Bone marrow fibrosis in primary myelofibrosis: pathogenic mechanisms and the role of TGF-β
Introduction
Essential thrombocythemia (ET), polycythemia vera (PV) and primary myelofibrosis (PMF) constitute the classical group of BCR-ABL (Ph) negative chronic myeloproliferative disorders, now termed myeloproliferative neoplasms (MPN) (1). JAK2V617F is the best characterized mutation seen in these disorders with prevalence of more than 95% in PV, 50–70% in ET, and 40–50% in PMF (2). JAK2V617F mutation negative cases of PV can have JAK2 mutations in exon 12 present at a frequency of approximately 2–3% (3). JAK2V617F mutation negative cases of ET and PML can contain MPL mutations occurring at the frequency of 5% to 10% (4). Recently, a mutation in the gene encoding CALR was found to occur in the majority of MPN patients with nonmutated JAK2 or MPL (5,6) (Figure 1). The mutations JAK2 and MPL kinases lead to constitutive stimulation of the JAK-STAT pathway leading to cytokine independent growth in PV and ET (7). Furthermore, there are a number of other genetic abnormalities being discovered like CSF3R and SETBP1 mutations in other MPNs like chronic neutrophilic leukemia indicating the multi-step pathogenesis and heterogeneity in this group of disorders (8,9). Even though these mutations can explain the pathogenesis of increased proliferation in MPNs, the pathogenesis of fibrosis in PMF is still very well elucidated.
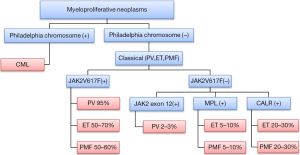
PMF carries the worst prognosis among the MPNs (10). The disease can start as PMF or present as the burnt out phase of PV (post-PV MF) or ET (post-ET MF) (11,12). It presents with anemia, splenomegaly and constitutional symptoms and is associated with a median survival of 6.5 years (10,11,13). Extramedullary hematopoiesis is seen not only in the liver and spleen but also in the lymph nodes, serosal surfaces, urogenital system and epidural and paraspinal spaces (12). Abnormal stem cell trafficking and extramedullary hematopoiesis occurs due to abnormalities in the CXCL12/CXCR4 axis (14). Bone marrow histology shows fibrosis, angiogenesis and osteosclerosis (15). Advanced reticulin or collagen fibrosis is associated with classic stages of PMF, but a diagnosis of PMF can be established without obvious fibrosis (15). Bone marrow fibrosis is the most important feature causing increased morbidity and mortality in these patients.
Pathogenesis of fibrosis in primary myelofibrosis (PMF)
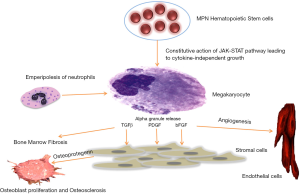
The pathogenesis of MPNs is poorly understood (12). Even though mutations in JAK2, MPL and CALR are seen and can lead to constitutive action of the JAK-STAT pathway, it has not been elucidated how these mutations can cause different clinical phenotypes (16). In all MPNs, megakaryocytes proliferate, acquire multilobulated nuclei and exhibit clustering in the marrow (17). These megakaryocytes have abnormal location of P selectin on their intracytoplasmic vacuoles and demarcation membrane system (DMS) that leads to the increased emperipolesis (the passage of a cell into the cytoplasm of another cell) of neutrophils (18). The neutrophils release their enzymes in the megakaryocytes leading to the release of cytokines such as transforming growth factor beta (TGF-β); platelet derived growth factor (PDGF) and fibroblast growth factor (FGF) from their alpha granules (18). These growth factors then stimulate fibroblasts to cause fibrosis and endothelial cells to cause neoangiogenesis (11). Increased production of osteoprotegerin by stromal and endothelial cells leads to unbalanced osteoblast proliferation, resulting in osteosclerosis and neoangiogenesis (19). Therefore, the disease is considered to be a clonal disorder of a hematopoietic/stem cell progenitor cell and the fibrosis that occurs is thought to be secondary due to cytokines released from the progenitor cells (20). Figure 2 illustrated the proposed mechanism of fibrosis in myelofibrosis.
Bone marrow fibrosis is a secondary process
The prevailing hypothesis is that bone marrow fibrosis is a secondary process. It is believed that the mutant clone stimulates the production of fibrous tissue by fibroblasts that are polyclonal in nature as evident from cytogenetics and G-6PD expression analysis of these cells (20-22). The bone marrow fibroblasts have been shown to behave normally in culture (23) and do not carry the JAK2 mutation (24). Bone marrow transplantation is thought to be effective since it can remove the mutant clone and causes reversal of fibrosis (25). However, there have been recent data that challenges this concept. Some of the cells especially endothelial cells lining the hepatic sinusoids, micro vessels and large splenic veins of the spleen, have been shown to contain the JAK2 mutation (26,27). Endothelial progenitor cells have also been shown to be clonal (28). These data raise the possibility that some of the effector cells in marrow fibrosis might be related to the mutant clone.
A defective niche might contribute to the pathogenesis
The hematopoietic stem cells (HSCs) reside in the bone marrow microenvironment and have tightly regulated interactions with various cells in this niche (29). The bone marrow niche consist of fibroblasts, osteocytes, and adipocytes derived from mesenchymal stem cells (MSCs), osteoclasts derived from hematopoietic cells and endothelial stem cells in addition to different growth factors and adhesion molecules (30). Two types of niches are described; the vascular niche and the endosteal niche (31). Whether or not there are two distinct niches or a combined hematopoietic environment is under debate, but there is evidence that there are distinct functional units. The vascular niche is located in the oxygen rich area close to the endosteum and regulates proliferation, differentiation and mobilization of the stem cells .The endosteal niche is located in the bone edge and keeps the stem cells in quiescence. An imbalance between the two niches has been suggested as a cause of myeloproliferative syndrome (31).
Two mouse models described by Walkley et al. gave us further insight into the importance of the microenvironment in causing myelofibrosis (32,33). Germ line deletion of retinoic acid receptor subtype-γ (RARγ) gene or the conditional knockout of the retinoblastoma (Rb) protein in the HSC compartment of the mice led to a myeloproliferative syndrome. Rb and RARγ are regulators of the cell cycle. In both mutant mice types, transplantation of the wild type HSCs did not lead to remission from the disease. When mutated HSCs were transplanted into wild-type mice, it did not cause myeloproliferation. Furthermore, only myeloid-restricted (compared to HSC) deletion of Rb also did not lead to myelofibrosis, hence highlighting the importance of microenvironment in the pathogenesis of this disease.
The hedgehog (Hh) signaling pathway has also been implicated in the pathogenesis of myelofibrosis using mouse models. Hh signaling pathway plays an important role in normal hematopoiesis and in the oncogenesis of hematologic malignancies. Inhibitors of the Hh pathway have been shown to inhibit growth and self-renewal capacity in preclinical models of MF. In a mouse model of MF, combined inhibition of the Hh and JAK pathways reduced JAK2 mutant allele burden, reduced bone marrow fibrosis, and reduced white blood cell and platelet counts (34).
Transforming growth factor beta (TGF-β) in bone marrow fibrosis
During hematopoiesis, TGF-β signaling pathway plays important roles in stem cell quiescence as well as in progenitor cell differentiation. This cytokine has been studied extensively as a regulator of fibrosis in myeloproliferative diseases (15). TGF-β occurs in 3 isoforms: TGFβ1, TGFβ2 and TGFβ3 (34). TGFβ1 is the most abundant of all these isoforms and platelets, megakaryocytes and bone marrow cells are sources of TGF-β production (35,36).
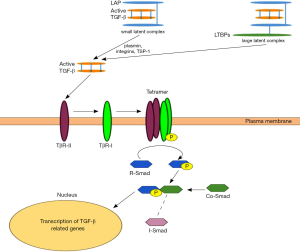
TGFβ1 is secreted as latent protein and is stored in the extracellular matrix. Latent complexes are classified as either small or large. The small complex consists of TGF-β noncovalently associated with a latency-associated protein (LAP). In the large complex, LAP is also linked to latent TGF-β-binding proteins (LTBPs) (37). Reactive oxygen species and a number of proteases including plasmin, integrins (38) and thrombospondin-1 (TSP-1) convert the inactive latent complexes to the active forms by releasing it from LTBP and LAP (37).
The active protein binds to two ubiquitous cell surface receptors—type I receptor (TBRI) and type II receptor (TBRII) with serine/threonine protein kinases in their intracellular domains (35). TGF-β binds to the TBRII which then recruits, transphosphorylates and binds to TBRI (31). Activated TBRI phosphorylates the Smad transcription factors (R-Smads 2/3) which then bind to a common Smad 4 protein (15,35,37). R-Smad/Co-Smad complexes translocate to the nucleus, where they associate with DNA-binding partners and then regulate the transcriptional response of the TGF-β target genes (35,39,40) (Figure 3). Regulation of the TGF-β pathway is accomplished by multiple negative feedback mechanisms including: down-regulating expression of cell surface receptors, increasing inhibitory Smad 6 and 7 which repress TGF-β responses, activating latent proteins, and initiating interaction of multiple transcriptional co-repressors and co-activators inside the nucleus (35).
Role of TGF-β in bone marrow fibrosis in Philadelphia negative myeloproliferative disorders
Bone marrow pathology in PMF is characterized by fibrosis, neoangiogenesis and osteosclerosis (15). Fibrosis is primarily due to an increase in production of total collagen, including types I, III, IV, and V collagens (41). Increased deposition of laminin and adhesive glycoproteins (vitronectin, fibronectin and tenascin) is seen in advanced stages of the disease. TGFβ1 increases the synthesis of types I, III and Type IV collagen in addition to the deposition of fibronectin, proteoglycans and tenascin (42). The fibrotic process induced by TGFβ1 is the combination of an increase in matrix biosynthesis, accompanied by a decrease in matrix degradation. TGFβ1 does this by decreasing the amount of matrix metalloproteinases (MMP) particularly MMP3 and increasing the synthesis of tissue inhibitors of metalloproteinase (TIMP) particularly TIMP-1 (43).
In addition to effects on the microenvironment, TGF-β has direct effects on hematopoietic cells also and is a well-known negative regulator of granulocyte, erythroid, megakaryocyte and macrophage progenitor proliferation and promotes their differentiation (44). It has been seen that hematopoietic cells in Philadelphia chromosome negative myeloproliferative disorders frequently become resistant to the effects of TGF-β due to decreased levels of TGF-β receptors and regulators. ET, PV and PMF have been associated with decreased levels of type II receptors while decreased levels of SMAD4 have been seen in some cases of ET. These data suggest that effects of TGF-β may be dominant on the bone marrow microenvironment (45,46).
Evidence from mouse models of the disease
Mouse models of myelofibrosis have provided important insights into the role of TGF-β in the pathogenesis of fibrosis (47-55). Two widely studied mouse models include the thrombopoietin (TPO) high and the GATA1 low mice. High doses of TPO result in megakaryocyte abnormalities similar to PMF patients and lead to a myeloproliferative syndrome with deposition of reticulin fibers in the bone marrow (47-49). Consequently, mice exposed to high doses of TPO transfected via a retrovirus vector developed a myeloproliferative syndrome with proliferation of megakaryocytes, extramedullary hematopoiesis, splenic and medullary fibrosis and osteosclerosis similar to PMF in humans (49,50). TGF-β in these mice models was significantly increased in the extracellular fluid of the marrow, plasma and platelet extracts (49). To determine the role of TGF-beta in this process, bone marrow stem cells obtained from knockout models of TGF-β1 gene (TGF-β1−/−) and wild-type (WT) mice were infected with a retrovirus encoding the murine TPO protein. These marrow cells were then engrafted into lethally irradiated wild-type hosts for long-term reconstitution. Both groups of animals developed a myeloproliferative syndrome with thrombocytosis, leukocytosis, splenomegaly, extramedullary hematopoiesis and increased progenitors in the blood. However the wild type mice developed severe reticulin fibrosis in the bone marrow and spleen compared to the null (TGF-β1−/−) mice and had latent TGF-β1 levels in the plasma and spleen that were 4 and 6 fold higher. These data demonstrate the important role of TGF-β as an effector of fibrosis in PMF (51).
Another mouse model that has been utilized to study myelofibrosis and myeloproliferation is the GATA-1 low mice. GATA-1 is a transcription factor that has a well-established role in erythroid and megakaryocytic cell differentiation (52). Absence of GATA-1 can also lead to myelofibrosis which was proved by the GATA-1 low mouse model. There was delay in the development of myelofibrosis in the GATA-1 low mice, starting at 15 months of age (55) but GATA-1 low mice eventually developed bone marrow fibrosis, presumably from a block in the maturation of megakaryocytes into proplatelets, resulting in accumulation of megakaryocytes in the marrow and spleen in the presence of lower GATA-1 levels compared to WT littermate (53,55). TPO exposure as discussed above is a contributing factor in developing reticulin fibrosis in WT mice models. In contrast, GATA-1 low mice treated with TPO do not develop liver and spleen fibrosis and TPO treatment leads to restoration of GATA-1 level in the megakaryocytes and down regulation of the TGF-β gene (47). A recent paper studied the role of TGF-β in the bone marrow and spleen of GATA-1 low mice (54). Alterations of TGF-β1, Hh, and p53 signaling in marrow and spleen and of mammalian target of rapamycin (mTOR) in spleen only were identified in GATA-1 low mice. Inhibition of TGF-β1 signaling in these GATA-1 low mice by an inhibitor of the tyrosine kinase activity of TGF-β1 receptor type I, led to normal development of megakaryocytes, and these mice had reduced fibrosis, neovascularization, and osteogenesis in the bone marrow, thus reducing extramedullary hematopoiesis in the spleen (54).
Evidence from human studies
Involvement of TGF-β in myelofibrosis was first examined in patients with acute megakaryoblastic leukemia with bone marrow fibrosis. Megakaryocytes produce and secrete an active form of TGF-β which was able to stimulate collagen synthesis by myofibroblasts in vitro (56). Intraplatelet levels of TGF-β were found to be elevated 2–3 fold in PMF patients as compared to controls (57). In a trial of six subjects, interferon-γ therapy for 6 months reduced the intraplatelet levels of TGF-β to almost normal in 4 subjects (58). Increased TGF-β levels were also found to be elevated in peripheral blood mononuclear cells both at the mRNA and peptide level (59). While TGF-β levels in CD34+ cells with myelofibrosis were not elevated, the expression of TGF-β II receptor was significantly decreased. TGF-β has a negative regulatory effect on CD34+ hematopoietic progenitor cells, thus in decreasing the TGF-β type II receptor, the CD34+ cells escape regulatory controls (60). Hypermethylation of the promoter of the TGF-β II receptor appears to cause the decrease in the level of the receptor (61).
In this complex disease, elevation of TGF-β alone is not the only cytokine responsible for producing fibrosis seen in myelofibrosis. Numerous studies indicate that other cytokines such as substance P (62), bFGF (63), vascular endothelial growth factor VEGF (64), increase in TIMPS and decrease in MMP (43) are also likely to be important in the pathogenesis of bone marrow fibrosis, illustrating the complexity of this disease and its treatment.
Targeting TGF-β in myelofibrosis
The discovery of the JAK2 mutation and subsequently the development of new JAK2 inhibitors have been instrumental landmarks in the treatment of symptoms in MF (65). However, unlike the tyrosine kinase inhibitors for CML, the JAK2 inhibitors have been unable to eradicate the mutant clone (66). JAK2 inhibitors have been successful in reducing spleen size and decreasing constitutional symptoms, but have not been able to reduce bone marrow fibrosis (67).
TGF-β inhibition is a potential therapeutic strategy to decrease marrow fibrosis in MPNs. Inhibition of TGF-β is also being investigated in other clinical and experimental scenarios. The levels of active TGF-β can be decreased by targeting the conversion of the latent form to the active form. Integrin αvβ6 plays an important role in converting the inactive latent complex to the active form by releasing it from LTBP and LAP as described above. Monoclonal antibody that blocks α(v)β6 integrin was evaluated in murine model of pulmonary fibrosis and it showed reduction in pulmonary fibrosis (68). A number of neutralizing antibodies have also been used against TGF-β, for example Fresolimumab (GC1008) that targets all three TGF-β isoforms. This was used in a phase I trial of treatment resistant primary focal segmental glomerulosclerosis (69) and myelofibrosis (70). Another method is to prevent the binding of TGF-β to cell surface receptors. This was demonstrated by topical use of P144, a peptide inhibitor of TGF-β1, that ameliorated skin fibrosis in a well-characterized murine model of human scleroderma (71).
The intracellular effects of TGF-β can be blocked by preventing receptor kinase activity. A small-molecule kinase inhibitor that blocks activin-like receptor kinase 5, SM305, was used in an experimental study. It showed that it blocked receptor kinase SMAD dependent activation of fibroblasts both in cell culture and mice (72).
Recent development of inhibitors of TGF-β receptor I kinase is very promising. A new molecule Galunisertib (LY2157299) is a specific inhibitor of TGF-β receptor I kinase and abrogates the phosphorylation of SMAD2. This was studied in a mouse model generated by transgenic expression of JAK2V617F that display PV-like at the age of 6 weeks and develop bone marrow and spleen fibrosis at 25–30 weeks. When these mice with established myelofibrosis were treated with LY2157299 daily for 4 weeks, a significant decrease in fibrosis was seen. This was also validated in MPLW515L transplantation mouse model with myelofibrosis, hence proving the concept of reversal of myelofibrosis with TGF-β inhibition (73).
Gene transfer of negative regulator SMAD 7 has been used in experimental renal fibrosis in mice (74). Non SMAD pathways like c-Abl that leads to TGF-β mediated fibrotic response have also been targeted and the use of Imatinib (Abl kinase inhibitor) for this purpose is a classic example (75).
Another way of targeting TGF-β is by inhibition of Aurora kinase. Megakaryocytes in PMF show impaired differentiation and Aurora kinase inhibition induces megakaryocyte maturation, thereby reducing the burden of immature megakaryocytes. This ameliorates the characteristics of PMF, including bone marrow fibrosis. This concept was proven in murine model by treatment with MLN8237, a selective AURKA inhibitor. MLN8237 promoted polyploidization and differentiation of megakaryocytes with PMF-associated mutations and had potent antifibrotic and antitumor activity in vivo in mouse models of PMF (76).
These studies have shown very encouraging results so far. Our hope is to have a greater comprehension of development of fibrosis in MPNs, leading to an era of exciting therapeutic development in this very difficult to treat disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Vakil E, Tefferi A.. BCR-ABL1--negative myeloproliferative neoplasms: a review of molecular biology, diagnosis, and treatment. Clin Lymphoma Myeloma Leuk 2011;11 Suppl 1:S37-45. [PubMed]
- Them NC, Kralovics R. Genetic basis of MPN: beyond JAK2-V617F. Curr Hematol Malig Rep 2013;8:299-306. [PubMed]
- Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007;356:459-68. [PubMed]
- Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270. [PubMed]
- Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391-405. [PubMed]
- Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379-90. [PubMed]
- Levine RL. Another piece of the myeloproliferative neoplasms puzzle. N Engl J Med 2013;369:2451-2. [PubMed]
- Li B, Gale RP, Xiao Z. Molecular genetics of chronic neutrophilic leukemia, chronic myelomonocytic leukemia and atypical chronic myeloid leukemia. J Hematol Oncol 2014;7:93. [PubMed]
- Cui Y, Li B, Gale RP, et al. CSF3R, SETBP1 and CALR mutations in chronic neutrophilic leukemia. J Hematol Oncol 2014;7:77. [PubMed]
- Komrokji RS, Verstovsek S, Padron E, et al. Advances in the management of myelofibrosis. Cancer Control 2012;19:4-15. [PubMed]
- Cervantes F, Martinez-Trillos A.. Myelofibrosis: an update on current pharmacotherapy and future directions. Expert Opin Pharmacother 2013;14:873-84. [PubMed]
- Tefferi A.. Myelofibrosis with myeloid metaplasia. N Engl J Med 2000;342:1255-65. [PubMed]
- Cervantes F, Dupriez B, Passamonti F, et al. Improving survival trends in primary myelofibrosis: an international study. J Clin Oncol 2012;30:2981-7. [PubMed]
- Bogani C, Ponziani V, Guglielmelli P, et al. Hypermethylation of CXCR4 promoter in CD34+ cells from patients with primary myelofibrosis. Stem Cells 2008;26:1920-30. [PubMed]
- Tefferi A.. Pathogenesis of myelofibrosis with myeloid metaplasia. J Clin Oncol 2005;23:8520-30. [PubMed]
- James C.. The JAK2V617F mutation in polycythemia vera and other myeloproliferative disorders: one mutation for three diseases? Hematology Am Soc Hematol Educ Program 2008.69-75. [PubMed]
- Briere J, Kiladjian JJ, Peynaud-Debayle E. Megakaryocytes and platelets in myeloproliferative disorders. Baillieres Clin Haematol 1997;10:65-88. [PubMed]
- Schmitt A, Jouault H, Guichard J, et al. Pathologic interaction between megakaryocytes and polymorphonuclear leukocytes in myelofibrosis. Blood 2000;96:1342-7. [PubMed]
- Chagraoui H, Tulliez M, Smayra T, et al. Stimulation of osteoprotegerin production is responsible for osteosclerosis in mice overexpressing TPO. Blood 2003;101:2983-9. [PubMed]
- Jacobson RJ, Salo A, Fialkow PJ. Agnogenic myeloid metaplasia: a clonal proliferation of hematopoietic stem cells with secondary myelofibrosis. Blood 1978;51:189-94. [PubMed]
- Barosi G, Gale RP. Bone marrow fibrosis in myeloproliferative neoplasms-associated myelofibrosis: deconstructing a myth? Leuk Res 2011;35:563-5. [PubMed]
- Greenberg BR, Woo L, Veomett IC, et al. Cytogenetics of bone marrow fibroblastic cells in idiopathic chronic myelofibrosis. Br J Haematol 1987;66:487-90. [PubMed]
- Castro-Malaspina H, Gay RE, Jhanwar SC, et al. Characteristics of bone marrow fibroblast colony-forming cells (CFU-F) and their progeny in patients with myeloproliferative disorders. Blood 1982;59:1046-54. [PubMed]
- Mercier F, Monczak Y, François M, et al. Bone marrow mesenchymal stromal cells of patients with myeloproliferative disorders do not carry the JAK2-V617F mutation. Exp Hematol 2009;37:416-20. [PubMed]
- Kerbauy DM, Gooley TA, Sale GE, et al. Hematopoietic cell transplantation as curative therapy for idiopathic myelofibrosis, advanced polycythemia vera, and essential thrombocythemia. Biol Blood Marrow Transplant 2007;13:355-65. [PubMed]
- Rosti V, Villani L, Riboni R, et al. Spleen endothelial cells from patients with myelofibrosis harbor the JAK2V617F mutation. Blood 2013;121:360-8. [PubMed]
- Sozer S, Fiel MI, Schiano T, et al. The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood 2009;113:5246-9. [PubMed]
- Teofili L, Martini M, Iachininoto MG, et al. Endothelial progenitor cells are clonal and exhibit the JAK2(V617F) mutation in a subset of thrombotic patients with Ph-negative myeloproliferative neoplasms. Blood 2011;117:2700-7. [PubMed]
- Noll JE, Williams SA, Purton LE, et al. Tug of war in the haematopoietic stem cell niche: do myeloma plasma cells compete for the HSC niche? Blood Cancer J 2012;2:e91. [PubMed]
- Le Bousse-Kerdilès MC. Primary myelofibrosis and the "bad seeds in bad soil" concept. Fibrogenesis Tissue Repair 2012;5:S20. [PubMed]
- Lataillade JJ, Pierre-Louis O, Hasselbalch HC, et al. Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood 2008;112:3026-35. [PubMed]
- Walkley CR, Shea JM, Sims NA, et al. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 2007;129:1081-95. [PubMed]
- Walkley CR, Olsen GH, Dworkin S, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 2007;129:1097-110. [PubMed]
- Tibes R, Mesa RA. Targeting hedgehog signaling in myelofibrosis and other hematologic malignancies. J Hematol Oncol 2014;7:18. [PubMed]
- Dong M, Blobe GC. Role of transforming growth factor-beta in hematologic malignancies. Blood 2006;107:4589-96. [PubMed]
- Assoian RK, Komoriya A, Meyers CA, et al. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 1983;258:7155-60. [PubMed]
- Fortunel NO, Hatzfeld A, Hatzfeld JA. Transforming growth factor-beta: pleiotropic role in the regulation of hematopoiesis. Blood 2000;96:2022-36. [PubMed]
- Shi M, Zhu J, Wang R, et al. Latent TGF-β structure and activation. Nature 2011;474:343-9. [PubMed]
- Wrana JL, Attisano L, Wieser R, et al. Mechanism of activation of the TGF-beta receptor. Nature 1994;370:341-7. [PubMed]
- Nakao A, Imamura T, Souchelnytskyi S, et al. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J 1997;16:5353-62. [PubMed]
- Hasselbalch HC. Idiopathic myelofibrosis--an update with particular reference to clinical aspects and prognosis. Int J Clin Lab Res 1993;23:124-38. [PubMed]
- Le Bousse-Kerdilès MC, Martyré MC. Dual implication of fibrogenic cytokines in the pathogenesis of fibrosis and myeloproliferation in myeloid metaplasia with myelofibrosis. Ann Hematol 1999;78:437-44. [PubMed]
- Wang JC. Importance of plasma matrix metalloproteinases (MMP) and tissue inhibitors of metalloproteinase (TIMP) in development of fibrosis in agnogenic myeloid metaplasia. Leuk Lymphoma 2005;46:1261-8. [PubMed]
- Blank U, Karlsson S.. The role of Smad signaling in hematopoiesis and translational hematology. Leukemia 2011;25:1379-88. [PubMed]
- Rooke HM, Vitas MR, Crosier PS, et al. The TGF-beta type II receptor in chronic myeloid leukemia: analysis of microsatellite regions and gene expression. Leukemia 1999;13:535-41. [PubMed]
- Kuroda H, Matsunaga T, Terui T, et al. Decrease of Smad4 gene expression in patients with essential thrombocythaemia may cause an escape from suppression of megakaryopoiesis by transforming growth factor-beta1. Br J Haematol 2004;124:211-20. [PubMed]
- Vannucchi AM, Bianchi L, Paoletti F, et al. A pathobiologic pathway linking thrombopoietin, GATA-1, and TGF-beta1 in the development of myelofibrosis. Blood 2005;105:3493-501. [PubMed]
- Ulich TR, del Castillo J, Senaldi G, et al. Systemic hematologic effects of PEG-rHuMGDF-induced megakaryocyte hyperplasia in mice. Blood 1996;87:5006-15. [PubMed]
- Yanagida M, Ide Y, Imai A, et al. The role of transforming growth factor-beta in PEG-rHuMGDF-induced reversible myelofibrosis in rats. Br J Haematol 1997;99:739-45. [PubMed]
- Yan XQ, Lacey D, Fletcher F, et al. Chronic exposure to retroviral vector encoded MGDF (mpl-ligand) induces lineage-specific growth and differentiation of megakaryocytes in mice. Blood 1995;86:4025-33. [PubMed]
- Chagraoui H, Komura E, Tulliez M, et al. Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood 2002;100:3495-503. [PubMed]
- Ferreira R, Ohneda K, Yamamoto M, et al. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol Cell Biol 2005;25:1215-27. [PubMed]
- Vannucchi AM, Migliaccio AR, Paoletti F, et al. Pathogenesis of myelofibrosis with myeloid metaplasia: lessons from mouse models of the disease. Semin Oncol 2005;32:365-72. [PubMed]
- Zingariello M, Martelli F, Ciaffoni F, et al. Characterization of the TGF-β1 signaling abnormalities in the Gata1low mouse model of myelofibrosis. Blood 2013;121:3345-63. [PubMed]
- Vannucchi AM, Bianchi L, Cellai C, et al. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice). Blood 2002;100:1123-32. [PubMed]
- Terui T, Niitsu Y, Mahara K, et al. The production of transforming growth factor-beta in acute megakaryoblastic leukemia and its possible implications in myelofibrosis. Blood 1990;75:1540-8. [PubMed]
- Martyré MC, Magdelenat H, Bryckaert MC, et al. Increased intraplatelet levels of platelet-derived growth factor and transforming growth factor-beta in patients with myelofibrosis with myeloid metaplasia. Br J Haematol 1991;77:80-6. [PubMed]
- Martyré MC, Magdelenat H, Calvo F. Interferon-gamma in vivo reverses the increased platelet levels of platelet-derived growth factor and transforming growth factor-beta in patients with myelofibrosis with myeloid metaplasia. Br J Haematol 1991;77:431-5. [PubMed]
- Martyré MC, Romquin N, Le Bousse-Kerdiles MC, et al. Transforming growth factor-beta and megakaryocytes in the pathogenesis of idiopathic myelofibrosis. Br J Haematol 1994;88:9-16. [PubMed]
- Le Bousse-Kerdilès MC, Chevillard S, Charpentier A, et al. Differential expression of transforming growth factor-beta, basic fibroblast growth factor, and their receptors in CD34+ hematopoietic progenitor cells from patients with myelofibrosis and myeloid metaplasia. Blood 1996;88:4534-46. [PubMed]
- Hemavathy KC, Chang TH, Zhang H, et al. Reduced expression of TGF beta1RII in agnogenic myeloid metaplasia is not due to mutation or methylation. Leuk Res 2006;30:47-53. [PubMed]
- Chang VT, Yook C, Rameshwar P. Synergism between fibronectin and transforming growth factor-β1 in the production of substance P in monocytes of patients with myelofibrosis. Leuk Lymphoma 2013;54:631-8. [PubMed]
- Sayinalp N, Cinar H, Uner A, et al. Plasma basic fibroblast growth factor and bone marrow fibrosis in clonal myeloproliferative disorders. Clin Lab Haematol 2004;26:265-8. [PubMed]
- Steurer M, Zoller H, Augustin F, et al. Increased angiogenesis in chronic idiopathic myelofibrosis: vascular endothelial growth factor as a prominent angiogenic factor. Hum Pathol 2007;38:1057-64. [PubMed]
- Santos FP, Verstovsek S. Therapy with JAK2 inhibitors for myeloproliferative neoplasms. Hematol Oncol Clin North Am 2012;26:1083-99. [PubMed]
- Santos FP, Verstovsek S. What is next beyond janus kinase 2 inhibitors for primary myelofibrosis? Curr Opin Hematol 2013;20:123-9. [PubMed]
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012;366:787-98. [PubMed]
- Horan GS, Wood S, Ona V, et al. Partial inhibition of integrin alpha(v)beta6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med 2008;177:56-65. [PubMed]
- Trachtman H, Fervenza FC, Gipson DS, et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int 2011;79:1236-43. [PubMed]
- Mascarenhas J, Li T, Sandy L, et al. Anti-transforming growth factor-β therapy in patients with myelofibrosis. Leuk Lymphoma 2014;55:450-2. [PubMed]
- Santiago B, Gutierrez-Cañas I, Dotor J, et al. Topical application of a peptide inhibitor of transforming growth factor-beta1 ameliorates bleomycin-induced skin fibrosis. J Invest Dermatol 2005;125:450-5. [PubMed]
- Ishida W, Mori Y, Lakos G, et al. Intracellular TGF-beta receptor blockade abrogates Smad-dependent fibroblast activation in vitro and in vivo. J Invest Dermatol 2006;126:1733-44. [PubMed]
- Yue L, Bartenstein M, Zhao W, et al. Preclinical efficacy of TGF-beta receptor I kinase inhibitor, galunisertib, in myelofibrosis. Blood 2015;126:603.
- Lan HY, Mu W, Tomita N, et al. Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol 2003;14:1535-48. [PubMed]
- Daniels CE, Wilkes MC, Edens M, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest 2004;114:1308-16. [PubMed]
- Jeremy Wen Q, Yang Q, Goldenson B, et al. Targeting megakaryocytic-induced fibrosis in myeloproliferative neoplasms by AURKA inhibition. Nat Med 2015;21:1473-80. [PubMed]
Cite this article as: Agarwal A, Morrone K, Bartenstein M, Zhao ZJ, Verma A, Goel S. Bone marrow fibrosis in primary myelofibrosis: pathogenic mechanisms and the role of TGF-β. Stem Cell Investig 2016;3:5.


