-
PDF
- Split View
-
Views
-
Cite
Cite
Sami Damak, Minqing Rong, Keiko Yasumatsu, Zaza Kokrashvili, Cristian A. Pérez, Noriatsu Shigemura, Ryusuke Yoshida, Bedrich Mosinger, John I. Glendinning, Yuzo Ninomiya, Robert F. Margolskee, Trpm5 Null Mice Respond to Bitter, Sweet, and Umami Compounds, Chemical Senses, Volume 31, Issue 3, March 2006, Pages 253–264, https://doi.org/10.1093/chemse/bjj027
Close - Share Icon Share
Abstract
Trpm5 is a calcium-activated cation channel expressed selectively in taste receptor cells. A previous study reported that mice with an internal deletion of Trpm5, lacking exons 15–19 encoding transmembrane segments 1–5, showed no taste-mediated responses to bitter, sweet, and umami compounds. We independently generated knockout mice null for Trpm5 protein expression due to deletion of Trpm5′s promoter region and exons 1–4 (including the translation start site). We examined the taste-mediated responses of Trpm5 null mice and wild-type (WT) mice using three procedures: gustatory nerve recording [chorda tympani (CT) and glossopharyngeal (NG) nerves], initial lick responses, and 24-h two-bottle preference tests. With bitter compounds, the Trpm5 null mice showed reduced, but not abolished, avoidance (as indicated by licking responses and preference ratios higher than those of WT), a normal CT response, and a greatly diminished NG response. With sweet compounds, Trpm5 null mice showed no licking response, a diminished preference ratio, and absent or greatly reduced nerve responses. With umami compounds, Trpm5 null mice showed no licking response, a diminished preference ratio, a normal NG response, and a greatly diminished CT response. Our results demonstrate that the consequences of eliminating Trmp5 expression vary depending upon the taste quality and the lingual taste field examined. Thus, while Trpm5 is an important factor in many taste responses, its absence does not eliminate all taste responses. We conclude that Trpm5-dependent and Trpm5-independent pathways underlie bitter, sweet, and umami tastes.
Introduction
The sense of taste is comprised of at least five distinct qualities: sweet, bitter, salty, sour, and umami. Sweet, bitter, and umami compounds bind to and activate specific G protein–coupled receptors (GPCRs), which via their coupled G proteins, activate second messenger cascades. Multiple families of tastant-responsive GPCRs have been identified, including the T1rs, heterodimers of which form sweet-responsive (T1r2 + T1r3) and umami-responsive (T1r1 + T1r3) receptors, respectively (Bachmanov et al., 2001a; Kitagawa et al., 2001; Max et al., 2001; Montmayeur et al., 2001; Nelson et al., 2001, 2002; Sainz et al., 2001; Li et al., 2002; Damak et al., 2003; Zhao et al., 2003), the T2r family of bitter-responsive receptors (Adler et al., 2000; Chandrashekar et al., 2000; Matsunami et al., 2000; Bufe et al., 2002), and a truncated form of mGluR4 that has been implicated in umami taste (Chaudhari et al., 2000). Among the G proteins expressed in taste receptor cells (TRCs), gustducin has been implicated in responses to sweet, bitter, and umami compounds (McLaughlin et al., 1992; Wong et al., 1996; Ruiz-Avila et al., 2001; He et al., 2004), and rod transducin has been shown to play a role in umami responses secondary to gustducin (Ruiz-Avila et al., 1995; He et al., 2002, 2004).
Bifurcated transduction pathways downstream from gustducin, transducin, Gi, and Gs appear to transduce the taste responses to sweet, bitter, and umami compounds, leading to opening of cation channels, depolarization of the TRCs, and neurotransmitter release. In the Gβγ arm of the pathway, the βγ subunits of gustducin activate phospholipase Cβ2 (PLCβ2), which catalyzes the conversion of phosphatidyl inositol phosphate into inositol trisphosphate (IP3) and diacyl glycerol, leading to calcium release from internal stores and activation of ion channels. In the Gα arm of the pathway, gustducin's α subunit activates phosphodiesterase to decrease cyclic nucleotide monophosphate levels (bitter), or Gsα activates adenylyl cyclase to raise cyclic nucleotide monophosphate levels (sweet) (for more details see Lindemann, 2001; Margolskee, 2002).
The subsequent events following the changes in second messenger concentration are incompletely understood. Activity of the following ion channels may be altered in response to changes in the cellular concentration of second messenger: voltage-activated Na+ and K+ channels (Medler et al., 2003), a cyclic nucleotide-gated channel (Misaka et al., 1998), and a cyclic nucleotide-inhibited channel (Kolesnikov and Margolskee, 1995). Channel activity may be modulated directly by second messengers or indirectly by phosphorylation by one or more TRC protein kinases.
We previously determined that Trpm5, a member of the transient receptor potential (Trp) channel family, is expressed selectively in TRCs (Perez et al., 2002). In TRCs, Trpm5 is coexpressed with Gβ3γ13, an IP3 receptor (IP3R3), and PLCβ2 (Clapp et al., 2001; Perez et al., 2002; Zhang et al., 2003). Electrophysiological studies of heterologously expressed Trpm5 show it to be a Ca++-activated cation channel (Hoffman et al., 2003; Liu and Liman, 2003; Prawitt et al., 2003). Thus, it would appear that Trpm5 functions within the GPCR–Gβγ–PLCβ2–IP3 pathways to depolarize the TRC and lead to neurotransmitter release.
A prior study reported that mice with a partial deletion of Trpm5, such that they retained most of the amino terminal portion of the gene, lacked all behavioral and nerve responses to bitter, sweet, and umami compounds (Zhang et al., 2003). This amino terminal fragment of Trpm5, should it be expressed, may interfere with the function of other Trp channels in TRCs: such dominant-negative effects have been observed upon expression of the amino terminal portion of TrpC3 and TrpV6 (Balzer et al., 1999; Erler et al., 2004). To determine the role of Trpm5 in taste responses in vivo and to avoid any confounding effects exerted by potential expression of the amino terminus of Trpm5, we generated knockout (KO) mice null for Trpm5 protein expression. We found that the Trpm5 null mice had markedly diminished, but not abolished, responses to bitter, sweet, and umami compounds.
Materials and methods
Production of KO mice
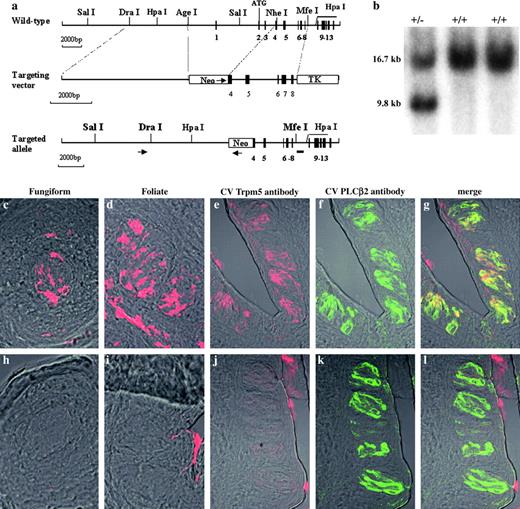
Trpm5-positive clones from a C57BL/6J mouse genomic DNA BAC library were used to construct the targeting vector. The left arm of the targeting vector consisted of a 6.1-kb DraI–AgeI fragment ending 2.4 kb upstream from the start of the first exon of the Trpm5 gene. The right arm consisted of a 3.2-kb NheI–MfeI fragment beginning 6.8 kb downstream from the start of the first exon and 1.8 kb downstream from the start codon. A neomycin resistance cassette (neo), for positive selection, with flanking Lox P sites was introduced between the two arms (Figure 1a). A thymidine kinase (TK) cassette, for negative selection, was cloned downstream from the right arm (Figure 1a). The targeting construct was designed to remove 2.4 kb of the Trpm5 gene's 5′-flanking region containing the promoter and exons 1–4, including the translation start site within exon 2.
Generation of Trpm5 null mice. (a) Top, map of the mouse Trpm5 locus showing the gene's first 13 exons (filled boxes), intervening, and flanking sequences (horizontal lines). Middle, map of the targeting vector showing PGKneo (Neo) and PGKTK (TK) casettes (open boxes) and the long and short arms of the targeting vector (within the dotted lines, which indicate homologies with the Trpm5 locus). PGKneo is flanked with Lox P sites for removal of neo by Cre recombinase. The arrow indicates the direction of transcription of Neo. The targeting vector was designed to remove 2.4 kb of Trpm5 5′-flanking region containing the promoter and exons 1–4, including the translation start site in exon 2. Bottom, diagram of the Trpm5 targeted allele. The primers and probe used to screen for G418 resistant ES colonies are indicated, respectively, by the arrows and short horizontal line below the map. (b) Southern blot of DNA from G418 resistant ES cell colonies digested with HpaI, using the probe shown in the bottom line of panel a. The first lane shows 16.7 and 9.8 kb bands, corresponding to the WT and targeted alleles, respectively. The next two lanes were from colonies in which the targeting vector had integrated nonhomologously. (c–l) Photomicrographs of frozen sections of taste bud–containing sections from fungiform (c, h), foliate (d, i) and circumvallate (e–g, j–l) papillae from WT (c–g), and Trpm5 null (h–l) mice imaged by indirect immunofluorescence with specific antibodies directed against Trpm5 (c–e, h–j, Cy3, red) or PLCb2 (f, k, fluorescein, green). Panels g and l are merged double images of panels e and f, and j and k, respectively. Note that Trpm5 protein is present in the WT mice (c–e, g), but absent in the Trpm5 null mice (h–j, l).
The targeting vector was electroporated into C57BL6 embryonic stem (ES) (Bruce 4) cells (Kontgen et al., 1993). Neomycin-resistant colonies were screened by Southern blot and polymerase chain reaction (PCR) for homologous recombination (see Figure 1 for location of PCR primers and probe). Positive clones were expanded and microinjected into Balb/c blastocysts to generate chimeras according to standard methods (Lemckert et al., 1997). Chimeras were bred to wild-type (WT) C57BL/6J mice to generate Trpm5 KO heterozygotes, which were interbred to generate homozygotes. To ensure that progeny carried the targeted allele and that they were 100% C57BL6, only those offspring derived from the ES cell contribution to the chimera were used.
Immunohistochemistry
The rabbit polyclonal antibody against Trpm5 (1028–1049 amino acids) was as described (Perez et al., 2002). The rabbit polyclonal antibody against PLCβ2 antibody was from Santa-Cruz Biotechnologies (Santa Cruz, CA) and was used as previously described (Perez et al., 2002). Ten micrometers thick frozen sections of murine lingual tissue (previously fixed in 4% paraformaldehyde and cryoprotected in 20% sucrose) were blocked in 3% bovine serum albumin, 0.3% Triton X-100, 2% goat serum, and 0.1% Na azide in phosphate-buffered saline for 1 h at room temperature and then single or double immunostained. Double immunofluorescence combined indirect immunofluorescence with direct immunofluorescence using labeled antibodies. Fixed sections were first incubated with the rabbit anti-Trpm5 primary antibody, followed by incubation with the Cy3-conjugated secondary anti-rabbit antibody (raised in goat). After washing and blocking, the sections were incubated with labeled primary antibody against PLCβ2 that had been treated with Alexa dyes Fabs (Alexa Fluor 488, Zenon Rabbit Antibody Labeling Kit, Molecular Probes, Eugene, OR). Control sections incubated without primary antibody did not show any fluorescence (Perez et al., 2002). Trpm5 preimmune serum did not show any immunoreactivity, and immunoreactivity could be blocked by preincubation with the immunizing peptide (Perez et al., 2002).
Two-bottle preference tests
Trpm5 null mice and heterozygous Trpm5 +/− littermate controls (10 each) were caged individually and given access for 48 h to two 25-ml bottles, one contained distilled water and the other a tastant solution. After 24 h, the bottle positions were switched to control for positional effects. The ratio of tastant volume to total liquid consumed was recorded for each tastant. A ratio of 0.5 indicates indifference, a ratio above 0.5 indicates preference, and a ratio below 0.5 indicates avoidance. For each tastant, presentation was within an ascending concentration series. Between tastant trials, the mice were given distilled water for 7 days. The order of testing was: monosodium glutamate (MSG), denatonium, sucrose, SC45647, quinine (one set of mice); NaCl (a second set of mice). All MSG solutions contained 10 μm amiloride. Data were analyzed using the general linear model repeated measures of the statistics package SPSS with tastant concentration as a within-subject factor and genotype as a between-subject factor. Significance of the between-subject factor indicates difference in the responses between null and WT, whereas significance of the within-subject factor for a given genotype indicates either preference and/or avoidance of certain concentrations of the tastant. Within subjects contrasts were calculated to determine the responses to concentrations that differed from that of the lowest concentration tested, which corresponds to indifference. The Bonferroni correction was applied to correct for multiple tests on related data sets. Preference responses of the Trpm5 +/− heterozygotes were similar to published results with C57BL6 Trpm5 +/+ mice (Bachmanov et al., 2001b), thus we refer to the Trpm5 +/− heterozygotes as “WT.”
Brief-access taste tests
Brief-access taste tests were conducted in a gustometer (Davis MS160-Mouse gustometer; DiLog Instruments, Tallahassee, FL). The training and testing procedures were as described (Glendinning et al., 2002). Two groups of 10 animals each were used: Trpm5 nulls and heterozygous Trpm5 +/− littermate controls. Both groups were tested for 3 days for bitter and umami tastants, and sucrose, or 1 day for NaCl, HCl, and SC45647. All solutions were dissolved in deionized water, autoclaved, and presented at room temperature. All MSG solutions contained 10 μm amiloride. When aversive stimuli were presented, the mice were water deprived for 23.5 h prior to the session to enhance drinking behavior. For normally preferred stimuli, mice were food and fluid restricted (given 1 g of food and 2 ml of water over the 23.5-h period prior to each test session) and allowed one recovery day between testing days. After each session, the mice were given access to distilled water for 1 h to allow rehydration. During each 30-min testing session, a mouse was presented with three to nine different concentrations of a taste stimulus (plus water alone). One solution was presented during each 5-s trial, and the different solutions were presented according to a randomized block design. A trial began once the mouse took its first lick. A lick ratio was calculated separately for each mouse and taste stimulus concentration (mean number of licks elicited by a taste stimulus divided by the mean number of licks elicited by water alone); a lick ratio of 1 indicates indifference. For most experiments, the control solution was autoclaved water; for MSG + 10 μM amiloride, the control solution was 10 μM amiloride. Mice that had been used in the two-bottle preference tests were also used in brief-access tests with sucrose and SC45647. The data were analyzed using the general linear model repeated measures of the statistics package SPSS as described above for the two-bottle preference test. Brief-access test responses of the Trpm5 +/− heterozygotes were similar to published results with C57BL6 Trpm5 +/+ mice (Boughter et al., 2005), thus we refer to the Trpm5 +/− heterozygotes as WT.
Nerve recordings
Whole-nerve responses to lingual application of tastants were recorded from the chorda tympani (CT) and the glossopharyngeal (NG) nerves as previously described (Kawai et al., 2000). Two groups of animals (n = 5–7) were used: Trpm5 nulls and heterozygous Trpm5 +/− littermate controls. Tastants were applied for 30 s (CT) or 60 s (NG). All MSG solutions contained 10 μm amiloride. Integrated whole-nerve response magnitudes (time constant = 1 s) were measured 5, 10, 15, 20, and 25 s (for the CT) and 5, 10, 20, 30, and 40 s (for the NG) after stimulus onset, averaged, and normalized to responses to 0.1 M NH4Cl to account for mouse to mouse variations in absolute responses. Prior to carrying out the normalization procedure, the responses of CT and NG nerves of WT and KO mice to NH4Cl were compared: no significant differences were found in WT versus KO responses to NH4Cl in either nerve. Data were analyzed with the general linear model repeated measures of the statistics package SPSS as described for the two-bottle preference test. CT and NG responses of the Trpm5 +/− heterozygotes were similar to our published results with C57BL6 Trpm5 +/+ mice (Damak et al., 2003), thus we refer to the Trpm5 +/− heterozygotes as WT. When single concentrations of a compound were tested, the t-test was used to compare the means of the responses of the null and WT mice and to compare the means of the responses of the null mice to the zero concentration points.
Results
Trpm5 KO mice lack Trpm5 protein
To determine the role of Trpm5 in taste responses in vivo, we used gene targeting via homologous recombination in ES cells to generate KO mice null for Trpm5 protein expression. The targeted allele has a neo cassette inserted in place of the deleted Trpm5 promoter and the first four exons of the Trpm5 gene (Figure 1a). Screening by PCR and Southern blotting of neomycin-resistant colonies obtained after electroporation of C57BL6 ES cells with the Trpm5 targeting construct identified seven independent colonies of ES cells with properly targeted alleles: in these cells, both the 5′ and 3′ arms of the targeting vector had recombined homologously with the endogenous gene, and no nonhomologous integration events had occurred (Figure 1a,b and data not shown). ES cells with the targeted Trpm5 gene were microinjected into Balb/c mouse blastocysts. The resulting chimeras were bred to C57BL/6J WT mice and the offspring derived from the C57BL6 ES cell contribution to the chimera were intercrossed to produce Trpm5 null mice in a 100% C57BL/6J background.
Indirect immunofluorescence using a rabbit polyclonal anti-Trpm5 antibody revealed strong and widespread expression of Trpm5 protein in WT mice in taste buds from fungiform, foliate, and circumvallate papillae (Figure 1c–e). These results are similar to earlier observations, indicating that Trpm5 is expressed in ∼50–60% of TRCs in anterior and posterior regions of the tongue (Clapp et al., 2001; Perez et al., 2002; Zhang et al., 2003). In contrast, there was no expression of Trpm5 protein in TRCs from the null mice (Figure 1h–j). Results obtained with two other polyclonal anti-Trpm5 antibodies similarly demonstrated the absence of Trpm5 protein in the null mice (data not shown). Inherent “stickiness” in the taste pores and cleft regions yielded nonspecific immunofluorescence in these two regions in both WT and null mice that was not within TRCs.
To determine if the absence of Trpm5 in the null mice may have led to any alteration in general taste bud morphology or loss of the subset of TRCs that normally express Trpm5, we carried out double immunofluorescence with antibodies directed against Trpm5 and PLCβ2. From earlier studies it was known that in WT mice, Trpm5 and PLCβ2 are coexpressed in the same subset of TRCs (Clapp et al., 2001; Perez et al., 2002; Zhang et al., 2003). Our double immunostaining confirms this pattern of coexpression in WT mice (Figure 1e–g). Although the Trpm5 null mice had a normal number of PLCβ2-positive TRCs (Figure 1k) because of the absence of Trpm5 protein, these mice had only PLCβ2 singly positive TRCs and no Trpm5/PLCβ2 doubly positive TRCs (Figure 1j–l). Thus, Trpm5 null mice lack Trpm5 protein in their TRCs but retain the PLCβ2-positive TRCs that normally express Trpm5 protein.
Trpm5 null mice have diminished responses to bitter compounds
To determine the contribution of Trpm5 to the taste responses of mice in vivo, we carried out behavioral tests with Trpm5 null mice and WT littermate controls using compounds representative of the five taste qualities. In two-bottle preference tests, the Trpm5 null mice showed greatly reduced, but not abolished, aversive responses to the bitter compounds quinine sulfate and denatonium benzoate (P < 0.001 comparing WT and null groups) (Figure 2a). The WT mice avoided quinine concentrations of 0.1 mM and higher, while the Trpm5 null mice were indifferent to concentrations of quinine up to 1 mM and avoided 3 mM. The WT mice avoided denatonium concentrations of 1 mM and higher. Surprisingly, the Trpm5 null mice preferred denatonium concentrations between 0.3 and 3 mM and avoided concentrations of 30 mM and above. Several concentrations of quinine and denatonium elicited responses from the null mice that were significantly different from indifference (points in Figure 2a marked by +). Thus, by this assay, the Trpm5 null mice retain the ability to detect and avoid high concentrations of these two bitter compounds, although their sensitivity is greatly diminished in comparison to WT.
Responses of Trpm5 null mice versus “WT” (+/− heterozygotes) to bitter compounds. (a) Mean preference ratios from 48-h two-bottle preference tests (tastant vs. water), comparing responses of Trpm5 null mice (filled squares) versus WT controls (open circles) to quinine sulfate (0.001, 0.003, 0.01, 0.03, 0.1, 0.3, 1, and 3 mM) and denatonium benzoate (0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, and 100 mM). For each group n = 10. (b) Brief-access lick ratios (tastant vs. water), comparing responses of Trpm5 null mice (filled squares) versus WT controls (open circles) to quinine sulfate (0.006, 0.01, 0.03, 0.1, 0.3, 1, 3, and 10 mM) and denatonium benzoate (0.1, 0.3, 1, 3, 10, 30, and 100 mM). For each group n = 10. (c) Whole-nerve recordings from CT nerves of Trpm5 null mice (filled squares) versus WT controls (open circles) stimulated by quinine hydrochloride (0.1, 1, 10, and 20 mM) and denatonium benzoate (0.1, 1, 10, and 20 mM). For each group n = 5. (d) Whole-nerve recordings from CT nerves of Trpm5 null mice (filled bars) versus WT controls (open bars) stimulated by the bitter compounds at the concentration indicated. For each group n = 5. (e) Whole-nerve recordings from NG nerves of Trpm5 null mice (filled squares) versus WT controls (open circles) stimulated by quinine hydrochloride (0.1, 1, 10, and 20 mM) and denatonium benzoate (0.1, 1, 10, and 20 mM). For each group n = 6. (f) Whole-nerve recordings from NG nerves of Trpm5 null mice (filled bars) versus WT controls (open bars) stimulated by the bitter compounds at the concentration indicated. For each group n = 5. Error bars are standard error of the mean. * indicates that the response of Trpm5 null mice was significantly different from that of WT mice. + indicates that the response of Trpm5 null mice was significantly different from indifference [preference ratio of 0.5 (a) or lick ratio of 1.0 (b)] or from baseline nerve response (c–f). The behavioral (a, b) and NG nerve (e, f) responses to most bitter compounds were strongly diminished in the Trpm5 null mice, although in most cases a detectable response remains. The CT nerve (c, d) responses of Trpm5 null mice to the several bitter compounds tested were indistinguishable to those of WT mice.
The behavioral responses of mice in the two-bottle preference test depend on peripheral nerve input, postingestive factors, hedonics, and central integration. To minimize the postingestive component, we recorded initial licking responses to quinine hydrochloride and denatonium benzoate in brief-access taste tests (Figure 2b). The WT mice showed decreased licking of quinine concentrations of 0.1 mM and higher, while the Trpm5 null mice were indifferent to all concentrations of quinine up to the maximum solubility of 10 mM. The WT mice showed decreased licking of denatonium concentrations of 1 mM and higher, while the Trpm5 null mice were indifferent to denatonium concentrations up to 10 mM and avoided 30 and 100 mM (points in Figure 2b marked by +). Thus, by this assay, the Trpm5 null mice retain the ability to detect and avoid denatonium but not quinine, and their sensitivity to denatonium is greatly diminished in comparison to WT.
To directly assess the peripheral taste inputs contributing to bitter detection, we recorded the responses of the NG and CT nerves in WT and Trpm5 null mice to lingual application of quinine hydrochloride, denatonium benzoate, and seven other bitter tastants (Figure 2c–f). The WT mice displayed strong NG responses to quinine hydrochloride concentrations of 1.0 mM and higher and to denatonium concentrations of 10 mM and higher. In comparison to WT, the NG responses of the Trpm5 null mice to quinine hydrochloride and denatonium were greatly diminished (P < 0.001 for quinine hydrochloride and P < 0.01 for denatonium). The null mice showed no NG responses to 1.0 mM quinine hydrochloride and minimal responses to 10 mM quinine hydrochloride, 10 mM denatonium, and 20 mM denatonium. Similar results were observed with three of the other bitter compounds: the NG responses of the Trpm5 null mice were markedly reduced, but not absent, when compared to those of WT for quinine sulfate (P < 0.05), magnesium sulfate (P < 0.01), and cylcoheximide (P < 0.01). The NG responses of WT and Trpm5 null mice to caffeine, sucrose octa-acetate (SOA), and phenyl-thio-carbamide (PTC) were small and statistically indistinguishable (P > 0.05). Several concentrations of quinine hydrochloride, denatonium benzoate, quinine sulfate, tetra-ethyl ammonium (TEA), magnesium sulfate, and cycloheximide elicited NG responses from the null mice that differed significantly from baseline (points in Figure 2e,f marked by +). In sum, the Trpm5 null mice had markedly diminished, but not absent, NG responses to quinine hydrochloride, denatonium benzoate, quinine sulfate, magnesium sulfate, and cycloheximide.
In rodents, the CT is less responsive than the NG to bitter compounds but more responsive to sweet and umami compounds (Ninomiya et al., 2000; Sako et al., 2000). In nerve recordings from WT mice, we found that the CT was indeed less responsive than the NG to the nine bitter compounds tested (compare Figure 2c,d to 2e,f). Furthermore, and in contrast to the NG recordings, the CT responses to these nine bitter compounds were identical in the WT and Trpm5 null mice (P > 0.05, Figure 2c,d). Several concentrations of quinine hydrochloride, quinine sulfate, TEA, and magnesium sulfate elicited CT responses from the WT and null mice that differed significantly from baseline (points in Figure 2c,d marked by +). There were no CT responses in WT or null mice to denatonium, caffeine, cycloheximide, SOA, or PTC. Thus, KO of Trpm5 had no effect on CT responses to these nine bitter compounds.
Trpm5 null mice have diminished responses to sweet compounds
In the 48-h two-bottle preference test, the Trpm5 null mice showed greatly diminished, but not abolished, preference for sucrose and the artificial sweetener SC45647 (P < 0.001, comparing WT and null) (Figure 3a). The WT mice strongly preferred sucrose concentrations of 20 mM and above and SC45647 concentrations of 10 μM and above. In contrast, the null mice were indifferent to sucrose concentrations of 100 mM and below and to SC45647 concentrations of 10 μM and below. The null mice strongly preferred sucrose concentrations of 150 mM and above and SC45647 concentrations of 30 μM and above. By this assay, the Trpm5 null mice retain the ability to detect and respond to high concentrations of these two sweet compounds, although their sensitivity is greatly diminished relative to WT.
Responses of Trpm5 null mice versus “WT” (+/− heterozygotes) to sweet compounds. (a) Mean preference ratios from 48-h two-bottle preference tests (tastant vs. water), comparing responses of Trpm5 null mice (filled squares) versus WT controls (open circles) to sucrose (5, 10, 20, 40, 80, 160, 320, and 640 mM) and SC45647 (0.003, 0.01, 0.03, 0.1, 0.3, 1, and 3 mM). For each group n = 10. (b) Brief-access lick ratios (tastant vs. water), comparing responses of Trpm5 null mice (filled squares) versus WT controls (open circles) to sucrose (30, 100, 200, 300, 600, and 1000 mM) and SC45647 (0.003, 0.01, 0.03, 0.1, 0.3, 1, 3, and 10 mM). For each group n = 10. (c) Whole-nerve recordings from CT nerves of Trpm5 null mice (filled squares) versus WT controls (open circles) stimulated by sucrose (10, 30, 100, 300, 500, and 1000 mM) and SC45647 (0.1, 0.3, 1, 3, and 8 mM). For each group n = 5–7. (d) Whole-nerve recordings from CT nerves of Trpm5 null mice (filled bars) versus WT controls (open bars) stimulated by the sweet compounds at the concentration indicated. For each group n = 5–7. (e) Whole-nerve recordings from NG nerves of Trpm5 null mice (filled squares) versus WT controls (open circles) stimulated by sucrose (10, 30, 100, 300, 500, and 1000 mM) and SC45647 (0.1, 0.3, 1, 3, and 8 mM). For each group n = 5–7. (f) Whole-nerve recordings from NG nerves of Trpm5 null mice (filled bars) versus WT controls (open bars) stimulated by the sweet compounds at the concentration indicated. For each group n = 5–7. Error bars are standard error of the mean. * indicates that the response of Trpm5 null mice was significantly different from that of WT mice. + indicates that the response of Trpm5 null mice was significantly different from indifference [preference ratio of 0.5 (a) or lick ratio of 1.0 (b)] or from baseline nerve response (c–f). The behavioral (a, b) and nerve (c–f) responses to sweet compounds were strongly diminished or in some cases absent in the Trpm5 null mice. Detectable responses to sucrose were seen in the Trpm5 null mice by behavior (a) and CT nerve recording (c). Detectable responses to glucose, maltose, fructose, sorbitol, saccharin, D-alanine, and glycine were seen in the Trpm5 null mice by CT nerve recording (d).
In the brief-access test, the Trpm5 null mice showed no concentration-dependent effect on licking to sucrose or SC45647 across the entire range of concentrations tested. In contrast, the WT mice showed strong concentration-dependent increases in licking to sucrose concentrations of 200 mM and above and SC45647 concentrations of 100 μM to 3 mM; at 10 mM SC45647, the licking response dropped toward baseline (Figure 3b). It is unclear why the Trpm5 null mice responded to both sweet compounds in the long access two-bottle preference test but not in the brief-access test. Potential contributing reasons for these differences could be postingestive effects in the long access test and/or decreased sensitivity of the brief-access test. Similar test-related differences have been noted in sweet responses of α-gustducin null mice (Glendinning et al., 2005).
We recorded the responses of the CT and NG nerves in WT and Trpm5 null mice to the application of sucrose, SC45647, and nine other sweet compounds (Figure 3c–f). The WT mice showed strong CT responses to sucrose concentrations of 300 mM and higher and to SC45647 concentrations of 1 mM and higher. In comparison to WT, the CT responses of the Trpm5 null mice to sucrose were greatly diminished, but not abolished (P < 0.01), with modest responses to 500 mM and 1 M concentrations. The CT nerves of the null mice did not respond to any concentration of SC45647 tested (P < 0.001). Similar results were obtained with CT responses to the other sweet compounds tested, that is, WT mice gave detectable responses to all of these compounds, whereas CT responses of the null mice to these sweet compounds were either absent (sucralose and D-tryptophan) or greatly diminished (glucose, maltose, fructose, sorbitol, saccharin, D-alanine, glycine). CT responses of the Trpm5 null mice to sucrose, glucose, and seven other sweet compounds were significantly different from baseline (Figure 3c,d, marked by +); thus, the Trpm5 null mice had markedly diminished, but not absent, CT responses to eight of 11 sweet compounds tested.
As noted above, in rodents, the NG is less responsive than the CT to sweet compounds. In our nerve recordings from WT mice, we found that the NG was generally less responsive to sweet compounds than was the CT (compare Figure 3e,f to 3c,d). In WT mice, nine of 11 sweet compounds gave smaller responses in the NG than in the CT, while two compounds (D-alanine and glycine) gave responses of equivalent magnitude in NG and CT nerves. In comparison to WT, the NG responses of the null mice were greatly diminished, but not absent, for saccharin, glycine (P < 0.05), alanine, and sorbitol (P < 0.001) (Figure 3e,f).
Trpm5 null mice have diminished responses to umami compounds
In the 48-h two-bottle preference test, the Trpm5 null mice showed diminished, but not abolished, preference for MSG (P < 0.01, comparing WT and null) (Figure 4a). The WT mice preferred MSG concentrations from 10 to 300 mM and avoided 1 M MSG. The Trpm5 null mice preferred MSG concentrations of 100 and 300 mM and avoided 1 M MSG, comparable to that of WT mice. By this assay, the Trpm5 null mice retain the ability to detect and respond to MSG from 100 mM to 1 M. Note that for behavioral tests and nerve recordings, all MSG solutions contained 10 μm amiloride to block the effects of the sodium ion.
Responses of Trpm5 null mice versus “WT” (+/− heterozygotes) to MSG, NaCl, and HCl. (a) Mean preference ratios from 48-h two-bottle preference tests (tastant vs. water), comparing responses of Trpm5 null mice (filled squares) versus WT controls (open circles) to MSG (1, 3, 10, 30, 100, 300, 600, and 1000 M) plus 10 μM amiloride, and NaCl (18, 37, 75, 150, 300, and 600 mM). For each group n = 10. (b) Brief-access lick ratios (tastant vs. water), comparing responses of Trpm5 null mice (filled squares) versus WT controls (open circles) to MSG (1, 3, 10, 30, 100, 300, 600, and 1000 mM) plus 10 μM amiloride, and NaCl (10, 20, 40, 80, 100, 200, 300, 600, and 1000 mM) and HCl (1, 10, and 100 mM). For each group n = 10. (c) Whole-nerve recordings from CT nerves of Trpm5 null mice (filled squares) versus WT controls (open circles) stimulated by MSG (10, 30, 100, 300, and 1000 mM) plus 10 μM amiloride, and NaCl (10, 30, 100, 300, and 1000 mM). For each group n = 6. (d) Whole-nerve recordings from CT nerves of Trpm5 null mice (filled bars) versus WT controls (open bars) stimulated by umami compounds (MPG, IMP, and MPG + IMP) and nonumami compounds [L-proline (L-Pro) and L-alanine (L-Ala)] at the concentration indicated. For each group n = 5–7. (e) Whole-nerve recordings from NG nerves of Trpm5 null mice (filled squares) versus WT controls (open circles) stimulated by MSG (10, 30, 100, 300, and 1000 mM) plus 10 μM amiloride, and NaCl (10 mM, 30 mM, 100 mM, 300 mM, and 1 M). For each group n = 5. (f) Whole-nerve recordings from NG nerves of Trpm5 null mice (filled bars) versus WT controls (open bars) stimulated by umami and nonumami compounds at the concentration indicated. For each group n = 5–6. Error bars are standard error of the mean. * indicates that the response of Trpm5 null mice was significantly different from that of WT mice. + indicates that the response of Trpm5 null mice was significantly different from indifference [preference ratio of 0.5 (a) or lick ratio of 1.0 (b)] or from baseline nerve response (c–f). The preference for MSG by Trpm5 null mice was strongly diminished (a) or absent (b). The CT nerve responses of Trpm5 null mice to MSG, MPG, IMP, and MPG + IMP were diminished but not absent (c,d). The NG nerve responses of Trpm5 null mice to MSG were reduced at high concentrations (e). The NG nerve responses of Trpm5 null mice to MPG, IMP, and MPG + IMP were indistinguishable from those of WT mice (f).
In the brief-access taste test, the Trpm5 null mice showed no concentration-dependent effect on licking of MSG. In contrast, the WT mice showed strong concentration-dependent increases in licking of MSG up to 100 mM and then progressively lower licking to higher MSG concentrations (Figure 4b). Why the Trpm5 null mice showed a preference response to intermediate concentrations of MSG in the long-term preference test but not in the brief-access test is unclear but is not dissimilar to the situation with the differences noted above with behavioral responses to sweet compounds.
In the CT nerve recordings, the Trpm5 null mice showed diminished, but not abolished, responses to umami stimuli [MSG (P < 0.05), inosine monophosphate (IMP) (P < 0.01), and monopotassium glutamate (MPG) + IMP (P < 0.001)], and to nonumami amino acids [L-proline (P < 0.05) and L-alanine (P < 0.001)] (Figure 4c,d). The CT response of WT mice to MPG + IMP was greater than the sums of CT responses of WT mice to either stimulus alone (Figure 4d). This additivity in WT mice, indicative of potentiation by 5′ ribonucleotides of responses to glutamate, was abolished in the Trpm5 null mice (Figure 4d).
The NG responses of the Trpm5 null mice to MSG, MPG, IMP, and MPG + IMP did not differ significantly from those of WT mice (Figure 4e,f). NG responses of the Trpm5 null mice to the nonumami amino acids were markedly diminished versus those of WT mice (P < 0.01 for L-proline and P < 0.001 for L-alanine) (Figure 4f).
Trpm5 null mice have diminished responses to NaCl and unaltered responses to HCl
In the 48-h two-bottle preference test, the Trpm5 null mice were indifferent to NaCl concentrations between 18 and 75 mM, whereas there was a trend for WT mice to prefer these concentrations of NaCl (P = 0.06) (Figure 4a). The WT and Trpm5 null mice avoided NaCl concentrations between 300 and 600 mM NaCl, although at these concentrations the Trpm5 null mice showed slightly diminished avoidance (P < 0.05, comparing WT and null) (Figure 4a). In the brief-access test, NaCl concentrations between 100 mM and 1.0 M were aversive to WT mice; and concentrations of 200 mM and higher were aversive to the Trpm5 null mice. The null mice showed decreased aversion to NaCl concentrations between 100 mM and 1.0 M (P < 0.001, comparing WT and null for these concentrations) (Figure 4b). In CT and NG nerve recordings, the Trpm5 null mice and WT controls gave similar responses to NaCl, although there were small but significant reductions in the nerve responses of the Trpm5 null mice to certain concentrations of NaCl [e.g., CT responses to 30 and 100 mM NaCl (P < 0.05)] (Figure 4c).
The behavioral and nerve responses of Trpm5 null and WT control mice to HCl were indistinguishable (Figure 4b and data not shown).
Discussion
We have shown previously that Trpm5, a Ca++-activated cation channel, is expressed selectively in a subset of TRCs along with Gβ3γ13, PLCβ2, and IP3R3 (Clapp et al., 2001; Perez et al., 2002). Signal transduction from taste GPCRs to Gβγ to PLCβ2 to IP3R3 would lead to Ca++ release from stores, which in turn would activate Trpm5 to let in cations, depolarize the TRC, and lead to neurotransmitter release. Thus, Trpm5 may act downstream of TRC-expressed GPCRs to play a role in signal detection/transduction for multiple taste qualities.
To gain insight into the role of Trpm5 in taste signaling in vivo, we and others have used Trpm5 KO mice. By targeted insertion of neo into the Trpm5 gene, Zhang et al. (2003) generated mice with a partial deletion of Trpm5; these mice lacked exons 15–19 encoding five of six transmembrane helices of Trpm5 but retained the gene's upstream promoter region along with exons 1–14 encoding most of the amino terminal portion of the gene (∼667 of 729 amino acids). Zhang et al. (2003) concluded that mice with this partial deletion of Trpm5 lack all behavioral and nerve responses to bitter, sweet, and umami compounds. However, their KO allele may not be neutral if the retained Trpm5 promoter in their construct drives expression of Trpm5 mRNA encoding an amino terminal fragment of Trpm5. Analogous deletion constructs of TrpC3 and TrpV6 expressing the amino terminal portion of the channel act as dominant negatives that interfere with the function of other Trp channels (Balzer et al., 1999; Erler et al., 2004). The hypothesized expression of Trpm5′s amino terminal fragment has not yet been tested in heterologous systems or in the mouse of Zhang et al. (2003) (e.g., by using an antibody directed against the amino terminus). Nevertheless, because of concerns that the expressed amino terminal portion of Trpm5 might act as a dominant negative to inhibit Trpm5′s partners and/or interfere with other Trp channels expressed in TRCs, we generated KO mice null for Trpm5 protein expression.
We found that Trpm5 null mice had diminished, but not abolished, behavioral and nerve responses to bitter, sweet, and umami compounds. The remaining taste responses, which in some cases were indistinguishable from those of WT, varied depending on the particular taste quality and the anterior versus posterior taste fields examined. Thus, there would appear to be Trpm5-dependent and Trpm5-independent pathways that underlie bitter, sweet, and umami tastes, and the relative contributions of these two pathways vary according to anterior versus posterior taste fields. The differences between our results and those of Zhang et al. (2003) may stem from dominant-negative effects of their construct in which the expressed truncated amino terminal portion of Trpm5 inhibits activity of other Trp channels expressed in TRCs. Another potential explanation for differences between our results and theirs are strain effects: our mice are 100% C57BL/6 (derived from C57BL6 ES cell progeny), whereas the mice of Zhang et al. (2003) are apparently a mixture of C57BL/6 and the unspecified strain of their ES cells (their mixed progeny were obtained by backcrossing the unspecified ES cell progeny with C57BL/6 mice).
With bitter compounds, Trpm5 null mice showed greatly reduced, but not abolished, aversive responses in two-bottle preference tests with quinine sulfate and denatonium benzoate. In the brief-access taste tests, licking of tastant solutions by Trpm5 null mice was unaffected by quinine hydrochloride and only diminished by the highest concentrations of denatonium benzoate. In NG nerve recording, Trpm5 null mice showed greatly reduced, but not abolished, responses to quinine hydrochloride, quinine sulfate, denatonium, magnesium sulfate, and cycloheximide. Thus, by these three methods, responses of Trpm5 null mice to several bitter compounds were greatly reduced, but not abolished, demonstrating the existence of Trpm5-dependent and Trpm5-independent mechanisms for the detection of these bitters in the NG and in the whole animal. In contrast, CT responses of Trpm5 null mice to nine bitter compounds were “identical” to those of WT mice, indicating that responses to these bitter compounds in this nerve are largely or entirely independent of Trpm5.
With the sweet compounds sucrose and SC45647, Trpm5 null mice showed greatly reduced, but not abolished, responses in two-bottle preference tests, and no enhancement of licking responses in the brief-access taste tests. CT nerve responses of Trpm5 null mice to sweet compounds were either absent (SC45647, sucralose, and D-tryptophan) or greatly diminished (sucrose, glucose, maltose, fructose, sorbitol, saccharin, D-alanine, and glycine). With WT mice, the magnitude of responses to all sweet compounds except D-alanine and glycine was less in the NG than in the CT. The pattern of NG responses of Trpm5 null mice to sweet compounds was in general similar to the pattern of CT responses, that is, mostly reduced, but not entirely absent. That behavioral and nerve responses of Trpm5 null mice to several sweet compounds were either greatly reduced, or in some cases abolished, demonstrates the importance of Trpm5-dependent pathways for the detection of sweet. Yet, the remaining CT and NG responses in the null mice to several sweet compounds indicate that Trpm5-independent mechanisms exist for the detection of sweet compounds.
With the umami compound MSG (at up to 300 mM), Trpm5 null mice showed greatly reduced, but not abolished, preference in two-bottle preference tests. At higher concentrations of MSG, Trpm5 null mice showed aversion in two-bottle preference tests that was slightly greater than that of WT mice. In brief-access taste tests, Trpm5 null mice showed no concentration-dependent increase in licking of MSG. There are several lines of evidence indicating that there are multiple transduction pathways for MSG taste and that preference and avoidance via distinct pathways both come to bear at high concentrations (Ninomiya et al., 2000; He et al., 2004). Nerve responses of Trpm5 null mice to MSG were lower in comparison to those of WT mice in both CT (10 mM to 1.0 M MSG) and NG (300 mM and 1.0 M MSG). Nerve responses of Trpm5 null mice to other umami compounds (MPG, IMP, and MPG + IMP) were unaltered versus WT in the NG, and greatly reduced, but not absent, in the CT. Thus, it would appear that there are Trpm5-dependent and Trpm5-independent means to detect umami in both anterior and posterior fields of the tongue, and in the posterior field the Trpm5-independent mechanisms predominate.
In most cases, the results of the two behavioral tests and the nerve recording data were in good agreement. However, for high concentrations of sucrose and SC45647 and moderate concentrations of MSG, Trpm5 null mice showed preference in long-term preference tests and indifference in brief-access taste tests. A possible explanation for this discrepancy is that postingestive products of digestion provide sensory cues or lead to positive or negative reinforcement for ingestion of the tastant (Mook, 1963; Rabe and Corbit, 1973). Although postingestive effects may contribute to the behavioral responses to tastants, with sucrose and MSG it is clear that the Trpm5 null mice display significant responses to these compounds in their gustatory nerves. Given the presence of these peripheral taste responses in the null mice, we think that for preferred compounds such as sucrose and MSG, the standard brief-access taste test may be less sensitive than either nerve recording or the 48-h two-bottle preference test. We recently found that a brief-access taste test similar to that used by Zhang et al. (2003) did not show differences between the responses to sweet compounds of α-gustducin null and WT mice, even though 48-h two-bottle preference tests, a brief-access no choice test, and CT and NG nerve recordings did show such a difference (Glendinning et al., 2005).
What is the nature of the Trpm5-independent pathways? One possibility is that Ca++ release or Ca++ entry acts on another Ca++-activated Trp channel expressed in TRCs, leading to cation influx, depolarization, and neurotransmitter release. Alternatively, the Trpm5-independent pathways may not utilize Trp channels at all: if sufficient Ca++ enters the TRC or is released from stores, then neurotransmitter may be released independently of Trp channels. In addition, some TRC signaling pathways may not rely on mobilization of calcium.
What is the role of Trpm5 in differential taste responses of anterior versus posterior lingual taste fields? Knocking out Trpm5 greatly attenuated NG (but not CT) nerve responses to most bitter compounds. In contrast, knocking out Trpm5 strongly diminished CT (but not NG) nerve responses to MSG. These results indicate that Trpm5-independent pathways transduce most of the responses to bitter compounds in the front of the tongue and most of the responses to MSG in the back of the tongue. Inferred differences in the transduction machinery between the front and the back of the tongue have been reported previously for MSG: T1R3, α-gustducin, and α-transducin are involved in the transduction of MSG taste in anterior but not posterior taste fields (He et al., 2004).
Does Trpm5 play a role in salty taste? Unexpectedly, we found that responses to NaCl were diminished in Trpm5 null mice. While the reduction of these responses was relatively small, it was significant and apparent in brief-access tests, two-bottle preference tests, and CT nerve recordings. Brief-access taste tests have revealed small but significant reductions in the responses of α-gustducin null and of PLCβ2 null mice to high concentrations of NaCl (Dotson et al., 2005; Glendinning et al., 2005). Together, these results suggest that a component of the salty taste response may be mediated by GPCRs and Trpm5.
We thank Deniliz Rodriquez and Shiying Zou for technical assistance. This work was supported in part by grants from the National Institutes of Health (DC003055 and DC003155 to R.F.M., DC004766 to S.D., DC007399 to B.M., and DC004475 to J.I.G.) and the Grant-in-Aid for scientific research from the Japan Society for the Promotion of Science (No. 15209061 and 17659588 to Y.N. and No. 16791127 to N.S.).
References
Adler, E., Hoon, M.A., Mueller, K.L., Chandrashekar, J., Ryba, N.J. and Zuker, C.S. (
Bachmanov, A.A., Li, X., Reed, D.R., Ohmen, J.D., Li, S., Chen, Z., Tordoff, M.G., de Jong, P.J., Wu, C., West, D.B., Chatterjee, A., Ross, D.A. and Beauchamp, G.K. (
Bachmanov, A.A., Tordoff, M.G. and Beauchamp, G.K. (
Balzer, M., Lintschinger, B. and Groschner, K. (
Boughter, J.D. Jr, Raghow, S., Nelson, T.M. and Munger, S.D. (
Bufe, B., Hofmann, T., Krautwurst, D., Raguse, J.D. and Meyerhof, W. (
Chandrashekar, J., Mueller, K.L., Hoon, M.A., Adler, E., Feng, L., Guo, W., Zuker, C.S. and Ryba, N.J. (
Chaudhari, N., Landin, A.M. and Roper, S.D. (
Clapp, T.R., Stone, L.M., Margolskee, R.F. and Kinnamon, S.C. (
Damak, S., Rong, M., Yasumatsu, K., Kokrashvili, Z., Varadarajan, V., Zou, S., Jiang, P., Ninomiya, Y. and Margolskee, R.F. (
Dotson, C.D., Roper, S.D. and Spector, A.C. (
Erler, I., Hirnet, D., Wissenbach, U., Flockerzi, V. and Niemeyer, B.A. (
Glendinning, J.I., Bloom, L.D., Onishi, M., Zheng, K.H., Damak, S., Margolskee, R.F. and Spector, A.C. (
Glendinning, J.I., Gresack, J. and Spector, A.C. (
He, W., Danilova, V., Zou, S., Hellekant, G., Max, M., Margolskee, R.F. and Damak, S. (
He, W., Yasumatsu, K., Varadarajan, V., Yamada, A., Lem, J., Ninomiya, Y., Margolskee, R.F. and Damak S. (
Hoffman, T., Chubanov, V., Gudermann, T. and Montell, C. (
Kawai, K., Sugimoto, K., Nakashima, K., Miura, H. and Ninomiya, Y. (
Kitagawa, M., Kusakabe, Y., Miura, H., Ninomiya, Y. and Hino, A. (
Kolesnikov, S.S. and Margolskee, R.F. (
Kontgen, F., Suss, G., Stewart, C., Steinmetz, M. and Bluethmann, H. (
Lemckert, F.A., Sedgwick, J.D. and Korner, H. (
Li, X., Staszewski, L., Xu, H., Durick, K., Zoller, M. and Adler, E. (
Liu, D. and Liman, E.R. (
Margolskee, R.F. (
Matsunami, H., Montmayeur, J.P. and Buck, L.B. (
Max, M., Shanker, Y.G., Huang, L., Rong, M., Liu, Z., Campagne, F., Weinstein H., Damak, S. and Margolskee, R.F. (
McLaughlin, S.K., McKinnon, P.J. and Margolskee, R.F. (
Medler, K.F., Margolskee, R.F. and Kinnamon, S.C. (
Misaka, T., Kusakabe, Y., Emori, Y., Arai, S. and Abe, K. (
Montmayeur, J.P., Liberles, S.D., Matsunami, H. and Buck, L.B. (
Mook, D.G. (
Nelson, G., Hoon, M.A., Chandrashekar, J., Zhang, Y., Ryba, N.J. and Zuker, C.S. (
Nelson, G., Chandrashekar, J., Hoon, M.A., Feng, L., Zhao, G., Ryba, N.J. and Zuker, C.S. (
Ninomiya, Y., Nakashima, K., Fukuda, A., Nishino, H., Sugimura, T., Hino, A., Danilova, V. and Hellekant, G. (
Perez, C.A., Huang, L., Rong, M., Kozak, J.A., Preuss, A.K., Zhang, H., Max, M. and Margolskee, R.F. (
Prawitt, D., Monteilh-Zoller, M.K., Brixel, L., Spangenberg, C., Zabel, B., Fleig, A. and Penner, R. (
Rabe, E.F. and Corbit, J.D. (
Ruiz-Avila, L., McLaughlin, S.K., Wildman, D., McKinnon, P.J., Robichon, A., Spickofsky, N. and Margolskee, R.F. (
Ruiz-Avila, L., Wong, G.T., Damak, S. and Margolskee, R.F. (
Sainz, E., Korley, J.N., Battey, J.F. and Sullivan, S. (
Sako, N., Harada, S. and Yamamoto, T. (
Wong, G.T., Gannon, K.S. and Margolskee, R.F. (
Zhang, Y., Hoon, M.A., Chandrashekar, J., Mueller, K.L., Cook, B., Wu, D., Zuker, C.S. and Ryba, N.J. (
Author notes
1Department of Physiology and Biophysics, Mount Sinai School of Medicine, 1 Gustave L. Levy Place, New York, NY 10029, USA, 4Section of Oral Neuroscience, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan, 5Department of Neuroscience, The Mount Sinai School of Medicine, 1 Gustave L. Levy Place, New York, NY 10029, USA and 7Department of Biological Sciences, Barnard College, Columbia University, 3009 Broadway, New York, NY 10027, USA


![Responses of Trpm5 null mice versus “WT” (+/− heterozygotes) to bitter compounds. (a) Mean preference ratios from 48-h two-bottle preference tests (tastant vs. water), comparing responses of Trpm5 null mice (filled squares) versus WT controls (open circles) to quinine sulfate (0.001, 0.003, 0.01, 0.03, 0.1, 0.3, 1, and 3 mM) and denatonium benzoate (0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, and 100 mM). For each group n = 10. (b) Brief-access lick ratios (tastant vs. water), comparing responses of Trpm5 null mice (filled squares) versus WT controls (open circles) to quinine sulfate (0.006, 0.01, 0.03, 0.1, 0.3, 1, 3, and 10 mM) and denatonium benzoate (0.1, 0.3, 1, 3, 10, 30, and 100 mM). For each group n = 10. (c) Whole-nerve recordings from CT nerves of Trpm5 null mice (filled squares) versus WT controls (open circles) stimulated by quinine hydrochloride (0.1, 1, 10, and 20 mM) and denatonium benzoate (0.1, 1, 10, and 20 mM). For each group n = 5. (d) Whole-nerve recordings from CT nerves of Trpm5 null mice (filled bars) versus WT controls (open bars) stimulated by the bitter compounds at the concentration indicated. For each group n = 5. (e) Whole-nerve recordings from NG nerves of Trpm5 null mice (filled squares) versus WT controls (open circles) stimulated by quinine hydrochloride (0.1, 1, 10, and 20 mM) and denatonium benzoate (0.1, 1, 10, and 20 mM). For each group n = 6. (f) Whole-nerve recordings from NG nerves of Trpm5 null mice (filled bars) versus WT controls (open bars) stimulated by the bitter compounds at the concentration indicated. For each group n = 5. Error bars are standard error of the mean. * indicates that the response of Trpm5 null mice was significantly different from that of WT mice. + indicates that the response of Trpm5 null mice was significantly different from indifference [preference ratio of 0.5 (a) or lick ratio of 1.0 (b)] or from baseline nerve response (c–f). The behavioral (a, b) and NG nerve (e, f) responses to most bitter compounds were strongly diminished in the Trpm5 null mice, although in most cases a detectable response remains. The CT nerve (c, d) responses of Trpm5 null mice to the several bitter compounds tested were indistinguishable to those of WT mice.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/chemse/31/3/10.1093/chemse/bjj027/2/m_chemsebjj027f02_lw.jpeg?Expires=1715786589&Signature=ZWYsRI-K106jOfx2JGUc2siO4Yy82DccIWSubamNtSPrg~39wP~6VVz1M02IR8ySX1KLI-ZHMNRGkpCy4ZSyWBnTqvI2XFfNSme9rhDIokQAAVxLLjF8BrcZ7tBM8AZcoHLco7C38748DaA9UprqLuvxMqN6xIW6fld0LHkQQT5ezsDrgPl8sQpG~Y~9tG9MmRjYX601R0HKsv~yIZ~689sf7CfbZifP2oL-GQPGWm6M~R4nEaxZOeIbLunMU7w1vVFJITn09fXGs-vVQcSv-DTICAqTrm-PImpUQWTC~XL~ASltMYaoRqphr4yW1fsVZO~wZkfOAKQTSKnga6RYJg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Responses of Trpm5 null mice versus “WT” (+/− heterozygotes) to sweet compounds. (a) Mean preference ratios from 48-h two-bottle preference tests (tastant vs. water), comparing responses of Trpm5 null mice (filled squares) versus WT controls (open circles) to sucrose (5, 10, 20, 40, 80, 160, 320, and 640 mM) and SC45647 (0.003, 0.01, 0.03, 0.1, 0.3, 1, and 3 mM). For each group n = 10. (b) Brief-access lick ratios (tastant vs. water), comparing responses of Trpm5 null mice (filled squares) versus WT controls (open circles) to sucrose (30, 100, 200, 300, 600, and 1000 mM) and SC45647 (0.003, 0.01, 0.03, 0.1, 0.3, 1, 3, and 10 mM). For each group n = 10. (c) Whole-nerve recordings from CT nerves of Trpm5 null mice (filled squares) versus WT controls (open circles) stimulated by sucrose (10, 30, 100, 300, 500, and 1000 mM) and SC45647 (0.1, 0.3, 1, 3, and 8 mM). For each group n = 5–7. (d) Whole-nerve recordings from CT nerves of Trpm5 null mice (filled bars) versus WT controls (open bars) stimulated by the sweet compounds at the concentration indicated. For each group n = 5–7. (e) Whole-nerve recordings from NG nerves of Trpm5 null mice (filled squares) versus WT controls (open circles) stimulated by sucrose (10, 30, 100, 300, 500, and 1000 mM) and SC45647 (0.1, 0.3, 1, 3, and 8 mM). For each group n = 5–7. (f) Whole-nerve recordings from NG nerves of Trpm5 null mice (filled bars) versus WT controls (open bars) stimulated by the sweet compounds at the concentration indicated. For each group n = 5–7. Error bars are standard error of the mean. * indicates that the response of Trpm5 null mice was significantly different from that of WT mice. + indicates that the response of Trpm5 null mice was significantly different from indifference [preference ratio of 0.5 (a) or lick ratio of 1.0 (b)] or from baseline nerve response (c–f). The behavioral (a, b) and nerve (c–f) responses to sweet compounds were strongly diminished or in some cases absent in the Trpm5 null mice. Detectable responses to sucrose were seen in the Trpm5 null mice by behavior (a) and CT nerve recording (c). Detectable responses to glucose, maltose, fructose, sorbitol, saccharin, D-alanine, and glycine were seen in the Trpm5 null mice by CT nerve recording (d).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/chemse/31/3/10.1093/chemse/bjj027/2/m_chemsebjj027f03_lw.jpeg?Expires=1715786589&Signature=hA9hHVTcxYcivu4Cd1GSCqBxveIyIkkUNNL5tyfh54pnlorhmkYa4g6ZvoE12cu9p-KgvT7p4DEFHDNQa21RMJC6zJGvkOkNsHLLeH5Th7k54B~SsC2JHx7vUHmTuHTgDTVJPuINrJU2ZhGS6w6s45B0keRDHtpLXkTUeLYxZyr~nWHQcnWR1eQmvqLTxJM9MmTvuMEsLXCyTzxO2yiC-MGtNXWao8DVA5Ji3HtY~yKw6nb9C9C5zGaeJsnYkMwGne~l1McQEbE2xxRONt8hVdc~GoquMzGvAItRzRYQ6THTqLi~SdVGgU9X~4Uj6i7cv~-Ic8DmVJnusES4BGdkCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Responses of Trpm5 null mice versus “WT” (+/− heterozygotes) to MSG, NaCl, and HCl. (a) Mean preference ratios from 48-h two-bottle preference tests (tastant vs. water), comparing responses of Trpm5 null mice (filled squares) versus WT controls (open circles) to MSG (1, 3, 10, 30, 100, 300, 600, and 1000 M) plus 10 μM amiloride, and NaCl (18, 37, 75, 150, 300, and 600 mM). For each group n = 10. (b) Brief-access lick ratios (tastant vs. water), comparing responses of Trpm5 null mice (filled squares) versus WT controls (open circles) to MSG (1, 3, 10, 30, 100, 300, 600, and 1000 mM) plus 10 μM amiloride, and NaCl (10, 20, 40, 80, 100, 200, 300, 600, and 1000 mM) and HCl (1, 10, and 100 mM). For each group n = 10. (c) Whole-nerve recordings from CT nerves of Trpm5 null mice (filled squares) versus WT controls (open circles) stimulated by MSG (10, 30, 100, 300, and 1000 mM) plus 10 μM amiloride, and NaCl (10, 30, 100, 300, and 1000 mM). For each group n = 6. (d) Whole-nerve recordings from CT nerves of Trpm5 null mice (filled bars) versus WT controls (open bars) stimulated by umami compounds (MPG, IMP, and MPG + IMP) and nonumami compounds [L-proline (L-Pro) and L-alanine (L-Ala)] at the concentration indicated. For each group n = 5–7. (e) Whole-nerve recordings from NG nerves of Trpm5 null mice (filled squares) versus WT controls (open circles) stimulated by MSG (10, 30, 100, 300, and 1000 mM) plus 10 μM amiloride, and NaCl (10 mM, 30 mM, 100 mM, 300 mM, and 1 M). For each group n = 5. (f) Whole-nerve recordings from NG nerves of Trpm5 null mice (filled bars) versus WT controls (open bars) stimulated by umami and nonumami compounds at the concentration indicated. For each group n = 5–6. Error bars are standard error of the mean. * indicates that the response of Trpm5 null mice was significantly different from that of WT mice. + indicates that the response of Trpm5 null mice was significantly different from indifference [preference ratio of 0.5 (a) or lick ratio of 1.0 (b)] or from baseline nerve response (c–f). The preference for MSG by Trpm5 null mice was strongly diminished (a) or absent (b). The CT nerve responses of Trpm5 null mice to MSG, MPG, IMP, and MPG + IMP were diminished but not absent (c,d). The NG nerve responses of Trpm5 null mice to MSG were reduced at high concentrations (e). The NG nerve responses of Trpm5 null mice to MPG, IMP, and MPG + IMP were indistinguishable from those of WT mice (f).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/chemse/31/3/10.1093/chemse/bjj027/2/m_chemsebjj027f04_lw.jpeg?Expires=1715786589&Signature=nxvDYCPaHGJZYZixZBkSFPrUcWcO1hRMZMNURRJkWOMGHRvtyo6wCDnj1k371RYEkcl3hN47rM60wM3tCJ5vPeMgtImGvbmzjBQW9ayW8exG3VByZUgaasxRQn8vMua8iUFGJvOOV10nMGBS6ulzbHvxwyQCYErlkuSr7gsrFPO5NuQ3pIKX-M9xSgulkuYS7n49J4feQAVT14d4gwvShESe~hIDz7G-vZJNMSDlFzlmKQuOLy-naG6E8DMYBA692pkHathBL41WyXX~YljHAZK7H0X4eaqN8e5amMcKsMbfITKEKhXISF7mKK1k08M52kCP-UK4ie94ddujWJGcww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
