Structure of metabotropic glutamate receptor C-terminal domains in contact with interacting proteins
- Emil-Fischer-Zentrum, Institut für Biochemie, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
Metabotropic glutamate receptors (mGluRs) regulate intracellular signal pathways that control several physiological tasks, including neuronal excitability, learning, and memory. This is achieved by the formation of synaptic signal complexes, in which mGluRs assemble with functionally related proteins such as enzymes, scaffolds, and cytoskeletal anchor proteins. Thus, mGluR associated proteins actively participate in the regulation of glutamatergic neurotransmission. Importantly, dysfunction of mGluRs and interacting proteins may lead to impaired signal transduction and finally result in neurological disorders, e.g., night blindness, addiction, epilepsy, schizophrenia, autism spectrum disorders and Parkinson's disease. In contrast to solved crystal structures of extracellular N-terminal domains of some mGluR types, only a few studies analyzed the conformation of intracellular receptor domains. Intracellular C-termini of most mGluR types are subject to alternative splicing and can be further modified by phosphorylation and SUMOylation. In this way, diverse interaction sites for intracellular proteins that bind to and regulate the glutamate receptors are generated. Indeed, most of the known mGluR binding partners interact with the receptors' C-terminal domains. Within the last years, different laboratories analyzed the structure of these domains and described the geometry of the contact surface between mGluR C-termini and interacting proteins. Here, I will review recent progress in the structure characterization of mGluR C-termini and provide an up-to-date summary of the geometry of these domains in contact with binding partners.
Introduction
The coordinated neuronal activity in the central nervous system (CNS) is largely guided by receptors for various neurotransmitters that are expressed at synaptic specializations. A correct localization and regulation of these neurotransmitter receptors is crucial for proper function of the neuronal network. This is mainly accomplished by proteins that interact with cytosolic receptor domains, thereby regulating localization, turnover and ligand affinity of these proteins (see e.g., Bard and Groc, 2011; Maurice et al., 2011). Thus, neurotransmitter receptors are central components of synaptically localized signal complexes, in which functionally related proteins, such as transmembrane proteins, enzymes, scaffolds, and anchor proteins assemble to secure a dynamic regulation of synaptic neurotransmission and neuronal excitability, both in space and time. Dysfunction of these signal complexes disturbs this coordinated interplay and can ultimately result in neurological diseases such as night blindness, addiction, depression, anxiety, epilepsy, schizophrenia, autism spectrum disorders, and Parkinson's disease (Szumlinski et al., 2006; Nicoletti et al., 2011).
Metabotropic Glutamate Receptors
Glutamate is the most important excitatory neurotransmitter in the CNS and binds to ionotropic (ion channel associated) and metabotropic (G-protein coupled) glutamate receptors (mGluRs). About 20 years ago, the first glutamate-gated G-protein coupled receptor (mGluR1) was discovered (Houamed et al., 1991; Masu et al., 1991). Today, eight mGluR types are described that are subdivided in three groups: Group I—mGluR1 and mGluR5, group II—mGluR2 and mGluR3, group III—mGluR4, mGluR6, mGluR7 and mGluR8 (Ferraguti and Shigemoto, 2006). Generally, group I receptors are expressed at the postsynapse, members of group II were found both pre- and postsynaptically and group III mGluR types show a clear preference for the presynaptic terminal. While ionotropic glutamate receptors are mostly expressed opposite to the active zone of neurotransmitter release, group I mGluR types were found perisynaptically, regulating the activity of their ionotropic counterparts (Baude et al., 1993; Nusser et al., 1994; Lujan et al., 1997). Interestingly, mGluR5 is also expressed on intracellular membranes of the endoplasmic reticulum and nucleus, where it is involved in the regulation of gene expression (Jong et al., 2009; Kumar et al., 2011).
Except mGluR6, group III mGluR types represent presynaptic auto-receptors that function as glutamate sensors for glutamatergic neurons and inhibit the release of this neurotransmitter upon activation. Interestingly, the localization of group III mGluR types in synapses of presynaptic terminal systems depends on the nature of the postsynaptic neuron contacted. Originally, an asymmetric distribution of mGluR7 was observed in presynaptic terminals of hippocampal pyramidal cells and bipolar cells of the mammalian retina (Brandstätter et al., 1996; Shigemoto et al., 1996). Later, a comparable receptor segregation was also reported for mGluR4 and mGluR8 in the hippocampus (Shigemoto et al., 1997). The authors suggest the existence of a retrograde signal that regulates the amount of group III mGluR types in the presynapse. In contrast to the presynaptic localization of mGluR4, mGluR7, and mGluR8, mGluR6 is exclusively expressed at postsynaptic dendritic specializations of “ON bipolar cells” (bipolar cells that respond with depolarization to light) in the retina and transmits the “light on” signal in vision (Masu et al., 1995; Vardi et al., 2000). A more detailed overview of the cellular and subcellular distribution of mGluR types can be found, e.g., in (Ferraguti and Shigemoto, 2006).
Today, more than 800 G-protein coupled receptor are known in humans, the majority of them functioning as sensors for environmental stimuli, such as olfaction, taste, and vision (Harmar et al., 2009). Being typical members of the G-protein coupled receptor class C, mGluRs contain seven transmembrane helices for membrane anchoring and rather large N- and C-terminal domains (Figure 1A). While the extracellular N-terminus forms the ligand-binding domain and mediates receptor dimerization, intracellular domains provide several interaction motifs for binding partners (Figure 1B). Intracellular C-termini of most mGluR types are subject to alternative splicing and post-translational modification, thereby increasing amount and diversity of available interaction sites for regulatory proteins.
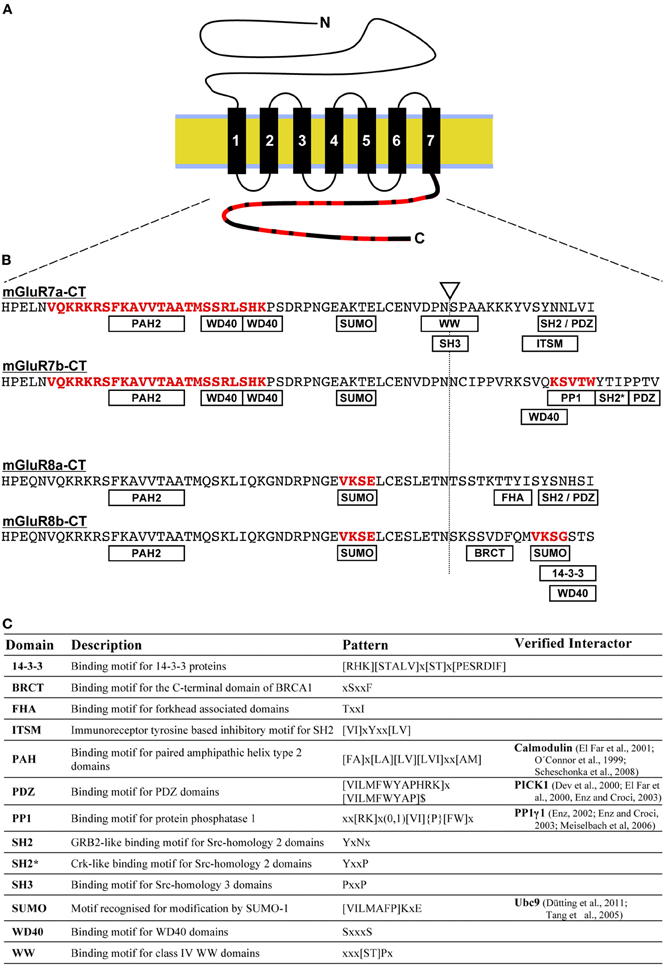
Figure 1. Distribution of short linear binding motifs in mGluR C-termini. (A) The sketch shows the membrane topology of mGluRs. Black rectangles represent the seven transmembrane domains that anchor the receptor in the lipid bilayer (yellow/blue). The intracellular C-terminus (bold line) contains short linear motifs (SLiMs) that function as interaction sites for intracellular proteins (indicated in red). (B) Amino acid sequences of mGluR7 and mGluR8 C-terminal domains are shown in the single letter code. The site for alternative splicing generating a and b variants is indicated by a triangle/dotted line and SLiMs are abbreviated in rectangles. Binding sequences for Calmodulin, PP1, and SUMO proteins that are listed in Figure 2B are highlighted in red (see main text for details). (C) Overview of predicted and experimentally verified sequence patterns of SLiMs shown in (B) and the description of corresponding binding domains. Motifs were identified using pattern-based searches in the ELM databank (Dinkel et al., 2012).
Binding Partners of Metabotropic Glutamate Receptors
MGluRs contact heterotrimeric G-proteins to regulate intracellular second messenger cascades. Group I mGluRs preferentially activate phospholipase C (PLC) and thereby stimulate the production of the second messenger molecules inositol-1,4,5-trisphosphate (IP3) and diacylglycerol. In contrast, mGluR types belonging to group II and III primarily reduce the concentration of cAMP by inhibiting adenylate cyclase (AC) activity. However, distinct coupling behavior was observed depending on the cell type analyzed. In cerebellar neurons, a dual mode of action has been suggested for the group III variant mGluR7a, in which the receptor reduces or stimulates the release of glutamate from the presynaptic terminal into the synaptic cleft by coupling to either AC or PLC pathways (Perroy et al., 2000; Martin et al., 2010).
Besides contacting heterotrimeric G-proteins, intracellular C-terminal domains of various mGluR types physically interact with several other proteins. These mGluR interactors include enzymes, ion channels, receptors, scaffolds, and cytoskeleton proteins that anchor the receptors at specific subcellular sites. MGluR interacting proteins regulate the efficacy of G-protein coupling, as well as G-protein independent tasks including targeting, localization, turnover, and glutamate affinity of the receptors. For example, G-protein coupled receptor kinase 2 (GRK2) and β-arrestin1 interact with the C-terminal domain of mGluR1a (Dale et al., 2001; Dhami et al., 2005). Receptor internalization is dependent on GRK2 activity and arrestin and an involvement of 25 amino acids located in the proximal region of the mGluR1a C-terminus has been suggested in this process (Mundell et al., 2003). In addition, binding of GRK2 to the second intracellular loop of mGluR1a promotes a phosphorylation and arrestin independent down-regulation of receptor mediated G-protein signaling (Dhami et al., 2005). The authors propose that the additional binding of GRK2 to the mGluR1a C-terminus may regulate the efficacy of this process. Some cytosolic mGluR binding partners physically link mGluR signaling with other receptor types, e.g., IP3 receptors or ionotropic glutamate receptors of the NMDA type (Shiraishi-Yamaguchi and Furuichi, 2007). In addition, mGluRs are directly contacted by other receptor types, including receptors for adenosine, dopamine, and serotonin (Ciruela et al., 2001; Ferre et al., 2002; Gonzalez-Maeso et al., 2008; Cabello et al., 2009). For a comprehensive overview of mGluR interacting proteins, mapped binding sites, and physiological functions of resulting signal complexes, I refer the reader to two recently published reviews (Enz, 2007, 2012).
Various mGluR types are associated with neurodegenerative diseases, such as Alzheimer's and Parkinson's disease (Nicoletti et al., 2011). In addition, it has been shown that an improper assembly of mGluR associated signal complexes can cause impaired signal transduction and ultimately may lead to neurological disorders, such as congenital night blindness, anxiety, addiction, depression, epilepsy, schizophrenia, and autism spectrum disorders (Szumlinski et al., 2006; Durand et al., 2007; Bourgeron, 2009; Cao et al., 2011). Interactions between mGluR C-termini and regulatory proteins are dynamic, which enables the design of molecules that interfere with the assembly of mGluR associated signal complexes. Thus, protein-protein interactions between mGluR C-termini and regulatory proteins represent attractive pharmaceutical targets (Enz, 2012).
Structure Investigation of Metabotropic Glutamate Receptors
In recent years, an increasing number of structural information for ionotropic and metabotropic neurotransmitter receptors has become available. For example, the three-dimensional structures of ligand-gated anion and cation channels describe detailed mechanism of ion specificity, conductance and gating mechanisms (see e.g., Sobolevsky et al., 2009; Hibbs and Gouaux, 2011). Furthermore, crystal structures of adenosine, adrenalin, dopamine, and glutamate gated G-protein coupled receptors were reported (Kunishima et al., 2000; Muto et al., 2007; Rosenbaum et al., 2009; Chien et al., 2010). Some of these studies analyzed in detail the purified extracellular N-terminal domains of mGluR1, mGluR3, and mGluR7. Besides functioning as ligand binding domains, these N-termini form disulphide bridges which results in homo- and heteromeric mGluR dimers (Doumazane et al., 2011). Heterodimerization was also observed between mGluR2 and the 5-HT2A serotonin receptor (Gonzalez-Maeso et al., 2008). A molecular dynamics simulation of 40 ns suggests that the formation of hydrogen bonds and hydrophobic microdomains between transmembrane helixes four and five of each receptor shape the geometry of the dimerization interface (Bruno et al., 2009).
Noteworthy, in all above mentioned crystal structures, intracellular receptor domains were deleted to allow efficient crystallization, or excluded from data evaluation. Thus, structural characteristics of mGluR intracellular domains has been analyzed by alternative methods. In addition, for some mGluR/interactor pairs, the geometry of the binding surface of intracellular sequences of the glutamate receptors in contact with binding partners was investigated by crystallography, NMR, and computational techniques. In the following, I will summarize recent progress in the structure determination of intracellular mGluR C-termini and review available structure information of these domains in complex with interacting proteins, as shown in Figure 2.
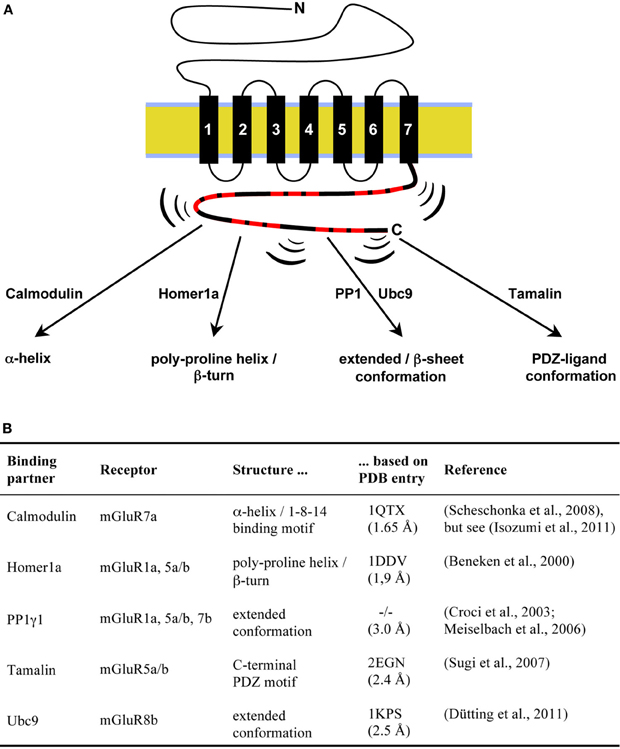
Figure 2. Inducible structure of mGluR C-terminal sequences in contact with interacting proteins. (A) Intrinsically disordered amino acid sequences within mGluR C-termini (symbolized by brackets) adopt defined confirmations upon interaction with the indicated proteins. (B) Overview of available structure information for mGluR C-terminal sequences in complex with binding partners. The resolution of the listed crystal structures in angstrom is given in parenthesis. A PDB entry for the PP1γ1/GM co-crystal is not available (−/−; Egloff et al., 1997).
Structural Analysis of mGluR C-Terminal Domains
Intracellular mGluR C-termini domains are the main targets for proteins regulating these receptors (Enz, 2007). Therefore, elucidation of their conformation is a prerequisite for designing drugs that interfere with specific protein-protein interactions (Enz, 2012). Recently, the structure of the purified intracellular C-termini from group III mGluR variants 6, 7a, and 8a has been analyzed using biochemical, biophysical, and computational techniques (Seebahn et al., 2011). Limited proteolysis, circular dichroism (CD), as well as one- and two-dimensional nuclear magnetic resonance (1H- and 1H-15N-NMR) spectroscopy consistently lead to the conclusion that the unliganded mGluR C-termini do not form secondary/tertiary structure elements that remain stable over time. C-terminal domains of G-protein coupled receptors are located in close proximity to the inner leaflet of the lipid bilayer that might induce regions of ordered secondary structure. Indeed, α-helical regions induced by lipids were observed in the C-termini of the β-adrenergic receptor and in the cannabinoid receptor 1 (Rasmussen et al., 2007; Ahn et al., 2009). However, recorded 1H-15N-NHSQC spectra of the mGluR8a C-terminus revealed no significant differences upon addition of dodecylphosphocholine (Seebahn et al., 2011). This observation is consistent with the disordered nature of the mGluR7a C-terminus in the presence of trifluoroethanol, a substance known to enhance the formation of secondary structure elements (Isozumi et al., 2011). Based on the available CD and NMR spectra, as well as due to their inability to form defined crystal structures, group III mGluR C-termini seem to be rather unstructured and may adopt defined three-dimensional conformations only upon binding to interacting proteins.
Indeed, unstructured/intrinsically disordered protein regions are capable to interact with other proteins or nucleic acids by induced fit (Figure 2; Cheng et al., 2006). Computational techniques predicted the existence of several short linear motifs (SLiMs) in mGluR C-termini (Figure 1C; Seebahn et al., 2011). Protein interactions involving short amino acid motifs occur more often in unstructured than structured protein regions. In the human genome, between 15% and 40% of all protein interactions are estimated to involve SliMs (Neduva and Russell, 2006). In most cases, SLiMs do not form stable secondary structures, but rather are highly flexible in order to adopt to the conformation defined by interacting proteins, e.g., by protein phosphatase 1 (see below). Within the C-terminal domains of group III mGluR types, most SLiMs are present in the distal, isoform specific regions, while some are overlapping the splice site (Figures 1B,C; Seebahn et al., 2011). Thus, the observed alternative splicing of most mGluR C-termini creates a large heterogeneity of interaction motifs accessible for regulatory proteins.
MGluR1a, mGluR5a/b, and mGluR7b in Contact with Protein Phosphatase 1
Several enzymes directly interact with the intracellular C-termini of mGluR types, such as kinases, phosphatases, and proteins of the SUMOylation machinery. In this way, the activity of mGluRs and associated proteins is regulated by post-translational modifications, including phosphorylation and SUMOylation. G-protein coupled receptor kinases phosphorylate intracellular mGluR domains, which allows binding of arrestin, uncouples receptor activation from G-protein signaling and stimulates receptor internalization (Dhami and Ferguson, 2006). Furthermore, PKA and PKC phosphorylate specific serine/threonine residues in mGluR C-termini (Airas et al., 2001; Cai et al., 2001; Mao et al., 2008). This kinase activity is antagonist by serine/threonine protein phosphatases, such as the protein phosphatase 1 (PP1). For proper physiological function, one PP1 catalytic subunit assembles with a regulatory subunit. In mammals there are four catalytic subunits known (α, β, γ1, and γ2) that interact with several hundreds of regulatory subunits that regulate enzymatic activity, substrate specificity and subcellular localization, thereby creating a large diversity of PP1 holoenzymes (Shi, 2009).
The two alternatively spliced PP1 gamma subunits (PP1γ1 and PP1γ2) interact with a linear stretch of five amino acids in the C-termini of mGluR1a, mGluR5a/b and mGluR7b (Croci et al., 2003; Meiselbach et al., 2006). This sequence (KSV[S/T]W; see Figure 1B) fulfills the consensus sequence of a so-called “PP1 docking motif” that is present in several PP1 interactors, including proteins that target the PP1 activity to ionotropic GABA receptors (Rose et al., 2008). In the literature, various consensus sequences for this docking motif are described, all of them showing different sensitivities and specificities, e.g., (RVxF or [HKR][ACHKMNQRSTV][V][CHKNQRST][FW]; x—any amino acid; Meiselbach et al., 2006; Bollen et al., 2010).
Based on a solved co-crystal formed by PP1γ1 and the interaction motif of the regulatory G-subunit that targets the phosphatase to glycogen particles in muscle (Egloff et al., 1997), the three-dimensional structures of the five amino acids forming the docking motifs present in mGluR C-termini (mGluR1a: KSVSW; mGluR5a/b and mGluR7b: KSVTW—see Figure 1B) were modeled applying structure based computational approaches. Homology based molecular modeling and molecular dynamics simulations showed that the backbone of the five amino acids adopts an extended conformation in contact with an acidic/hydrophobic groove on the PP1γ1 surface (Figure 3A; Croci et al., 2003; Meiselbach et al., 2006). Within this groove, Lys+1 and Ser+2 of the ligand form electrostatic contacts with Asp166, Glu167, Asp242, and Glu287 of PP1γ1 (Figure 3B). In contrast, Val+3 and Trp+5 are buried in two hydrophobic pockets formed mainly by the side chains of Ile169, Leu243, Leu289, and Cys291, or of Phe257, Met283, Cys291, and Phe293 of the phosphatase. The side chains of Ser/Thr+4 point away from the enzyme surface and use a water molecule to form a hydrogen-bonding network with the backbone carbonyl oxygen of Ser+2 and Thr288 of PP1γ1 (Croci et al., 2003).
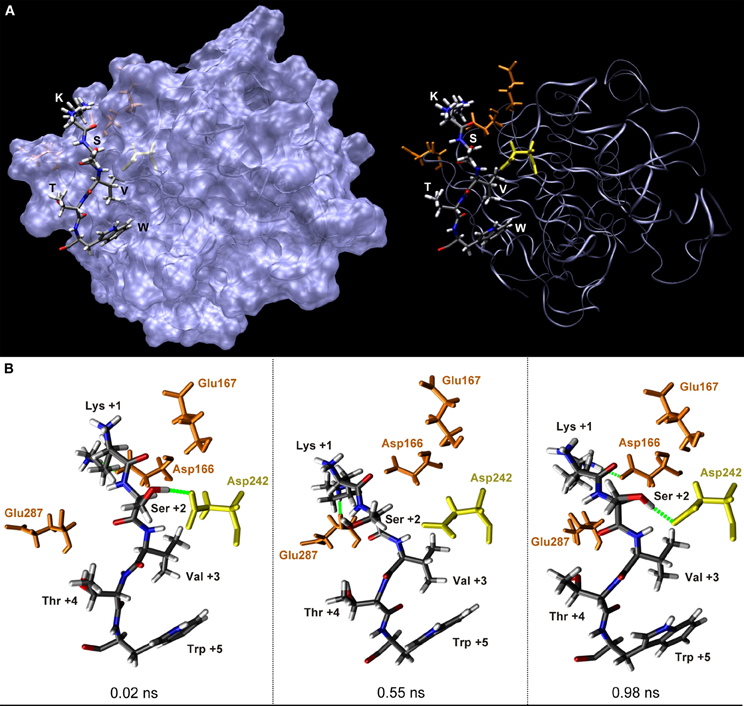
Figure 3. Structure of the mGluR7b C-terminus in contact with protein phosphatase 1 in the time course of a molecular dynamics simulation. (A) Stick presentation of five amino acids (KSVTW) in the mGluR7b C-terminus that contact the PP1γ1 surface (blue) in an extended conformation. Functional groups of the mGluR7b sequence are colored according to their atom types. Asp166, Glu167, and Glu287 of PP1γ1 forming polar interactions with Lys+1 are shown in brown, Asp242 of PP1γ1 contacting Ser+2 is marked in yellow. Val+3 and Trp+5 are buried in hydrophobic pockets of the PP1γ1 surface and the side chain of Thr+4 points to the opposite direction. (B) Enlarged views of the KSVTW ligand sequence at 0.02 ns, 0.55 ns, and 0.98 ns of molecular dynamics simulation time, as indicated. Green dotted lines represent important electrostatic interactions formed by Lys+1 and Ser+2 with PP1γ1.
In order to gain insight in the conformational variability of amino acids forming polar interactions, molecular dynamics simulations were applied. Within the simulation time of 1 ns, Lys+1 contacted various negatively charged amino acid side chains and backbone carbonyl groups on the PP1γ1 surface by hydrogen bonds and salt bridges (Figure 3B). In addition, the side chain hydroxyl group of Ser+2 formed hydrogen bonds with the two oxygen atoms of the carboxyl group of Asp242 in about 30% of the simulation time (Figure 3B; Croci et al., 2003; Meiselbach et al., 2006).
The described structural information can be translated into a binding mechanism for PP1 docking motifs present in mGluR C-terminal domains: binding is initiated by a general electrostatic attraction between the positively charged Lys+1 and a negatively charged surface patch of the phosphatase. Indeed, an increase of positive charges in the ligand by inserting one, two, or three lysine residues N-terminal of Lys+1 increased the PP1γ1 binding strength of the mGluR7b C-terminus by factors of 2, 4, and 6, respectively (Croci et al., 2003; Meiselbach et al., 2006). After electrostatic attraction, the hydrophobic interactions at position +3 and +5 of the docking motifs ensure the correct orientation of the mGluR C-terminal domains in respect to the PP1γ1 surface. Interestingly, Ser+2 of the mGluR1a docking motif can be phosphorylated by PKC (Mao et al., 2008), which might disrupt the PP1γ1/mGluR binding surface. Indeed, when phosphorylation of this serine was mimicked by mutation into an aspartate, the protein interaction was completely prevented (Meiselbach et al., 2006).
MGluR8b in Contact with the SUMO E2-Conjugating Enzyme Ubc9
Besides protein phosphorylation, SUMOylation represents another biological mechanism to change the surface characteristic of binding partners. Although originally described for nuclear proteins, the covalent attachment of SUMO to targets regulates a variety of physiological processes, including transport of proteins, synaptic excitability and protein-protein interactions (Wilkinson et al., 2010). Thus, as protein phosphorylation, also SUMOylation regulates neuronal function and indeed, enzymes of the SUMOylation machinery physically interact with neurotransmitter receptors and an association between SUMOylation and neurodegeneration is discussed (Tang et al., 2005; Martin et al., 2007; Wilkinson et al., 2010; Dütting et al., 2011).
For SUMOylation, SUMO proteins are first activated by an E1-activating enzyme and subsequently transferred to the E2-conjugating enzyme Ubc9 (Gareau and Lima, 2010). Ubc9 recognizes consensus sequences for SUMOylation in the target that all carry a lysine residue for covalent binding of the SUMO protein at position +2 (ψKx[D/E]; ψ—large hydrophobic amino acid, x—any amino acid). This enzymatic process can be guided/facilitated by E3-ligases, such as PC2, RanBP2, or members of the PIAS protein family. In addition to this enzymatically controlled covalent attachment of SUMO proteins, non-covalent SUMO interaction motifs were described (Perry et al., 2008).
SUMO E2- and E3-ligases interact with C-terminal domains of group III mGluR types and SUMOylate the purified mGluR7a and mGluR8a C-termini (Tang et al., 2005; Wilkinson and Henley, 2011). However, SUMOylation of the full-length mGluR7a was undetectable (Wilkinson and Henley, 2011). In contrast, two intracellular lysine residues located in the C-terminus of the complete mGluR8b receptor protein were SUMOylated in mammalian cells (Dütting et al., 2011). The mGluR8b C-terminus contains one bona-fide SUMOylation site (VKSE) that is located in the proximal region of the mGluR8b C-terminus, which is identical between the mGluR8a and mGluR8b isoforms (Figure 1B). In addition, mGluR8b—but not mGluR8a—carries a second non-canonical SUMOylation motif (VKSG) located in the isoform specific, distal part of the C-terminus. The lysine residues present at position +2 in both motifs were covalently linked to SUMO proteins and this process was enhanced in the presence of the E3-ligase PIAS1 (Dütting et al., 2011).
The three-dimensional structure of the interaction site between the VKSE and VKSG sequences in the mGluR8b C-terminus and the E2-conjugating enzyme Ubc9 was analyzed, based on the solved crystal structure of RanGAP1 in complex with Ubc9 (Bernier-Villamor et al., 2002). Structure-based computational techniques predicted an extended backbone conformation for the two mGluR8b motifs VKSE and VKSG after complex formation (Dütting et al., 2011). In both sequences, Val+1 interacts with a hydrophobic pocket on the Ubc9 surface, while the side chain of Ser+3 points away from the mGluR8b/Ubc9 interface. The exchange from Glu+4 to Gly+4 in the second, non-canonical mGluR8b motif disrupts the hydrogen bonds formed at this position between RanGAP1 and Ubc9 in the original crystal. Estimation of the binding energy by two independent methods suggested that the Ubc9 affinity of the VKSG motif is reduced by 1.2–1.6 kcal/mol, which corresponds to an 8–15-fold change (Dütting et al., 2011). Thus, compared to the VKSE motif, the Ubc9 binding affinity of the non-canonical VKSG motif is reduced by about one order of magnitude, being within the range of measured binding affinities of naturally occurring SUMOylation sites that show variations of up to three orders of magnitude (Macauley et al., 2006).
MGluR7a in Contact with Calmodulin
Calcium ions function as second messengers and, e.g., regulate the activity of PKC isoforms. The intracellular calcium concentration is controlled by calcium binding proteins and some of them were shown to interact with mGluR C-termini, e.g., Calmodulin. Interestingly, the binding of Calmodulin to group I and III mGluR types is regulated by both, calcium ions and PKC mediated phosphorylation (Minakami et al., 1997; Nakajima et al., 1999; O'Connor et al., 1999).
The three-dimensional structure of the mGluR7a C-terminus in contact with Calmodulin was investigated using biophysical and computational techniques (Scheschonka et al., 2008). Based on NMR spectroscopy with peptides that contain the Calmodulin binding region of mGluR7a, the authors suggest that amino acids 856–879 of mGluR7a, being identical between the mGluR7a and mGluR7b isoforms (see Figure 1B), adopt a α-helical structure that forms a 1-8-14 binding motif. This α-helix is embedded between the globular N- and C-terminal domains of Calmodulin in a “classical wraparound structure.” These data are supported by homology based molecular modeling using the solved crystal structure of Calmodulin in complex with the smooth muscle myosin light chain kinase (PDB entry 1QTX). Within the α-helical region of the mGluR7a C-terminus, polar side chains of amino acids 859–861 form electrostatic contacts, while four hydrophobic anchor residues (Phe863, Val867, Met872, and Leu876) interact with hydrophobic pockets of Calmodulin. The formation of an α-helical structure is further supported by the above described prediction of SLiMs that identified an amphipathic helix (PAH2 interaction motif) between Phe863 and Ala870 of the mGluR7a C-terminus (Figure 1B; Seebahn et al., 2011). Furthermore, the same SLiM was predicted in the C-termini of mGluR7b and both mGluR8 isoforms and indeed, binding of Calmodulin to these receptors has been demonstrated (O'Connor et al., 1999; El Far et al., 2001).
A recent study describes multiple conformations of the mGluR7a C-terminus in complex with Calmodulin, applying CD, and NMR measurements (Isozumi et al., 2011). Under these experimental conditions, the Calmodulin binding region of mGluR7a does not adopt α-helical structures that remain stable over time. Based on their data, the authors propose multiple conformations for the mGluR7a/Calmodulin protein complex and suggest an equilibrium between unstructured and α-helical geometries.
MGluR1a and mGluR5a/b in Contact with Homer
Homer proteins are scaffolds that interact with the group I receptors mGluR1a and mGluR5a/b. Scaffold proteins serve as molecular platforms that dynamically control the assembly of functionally related molecules within the postsynaptic density (Renner et al., 2008). Dysfunction of resulting protein complexes may lead to neurological diseases and indeed, Homer proteins are related to neuropsychiatric disorders, including addiction, depression, epilepsy, and schizophrenia (Szumlinski et al., 2006; Durand et al., 2007; Bourgeron, 2009). Besides their interaction with group I mGluR types, Homer isoforms also bind other proteins, e.g., IP3 receptors and Shank proteins (Tu et al., 1998, 1999; Kitano et al., 2002). Homer and Shank form a mesh-like structure in postsynaptic terminals needed for the maintenance of dendritic spines (Hayashi et al., 2009). Therefore, it is not surprising that disassembly of this mesh-like structure by amyloid-β, or mutations in Shank proteins are linked to CNS disorders, such as Alzheimer's disease or autism spectrum disorders (Durand et al., 2007; Roselli et al., 2009).
Today, more than 20 different Homer isoforms are described that originate form alternative splicing of three Homer genes (Shiraishi-Yamaguchi and Furuichi, 2007). Most Homer proteins are able to dimerize via a C-terminal coiled-coil region. Furthermore, an N-terminally located Ena/VASP homology 1 (EVH1) domain binds proline rich amino acid sequences, such as the PPxxF motif (x—any amino acid) present in the C-termini of mGluR1a and mGluR5a/b. Thus, Homer proteins that form dimers via their C-terminal coiled-coil regions physically link group I mGluR types to other functionally related proteins carrying proline rich motifs, e.g., Shank proteins and IP3 receptors (Tu et al., 1998, 1999). Interestingly, expression of a dominant-negative acting Homer isoform that lacks the C-terminal dimerization region (Homer 1a) is induced by neuronal activity and interferes with the formation of mGluR/Homer associated signal complexes (Brakeman et al., 1997; Kato et al., 1997).
The amino acid sequence TPPSPF is present in the C-termini of mGluR1a and both mGluR5 variants, fulfills the above described consensus sequence (PPxxF) and was co-crystallized in complex with an EVH1 domain containing region of Homer 1a (amino acids 1–111; Beneken et al., 2000). The two conserved proline residues within the four N-terminal amino acids of the binding motif (TPPS) adopt the conformation of a type II polyproline helix. The N-terminal amino acid (Thr+1) does not form a direct contact with the EVH1 domain and indeed, the authors found that position +1 is not conserved among different Homer interacting proteins. The two amino acids Pro+3 and Ser+4 also participate in a beta turn that is formed by the sequence PSPF. As a consequence, the side chains of the proline residues at position +2 and +3, as well as of Phe+6 all point to the mGluR/Homer interface and form direct contacts to the EVH1 domain. In contrast, side chains of Ser+4 and Pro+5 point away from the binding surface and are exposed to the solvent. As it is the case for Thr+1, also these amino acid positions are not conserved in Homer binding proteins.
MGluR5a/b in Contact with Tamalin
The scaffold protein Tamalin interacts with group I mGluR types and regulates surface expression and targeting of mGluR1a and mGluR5a/b in hippocampal neurons (Kitano et al., 2002). The C-terminal domains of group I mGluRs contact the PDZ-domain of Tamalin and a peptide representing the very C-terminal amino acids that are identical between mGluR5a and mGluR5b isoforms (SSSSL) was used to analyze the geometry of the protein complex (Sugi et al., 2007). Mostly, PDZ domain binding motifs contain the C-terminal three amino acids of the ligand, however binding of PDZ domains to internal protein sequences was also observed (Lee and Zheng, 2010). While recently up to 16 different classes of PDZ domains were suggested (Tonikian et al., 2008), traditionally C-terminally located PDZ domain binding motifs are subdivided in three classes according to the consensus sequences [ST]xΦ, ΦxΦ and [DE]xΦ (x—any amino acid; Φ—hydrophobic amino acid). In these consensus sequences, the amino acid type at position −1 is variable, indicating that the side chain of residue −1 does not form specific interactions with the PDZ domain binding pocket. Therefore, it is interesting that the side chain of Ser−1 of the PDZ domain binding motif SSSSL in the mGluR5 C-terminus contributes to a hydrogen bonding network in which a water molecule bridges its hydroxyl group to two polar groups of Tamalin (Sugi et al., 2007). Thus, amino acids not being part of consensus sequences for PDZ domain binding motifs can contact the PDZ domain binding pocket and regulate binding affinities. Indeed, binding of the mGluR7b C-terminal domain to the PDZ domains of PICK1 and Syntenin required amino acids located at positions −6 and −7 of the receptors' C-terminus (Enz and Croci, 2003).
Without an interaction partner, Tamalin adopts an auto-inhibited state in which an intrinsic C-terminal sequence (EESQL) occupies the internal PDZ domain of the scaffold (Sugi et al., 2007). The mGluR5 C-terminal peptide competes with the intrinsic ligand of Tamalin for PDZ domain binding. Variations in electrostatic forces between the two ligands and the PDZ domain discriminate between interactors. These variations are due to the above described hydrogen network of Ser−1 and additional hydrogen bond formation of Ser−3 of the mGluR5 ligand. Both interactions cannot be realized by the intrinsic ligand of Tamalin due to its different amino acid sequence. After binding of the mGluR5 C-terminal PDZ motif, the intrinsic ligand of Tamalin is displaced and now able to bind a receptor for kinesin motor proteins, the synaptic scaffolding molecule S-SCAM, thereby regulating subcellular trafficking of interacting mGluR types (Kitano et al., 2003). Both binding partners, mGluR5 and the PDZ domain of Tamalin are able to dimerize, resulting in a tetrameric protein complex (Sugi et al., 2007). Thus, besides regulating trafficking and surface expression of group I mGluRs, scaffold proteins might also facilitate receptor dimerization.
Conclusion
Besides the well-known binding of heterotrimeric G-Proteins to mGluRs, within the last decade a huge diversity of additional mGluR interactors were identified, the majority of them binding to the receptors' intracellular C-termini. While increasing information is available describing the conformation of extracellular and transmembrane domains of G-protein coupled receptors, structural data elucidating the nature of intracellular receptor regions is rather sparse. Recently, structural characteristics of different mGluR C-terminal domains without, or in complex with binding proteins were reported. From these data a picture emerges, in which group III mGluR C-termini are intrinsically disordered and do not seem to form secondary or tertiary structures that remain stable over time. Rather, these domains adopt a defined three-dimensional conformation only upon interaction with mGluR binding proteins. MGluR C-termini contain multiple and sometimes overlapping short linear binding motifs, most of them clustered in the isoform specific, distal regions of the receptor domains. In contrast to preformed folds, the use of short linear binding motifs allows the regulation of the glutamate receptors by a high number of diverse binding partners. In conclusion, the principle idea that biological function may require the absence of a defined three-dimensional structure is evolving as a general feature in living systems and indeed, about 70% of signal proteins contain intrinsically disordered protein regions (Uversky and Dunker, 2010).
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I thank Heike Meiselbach for providing graphics assembled in Figure 3 and Heinrich Sticht for critically reading the manuscript. Work in my laboratory is supported by grants from the Deutsche Forschungsgemeinschaft (DFG), the Bundesministerium für Bildung und Forschung (BMBF) and the Interdisciplinary Centre for Clinical Research (IZKF) at the university hospital of the Friedrich-Alexander-Universität Erlangen-Nürnberg.
Abbreviations
Å, angstrom; AC, adenylate cyclase; cAMP, cyclic adenosine monophosphate; CD, circular dichroism; CNS, central nervous system; CT, C-terminus; ELM, eukaryotic linear motif; EVH1, enabled/VASP homology type 1; HSQC, heteronuclear single quantum coherence; IP3, inositol-1,4,5-trisphosphate; mGluR, metabotropic glutamate receptor; NMDA, N-methyl-D-aspartate; NMR, nuclear magnetic resonance; ns, nano second; PC2, polycomb protein; PDZ, postsynaptic density 95/discs-large/zona occludens 1; PIAS, protein inhibitor of activated STAT; PICK, protein interacting with C-kinase; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; PP1, protein phosphatase 1; RanBP2, Ran binding protein 2; RanGAP1, Ran GTPase activating protein 1; SLiM, short linear motif; SUMO, small ubiquitin-like modifier; S-SCAM, synaptic scaffolding molecule.
References
Ahn, K. H., Pellegrini, M., Tsomaia, N., Yatawara, A. K., Kendall, D. A., and Mierke, D. F. (2009). Structural analysis of the human cannabinoid receptor one carboxyl-terminus identifies two amphipathic helices. Biopolymers 91, 565–573.
Airas, J. M., Betz, H., and El Far, O. (2001). PKC phosphorylation of a conserved serine residue in the C-terminus of group III metabotropic glutamate receptors inhibits calmodulin binding. FEBS Lett. 494, 60–63.
Bard, L., and Groc, L. (2011). Glutamate receptor dynamics and protein interaction: lessons from the NMDA receptor. Mol. Cell. Neurosci. 48, 298–307.
Baude, A., Nusser, Z., Roberts, J. D., Mulvihill, E., McIlhinney, R. A., and Somogyi, P. (1993). The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron 11, 771–787.
Beneken, J., Tu, J. C., Xiao, B., Nuriya, M., Yuan, J. P., Worley, P. F., and Leahy, D. J. (2000). Structure of the Homer EVH1 domain-peptide complex reveals a new twist in polyproline recognition. Neuron 26, 143–154.
Bernier-Villamor, V., Sampson, D. A., Matunis, M. J., and Lima, C. D. (2002). Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell 108, 345–356.
Bollen, M., Peti, W., Ragusa, M. J., and Beullens, M. (2010). The extended PP1 toolkit: designed to create specificity. Trends Biochem. Sci. 35, 450–458.
Brakeman, P. R., Lanahan, A. A., O'Brien, R., Roche, K., Barnes, C. A., Huganir, R. L., and Worley, P. F. (1997). Homer: a protein that selectively binds metabotropic glutamate receptors. Nature 386, 284–288.
Brandstätter, J. H., Koulen, P., Kuhn, R., van der Putten, H., and Wässle, H. (1996). Compartmental localization of a metabotropic glutamate receptor (mGluR7): two different active sites at a retinal synapse. J. Neurosci. 16, 4749–4756.
Bruno, A., Guadix, A. E., and Costantino, G. (2009). Molecular dynamics simulation of the heterodimeric mGluR2/5HT(2A) complex. An atomistic resolution study of a potential new target in psychiatric conditions. J. Chem. Inf. Model. 49, 1602–1616.
Cabello, N., Gandia, J., Bertarelli, D. C., Watanabe, M., Lluis, C., Franco, R., Ferre, S., Lujan, R., and Ciruela, F. (2009). Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells. J. Neurochem. 109, 1497–1507.
Cai, Z., Saugstad, J. A., Sorensen, S. D., Ciombor, K. J., Zhang, C., Schaffhauser, H., Hubalek, F., Pohl, J., Duvoisin, R. M., and Conn, P. J. (2001). Cyclic AMP-dependent protein kinase phosphorylates group III metabotropic glutamate receptors and inhibits their function as presynaptic receptors. J. Neurochem. 78, 756–766.
Cao, Y., Posokhova, E., and Martemyanov, K. A. (2011). TRPM1 forms complexes with nyctalopin in vivo and accumulates in postsynaptic compartment of ON-bipolar neurons in mGluR6-dependent manner. J. Neuroscience. 31, 11521–11526.
Cheng, Y., LeGall, T., Oldfield, C. J., Mueller, J. P., Van, Y. Y., Romero, P., Cortese, M. S., Uversky, V. N., and Dunker, A. K. (2006). Rational drug design via intrinsically disordered protein. Trends Biotechnol. 24, 435–442.
Chien, E. Y., Liu, W., Zhao, Q., Katritch, V., Han, G. W., Hanson, M. A., Shi, L., Newman, A. H., Javitch, J. A., Cherezov, V., and Stevens, R. C. (2010). Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 330, 1091–1095.
Ciruela, F., Escriche, M., Burgueno, J., Angulo, E., Casado, V., Soloviev, M. M., Canela, E. I., Mallol, J., Chan, W. Y., Lluis, C., McIlhinney, R. A., and Franco, R. (2001). Metabotropic glutamate 1alpha and adenosine A1 receptors assemble into functionally interacting complexes. J. Biol. Chem. 276, 18345–18351.
Croci, C., Sticht, H., Brandstätter, J. H., and Enz, R. (2003). Group I metabotropic glutamate receptors bind to protein phosphatase 1C. Mapping and modeling of interacting sequences. J. Biol. Chem. 278, 50682–50690.
Dale, L. B., Bhattacharya, M., Seachrist, J. L., Anborgh, P. H., and Ferguson, S. S. (2001). Agonist-stimulated and tonic internalization of metabotropic glutamate receptor 1a in human embryonic kidney 293 cells: agonist-stimulated endocytosis is beta-arrestin1 isoform-specific. Mol. Pharmacol. 60, 1243–1253.
Dev, K. K., Nakajima, Y., Kitano, J., Braithwaite, S. P., Henley, J. M., and Nakanishi, S. (2000). PICK1 interacts with and regulates PKC phosphorylation of mGLUR7. J. Neurosci. 20, 7252–7257.
Dhami, G. K., Babwah, A. V., Sterne-Marr, R., and Ferguson, S. S. (2005). Phosphorylation-independent regulation of metabotropic glutamate receptor 1 signaling requires g protein-coupled receptor kinase 2 binding to the second intracellular loop. J. Biol. Chem. 280, 24420–24427.
Dhami, G. K., and Ferguson, S. S. (2006). Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol. Ther. 111, 260–271.
Dinkel, H., Michael, S., Weatheritt, R. J., Davey, N. E., Van Roey, K., Altenberg, B., Toedt, G., Uyar, B., Seiler, M., Budd, A., Jodicke, L., Dammert, M. A., Schroeter, C., Hammer, M., Schmidt, T., Jehl, P., McGuigan, C., Dymecka, M., Chica, C., Luck, K., Via, A., Chatr-Aryamontri, A., Haslam, N., Grebnev, G., Edwards, R. J., Steinmetz, M. O., Meiselbach, H., Diella, F., and Gibson, T. J. (2012). ELM–the database of eukaryotic linear motifs. Nucleic Acids Res. 40, D242–D251.
Doumazane, E., Scholler, P., Zwier, J. M., Eric, T., Rondard, P., and Pin, J. P. (2011). A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 25, 66–77.
Durand, C. M., Betancur, C., Boeckers, T. M., Bockmann, J., Chaste, P., Fauchereau, F., Nygren, G., Rastam, M., Gillberg, I. C., Anckarsater, H., Sponheim, E., Goubran-Botros, H., Delorme, R., Chabane, N., Mouren-Simeoni, M. C., de Mas, P., Bieth, E., Roge, B., Heron, D., Burglen, L., Gillberg, C., Leboyer, M., and Bourgeron, T. (2007). Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat. Genet. 39, 25–27.
Dütting, E., Schröder-Kress, N., Sticht, H., and Enz, R. (2011). SUMO E3 ligases are expressed in the retina and regulate SUMOylation of the metabotropic glutamate receptor 8b. Biochem. J. 435, 365–371.
Egloff, M. P., Johnson, D. F., Moorhead, G., Cohen, P. T., Cohen, P., and Barford, D. (1997). Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 16, 1876–1887.
El Far, O., Airas, J., Wischmeyer, E., Nehring, R. B., Karschin, A., and Betz, H. (2000). Interaction of the C-terminal tail region of the metabotropic glutamate receptor 7 with the protein kinase C substrate PICK1. Eur. J. Neurosci. 12, 4215–4221.
El Far, O., Bofill-Cardona, E., Airas, J. M., O'Connor, V., Boehm, S., Freissmuth, M., Nanoff, C., and Betz, H. (2001). Mapping of calmodulin and Gbetagamma binding domains within the C-terminal region of the metabotropic glutamate receptor 7A. J. Biol. Chem. 276, 30662–30669.
Enz, R. (2007). The trick of the tail: protein-protein interactions of metabotropic glutamate receptors. Bioessays 29, 60–73.
Enz, R. (2012). Metabotropic glutamate receptors and interacting proteins: evolving drug target. Curr. Drug Targets 13, 145–156.
Enz, R., and Croci, C. (2003). Different binding motifs in metabotropic glutamate receptor type 7b for filamin A, protein phosphatase 1C, protein interacting with protein kinase C (PICK) 1 and syntenin allow the formation of multimeric protein complexes. Biochem. J. 372, 183–191.
Ferraguti, F., and Shigemoto, R. (2006). Metabotropic glutamate receptors. Cell Tissue Res. 326, 483–504.
Ferre, S., Karcz-Kubicha, M., Hope, B. T., Popoli, P., Burgueno, J., Gutierrez, M. A., Casado, V., Fuxe, K., Goldberg, S. R., Lluis, C., Franco, R., and Ciruela, F. (2002). Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc. Natl. Acad. Sci. U.S.A. 99, 11940–11945.
Gareau, J. R., and Lima, C. D. (2010). The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat. Rev. Mol. Cell Biol. 11, 861–871.
Gonzalez-Maeso, J., Ang, R. L., Yuen, T., Chan, P., Weisstaub, N. V., Lopez-Gimenez, J. F., Zhou, M., Okawa, Y., Callado, L. F., Milligan, G., Gingrich, J. A., Filizola, M., Meana, J. J., and Sealfon, S. C. (2008). Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452, 93–97.
Harmar, A. J., Hills, R. A., Rosser, E. M., Jones, M., Buneman, O. P., Dunbar, D. R., Greenhill, S. D., Hale, V. A., Sharman, J. L., Bonner, T. I., Catterall, W. A., Davenport, A. P., Delagrange, P., Dollery, C. T., Foord, S. M., Gutman, G. A., Laudet, V., Neubig, R. R., Ohlstein, E. H., Olsen, R. W., Peters, J., Pin, J. P., Ruffolo, R. R., Searls, D. B., Wright, M. W., and Spedding, M. (2009). IUPHAR-DB: the IUPHAR database of G protein-coupled receptors and ion channels. Nucleic Acids Res. 37, D680–D685.
Hayashi, M. K., Tang, C., Verpelli, C., Narayanan, R., Stearns, M. H., Xu, R. M., Li, H., Sala, C., and Hayashi, Y. (2009). The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell 137, 159–171.
Hibbs, R. E., and Gouaux, E. (2011). Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474, 54–60.
Houamed, K. M., Kuijper, J. L., Gilbert, T. L., Haldeman, B. A., O'Hara, P. J., Mulvihill, E. R., Almers, W., and Hagen, F. S. (1991). Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science 252, 1318–1321.
Isozumi, N., Iida, Y., Nakatomi, A., Nemoto, N., Yazawa, M., and Ohki, S. (2011). Conformation of the calmodulin-binding domain of metabotropic glutamate receptor subtype 7 and its interaction with calmodulin. J. Biochem. 149, 463–474.
Jong, Y. J., Kumar, V., and O'Malley, K. L. (2009). Intracellular metabotropic glutamate receptor 5 (mGluR5) activates signaling cascades distinct from cell surface counterparts. J. Biol. Chem. 284, 35827–35838.
Kato, A., Ozawa, F., Saitoh, Y., Hirai, K., and Inokuchi, K. (1997). vesl, a gene encoding VASP/Ena family related protein, is upregulated during seizure, long-term potentiation and synaptogenesis. FEBS Lett. 412, 183–189.
Kitano, J., Kimura, K., Yamazaki, Y., Soda, T., Shigemoto, R., Nakajima, Y., and Nakanishi, S. (2002). Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins. J. Neurosci. 22, 1280–1289.
Kitano, J., Yamazaki, Y., Kimura, K., Masukado, T., Nakajima, Y., and Nakanishi, S. (2003). Tamalin is a scaffold protein that interacts with multiple neuronal proteins in distinct modes of protein-protein association. J. Biol. Chem. 278, 14762–14768.
Kumar, V., Fahey, P. G., Jong, Y. J., Ramanan, N., and O'Malley, K. L. (2011). Activation of the intracellular metabotropic glutamate receptor 5 in striatal neurons leads to upregulation of genes associated with sustained synaptic transmission including Arc/Arg3.1. J. Biol. Chem. 287, 5412–5425.
Kunishima, N., Shimada, Y., Tsuji, Y., Sato, T., Yamamoto, M., Kumasaka, T., Nakanishi, S., Jingami, H., and Morikawa, K. (2000). Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407, 971–977.
Lee, H. J., and Zheng, J. J. (2010). PDZ domains and their binding partners: structure, specificity, and modification. Cell Commun. Signal. 8, 8.
Lujan, R., Roberts, J. D., Shigemoto, R., Ohishi, H., and Somogyi, P. (1997). Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J. Chem. Neuroanat. 13, 219–241.
Macauley, M. S., Errington, W. J., Scharpf, M., Mackereth, C. D., Blaszczak, A. G., Graves, B. J., and McIntosh, L. P. (2006). Beads-on-a-string, characterization of ETS-1 sumoylated within its flexible N-terminal sequence. J. Biol. Chem. 281, 4164–4172.
Mao, L. M., Liu, X. Y., Zhang, G. C., Chu, X. P., Fibuch, E. E., Wang, L. S., Liu, Z., and Wang, J. Q. (2008). Phosphorylation of group I metabotropic glutamate receptors (mGluR1/5) in vitro and in vivo. Neuropharmacology 55, 403–408.
Martin, R., Durroux, T., Ciruela, F., Torres, M., Pin, J. P., and Sanchez-Prieto, J. (2010). The metabotropic glutamate receptor mGlu7 activates phospholipase C, translocates munc-13-1 protein, and potentiates glutamate release at cerebrocortical nerve terminals. J. Biol. Chem. 285, 17907–17917.
Martin, S., Nishimune, A., Mellor, J. R., and Henley, J. M. (2007). SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature 447, 321–325.
Masu, M., Iwakabe, H., Tagawa, Y., Miyoshi, T., Yamashita, M., Fukuda, Y., Sasaki, H., Hiroi, K., Nakamura, Y., Shigemoto, R., Takada, M., Nakamura, K., Nakao, K., Katsuki, M., and Nakanishi, S. (1995). Specific deficit of the ON response in visual transmission by targeted disruption of the mGluR6 gene. Cell 80, 757–765.
Masu, M., Tanabe, Y., Tsuchida, K., Shigemoto, R., and Nakanishi, S. (1991). Sequence and expression of a metabotropic glutamate receptor. Nature 349, 760–765.
Maurice, P., Guillaume, J. L., Benleulmi-Chaachoua, A., Daulat, A. M., Kamal, M., and Jockers, R. (2011). GPCR-interacting proteins, major players of GPCR function. Adv. Pharmacol. 62, 349–380.
Meiselbach, H., Sticht, H., and Enz, R. (2006). Structural analysis of the protein phosphatase 1 docking motif: molecular description of binding specificities identifies interacting proteins. Chem. Biol. 13, 49–59.
Minakami, R., Jinnai, N., and Sugiyama, H. (1997). Phosphorylation and calmodulin binding of the metabotropic glutamate receptor subtype 5 (mGluR5) are antagonistic in vitro. J. Biol. Chem. 272, 20291–20298.
Mundell, S. J., Pula, G., Carswell, K., Roberts, P. J., and Kelly, E. (2003). Agonist-induced internalization of metabotropic glutamate receptor 1A: structural determinants for protein kinase C- and G protein-coupled receptor kinase-mediated internalization. J. Neurochem. 84, 294–304.
Muto, T., Tsuchiya, D., Morikawa, K., and Jingami, H. (2007). Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc. Natl. Acad. Sci. U.S.A. 104, 3759–3764.
Nakajima, Y., Yamamoto, T., Nakayama, T., and Nakanishi, S. (1999). A relationship between protein kinase C phosphorylation and calmodulin binding to the metabotropic glutamate receptor subtype 7. J. Biol. Chem. 274, 27573–27577.
Neduva, V., and Russell, R. B. (2006). Peptides mediating interaction networks: new leads at last. Curr. Opin. Biotechnol. 17, 465–471.
Nicoletti, F., Bockaert, J., Collingridge, G. L., Conn, P. J., Ferraguti, F., Schoepp, D. D., Wroblewski, J. T., and Pin, J. P. (2011). Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60, 1017–1041.
Nusser, Z., Mulvihill, E., Streit, P., and Somogyi, P. (1994). Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience 61, 421–427.
O'Connor, V., El Far, O., Bofill-Cardona, E., Nanoff, C., Freissmuth, M., Karschin, A., Airas, J. M., Betz, H., and Boehm, S. (1999). Calmodulin dependence of presynaptic metabotropic glutamate receptor signaling. Science 286, 1180–1184.
Perroy, J., Prezeau, L., De Waard, M., Shigemoto, R., Bockaert, J., and Fagni, L. (2000). Selective blockade of P/Q-type calcium channels by the metabotropic glutamate receptor type 7 involves a phospholipase C pathway in neurons. J. Neurosci. 20, 7896–7904.
Perry, J. J., Tainer, J. A., and Boddy, M. N. (2008). A SIM-ultaneous role for SUMO and ubiquitin. Trends Biochem. Sci. 33, 201–208.
Rasmussen, S. G., Choi, H. J., Rosenbaum, D. M., Kobilka, T. S., Thian, F. S., Edwards, P. C., Burghammer, M., Ratnala, V. R., Sanishvili, R., Fischetti, R. F., Schertler, G. F., Weis, W. I., and Kobilka, B. K. (2007). Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature 450, 383–387.
Renner, M., Specht, C. G., and Triller, A. (2008). Molecular dynamics of postsynaptic receptors and scaffold proteins. Curr. Opin. Neurobiol. 18, 532–540.
Rose, M., Dütting, E., Schröder, N., Sticht, H., Brandstätter, J. H., and Enz, R. (2008). PNUTS forms a trimeric protein complex with GABA(C) receptors and protein phosphatase 1. Mol. Cell. Neurosci. 37, 808–819.
Roselli, F., Hutzler, P., Wegerich, Y., Livrea, P., and Almeida, O. F. (2009). Disassembly of shank and homer synaptic clusters is driven by soluble beta-amyloid(1-40) through divergent NMDAR-dependent signalling pathways. PLoS One 4:e6011. doi: 10.1371/journal.pone.0006011
Rosenbaum, D. M., Rasmussen, S. G., and Kobilka, B. K. (2009). The structure and function of G-protein-coupled receptors. Nature 459, 356–363.
Scheschonka, A., Findlow, S., Schemm, R., El Far, O., Caldwell, J. H., Crump, M. P., Holden-Dye, K., O'Connor, V., Betz, H., and Werner, J. M. (2008). Structural determinants of calmodulin binding to the intracellular C-terminal domain of the metabotropic glutamate receptor 7A. J. Biol. Chem. 283, 5577–5588.
Seebahn, A., Dinkel, H., Mohrluder, J., Hartmann, R., Vogel, N., Becker, C. M., Sticht, H., and Enz, R. (2011). Structural characterization of intracellular C-terminal domains of group III metabotropic glutamate receptors. FEBS Lett. 585, 511–516.
Shigemoto, R., Kinoshita, A., Wada, E., Nomura, S., Ohishi, H., Takada, M., Flor, P. J., Neki, A., Abe, T., Nakanishi, S., and Mizuno, N. (1997). Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J. Neurosci. 17, 7503–7522.
Shigemoto, R., Kulik, A., Roberts, J. D., Ohishi, H., Nusser, Z., Kaneko, T., and Somogyi, P. (1996). Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature 381, 523–525.
Sobolevsky, A. I., Rosconi, M. P., and Gouaux, E. (2009). X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature 462, 745–756.
Sugi, T., Oyama, T., Muto, T., Nakanishi, S., Morikawa, K., and Jingami, H. (2007). Crystal structures of autoinhibitory PDZ domain of Tamalin: implications for metabotropic glutamate receptor trafficking regulation. EMBO J. 26, 2192–2205.
Szumlinski, K. K., Kalivas, P. W., and Worley, P. F. (2006). Homer proteins: implications for neuropsychiatric disorders. Curr. Opin. Neurobiol. 16, 251–257.
Tang, Z., El Far, O., Betz, H., and Scheschonka, A. (2005). Pias1 interaction and sumoylation of metabotropic glutamate receptor 8. J. Biol. Chem. 280, 38153–38159.
Tonikian, R., Zhang, Y., Sazinsky, S. L., Currell, B., Yeh, J. H., Reva, B., Held, H. A., Appleton, B. A., Evangelista, M., Wu, Y., Xin, X., Chan, A. C., Seshagiri, S., Lasky, L. A., Sander, C., Boone, C., Bader, G. D., and Sidhu, S. S. (2008). A specificity map for the PDZ domain family. PLoS Biol. 6:e239. doi: 10.1371/journal.pbio.0060239
Tu, J. C., Xiao, B., Naisbitt, S., Yuan, J. P., Petralia, R. S., Brakeman, P., Doan, A., Aakalu, V. K., Lanahan, A. A., Sheng, M., and Worley, P. F. (1999). Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23, 583–592.
Tu, J. C., Xiao, B., Yuan, J. P., Lanahan, A. A., Leoffert, K., Li, M., Linden, D. J., and Worley, P. F. (1998). Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 21, 717–726.
Uversky, V. N., and Dunker, A. K. (2010). Understanding protein non-folding. Biochim. Biophys. Acta 1804, 1231–1264.
Vardi, N., Duvoisin, R., Wu, G., and Sterling, P. (2000). Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J. Comp. Neurol. 423, 402–412.
Wilkinson, K. A., and Henley, J. M. (2011). Analysis of metabotropic glutamate receptor 7 as a potential substrate for SUMOylation. Neurosci. Lett. 491, 181–186.
Keywords: binding surface, metabotropic glutamate receptor, G-protein coupled receptor, mGluR, protein-protein interaction, signaling complex, short linear motif, structure
Citation: Enz R (2012) Structure of metabotropic glutamate receptor C-terminal domains in contact with interacting proteins. Front. Mol. Neurosci. 5:52. doi: 10.3389/fnmol.2012.00052
Received: 26 January 2012; Paper pending published: 26 February 2012;
Accepted: 02 April 2012; Published online: 23 April 2012.
Edited by:
Hans-Georg Breitinger, The German University in Cairo, EgyptReviewed by:
Frantisek Jursky, Institute of Molecular Biology, Slovak RepublicRalf Jockers, University of Paris, France
Copyright: © 2012 Enz. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Ralf Enz, Emil-Fischer-Zentrum, Institut für Biochemie, Friedrich-Alexander-Universität Erlangen-Nürnberg, Fahrstrasse 17, 91054 Erlangen, Germany. e-mail: ralf.enz@biochem.uni-erlangen.de