg effect is also associated with other cellular activities, including the activation of immune cells (18), and induction of aerobic glycolysis generates products required for host cell activation and maturation (56). A transition in host cell metabolism is a feature of the induction of inflammation. Specifically, induction of aerobic glycolysis is a requirement for macrophages and dendritic cells to activate production of pro-inflammatory cytokines (10,57,58). Since, in this study, we found that arsenite induces glycolysis and inflammation, we determined whether elevated glycolysis induces the inflammatory response. Indeed, 2-DG (a glycolysis inhibitor) inhibits arsenite-induced inflammation, which blocks the increases of pro-inflammatory cytokines. By exporting newly formed lactate, MCT-4 maintains the continuous conversion of pyruvate to lactate and therefore continuous glycolysis (59). MCT-4 is required for sustaining the high levels of glycolysis that are necessary for the expression of pro-inflammatory mediators (18). In the present investigation, we found that MCT-4 is responsible for increases of IL-6, IL-8 and iNOS caused by arsenite. The increase of TNF-α, however, was not appreciably altered by knockdown of MCT-4. The expression of pro-inflammatory cytokines is regulated by various transcription factors, including NF-κB and ERK1/2 (60,61), and TNF-α is regulated by NF-κB (62). Thus, it appears that arsenite induces the expression of TNF-α via a pathway independent of MCT-4 but dependent on other transcription factors. Thus, the present results suggest that arsenite-induced up-regulation of MCT-4 maintains high levels of glycolysis, which are involved in inflammatory manifestations of arsenite-induced liver diseases.
Inflammation is involved in the development and exacerbation of some cancers, and cytokines released during inflammation influence carcinogenesis (28). IL-6, a cytokine mediating immunological and inflammatory events, is involved in the development and progression of several types of tumors (63). Another protein associated with development of inflammation is IL-8, a multifunctional cytokine that participates in development of inflammation-associated cancers (64). In our previous study, we found that a low level of arsenite (2 μM) increased cell proliferation and enhanced neoplastic transformation, as determined by anchorage-independent growth in soft agar and tumorigenesis in nude mice (25). In the present investigation, we find that MCT-4 maintains a high level of glycolysis and is responsible for the expression of IL-6 and IL-8. To establish that up-regulation of MCT-4 maintains the high levels of glycolysis, IL-6, and IL-8 and thereby the malignant behavior of cells and arsenite-induced carcinogenesis, we found that inhibition of glycolysis by 2-DG or knockdown of MCT-4 decreased the number of colonies and invasive and metastatic cells. These effects were reversed by up-regulation of IL-6 and IL-8, indicating the involvement of these chemokines in arsenite-induced carcinogenesis. This conclusion is consistent with reports that IL-6 and IL-8 are engaged in cell transformation caused by other environmental chemicals (65,66).
In 1976, villagers from Guizhou province in southwestern China were found to be suffering severe symptoms of arsenicosis, which was attributed to exposure to high levels of arsenic in food, especially in corn and chili peppers, and to a lesser extent by breathing arsenic-laden air (22,67). The final product of glycolysis, lactate, present in the serum has been considered as a potential biomarker for development of CCl4-induced acute liver injury (68). Of note, increased serum lactate levels have been suggested as a biomarker of organ failure and mortality in patients with sepsis, trauma, and other critical illnesses (69). Lactate stimulates macrophages to release HMGB1, a protein in chromatin, providing an explanation for lactate clearance as a therapy for sepsis and other inflammatory diseases (33). In this study, we collected sera from 150 villagers in January 2014 and found that, in hair samples obtained from those with arsenic exposure, the arsenic concentrations in hair were higher than in hair of those not exposed and that there were various degrees of liver damage. Further, the serum levels of lactate and cytokines IL-6, IL-8 and TNF-α were higher in the mild, intermediate and severe groups compared with the external and internal control groups. Moreover, a statistically significant positive correlation was observed between serum lactate, the cytokines IL-6 and IL-8 and TNF-α. Moreover, there were positive relationships between these factors (except for TNF-α) with arsenic concentrations in hair. These data indicate that serum lactate and pro-inflammatory cytokines may serve as biomarkers for diagnosing exposure to arsenic.
In summary, upon arsenite exposure of cells, over-expression of HIF-1α regulates MCT-4, and MCT-4 upregulation maintains high levels of glycolysis to enhance arsenite-induced inflammatory properties, which are involved in arsenite carcinogenesis (Graphical abstract). Serum lactate and cytokines are higher in arsenic-exposed subjects, and there is a statistically significant positive correlation between them. Moreover, there is a positive relationship between lactate and cytokines with arsenic in hair. The results demonstrate the toxic potential of arsenite by identifying a mechanism involving regulation of glycolysis and inflammation and expand understanding of the carcinogenic potential of arsenic.
Supplementary material
Supplementary Figures 1–6 are available at Carcinogenesis online.
Funding
This work was supported by the Natural Science Foundations of China (81430077, 81273114, 81302467), the open project of the Key Laboratory of Environmental Pollution Monitoring and Disease Control, Ministry of Education, Guizhou Medical University, China (GMU-2015-HJZ05), the Postgraduate Innovation Project of Jiangsu province (CXZZ14_0421, CXZZ14_0951 and KYLX15_0974), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (2014).
Abbreviations
- 2-DG
2-deoxyglucose
- ELISA
enzyme-linked immunosorbent assay
- FBS
fetal bovine serum
- HRE
hypoxia response element
- HIF-1α
hypoxia inducible factor-1α
- IL
interleukin
- MCT-4
monocarboxylate transporter-4
- TNF-α
tumor necrosis factor alpha
Acknowledgements
The authors wish to thank Donald L. Hill (University of Alabama at Birmingham, USA), an experienced, English-speaking scientific editor for editing.
Conflict of Interest Statement: None declared.
References
Author notes
These authors contributed equally to this work.