Article Text
Abstract
Aim A first in human study to evaluate tolerability and pharmacokinetics followed by an early proof of mechanism (POM) study to determine whether the small orally, available molecule, Posiphen tartrate (Posiphen), lowers secreted (s) amyloid-β precursor protein (APP) α and -β, amyloid-β peptide (Aβ), tau (τ) and inflammatory markers in CSF of patients with mild cognitive impairment (MCI).
Study design Posiphen single and multiple ascending dose phase 1 randomised, double blind, placebo-controlled safety, tolerance, pharmacokinetic studies were undertaken in a total of 120 healthy volunteers to define a dose that was then used in a small non-randomised study of five MCI subjects, used as their own controls, to define target engagement.
Main outcome measures Pharmacodynamic: sAPPα, sAPPβ, Aβ42, τ (total (t) and phosphorylated (p)) and inflammatory marker levels were time-dependently measured over 12 h and compared prior to and following 10 days of oral Posiphen treatment in four MCI subjects who completed the study. Pharmacokinetic: plasma and CSF drug and primary metabolite concentrations with estimated brain levels extrapolated from steady-state drug administration in rats.
Results Posiphen proved well tolerated and significantly lowered CSF levels of sAPPα, sAPPβ, t-τ, p-τ and specific inflammatory markers, and demonstrated a trend to lower CSF Aβ42.
Conclusions These results confirm preclinical POM studies, demonstrate that pharmacologically relevant drug/metabolite levels reach brain and support the continued clinical optimisation and evaluation of Posiphen for MCI and Alzheimer's disease.
- Posiphen
- amyloid precursor protein
- amyloid-β peptide
- inflammatory markers
- mild cognitive impairment
- genetics
- B12 deficiency
- neurochemistry
- Alzheimer's disease
- amyloid
- head injury
- Parkinson's disease
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-commercial License, which permits use, distribution, and reproduction in any medium, provided the original work is properly cited, the use is non commercial and is otherwise in compliance with the license. See: http://creativecommons.org/licenses/by-nc/2.0/ and http://creativecommons.org/licenses/by-nc/2.0/legalcode.
Statistics from Altmetric.com
- Posiphen
- amyloid precursor protein
- amyloid-β peptide
- inflammatory markers
- mild cognitive impairment
- genetics
- B12 deficiency
- neurochemistry
- Alzheimer's disease
- amyloid
- head injury
- Parkinson's disease
Introduction
The treatment of Alzheimer's disease (AD), the most common dementing disorder of the elderly, remains an unmet medical need.1 Its hallmarks are neurodegeneration, brain atrophy and abnormal protein depositions, particularly of amyloid plaques and neurofibrillary tangles deriving from amyloid-β peptide (Aβ) and hyperphosphorylated τ, respectively,2–4 resulting in progressive cognitive decline. Current approved AD drugs provide symptomatic relief and temporarily delay loss of cognition, but do not halt or modify disease progression.5
The key AD drug target, Aβ, is a proteolytic product of amyloid-β precursor protein (APP) cleavage: an integral transmembrane protein concentrated at the synapse of neurons.4 APP is cleaved by β- and γ-secretases to generate Aβ,2 ,6 which assembles into oligomers that cause inflammation and target synapses to induce cellular dysfunction and impair memory.7–9 APP is additionally cleaved into a number of other bioactive N- and C-terminal fragments, including N-APP10 and C3111 ,12 (figure 1). These fragments, likewise, may contribute to AD pathogenesis, making APP an interesting AD drug target to regulate. Posiphen® tartrate (Posiphen (figure 2), also known as (+)-phenserine), an APP synthesis inhibitor,13 interacts via the 5′-untranslated region of APP mRNA14 to inhibit ribosomal access and block APP translation.14–16 Posiphen is the chirally pure positive enantiomer of (−)-phenserine (phenserine).17 However, whereas phenserine is an acetylcholinesterase inhibitor, Posiphen lacks acetylcholinesterase activity; instead, it inhibits the translation of APP. In neuronal cultures and brain of wild type and AD transgenic mice, Posiphen lowered APP and Aβ levels13 ,18 in a dose-dependent manner and hence represents an interesting candidate drug to reduce APP toxic products in humans.
Schematic of amyloid-β precursor protein (APP) processing pathway leading to AD. Amyloidogenic processing of APP generates Aβ, a hydrophobic, neurotoxic, self-aggregating 40–42 amino acid peptide that accumulates preferentially within amyloid plaques.4 ,6 Recent research suggests that APP processing can result in a number of toxic fragments, including N- and C-terminal peptides10 ,11 that may induce neuronal dysfunction, degeneration and death leading to the hypothesis that a number of APP fragments are toxic to nerve cells.
Chemical structures of Posiphen and primary metabolites, and their plasma, brain and CSF concentrations in (A) rats and (B) mild cognitive impairment (MCI) subjects following Posiphen administration. (A) Simultaneous plasma (ng/ml), brain (ng/g wet weight) and CSF (ng/ml) levels of Posiphen and metabolites measured under steady-state conditions following Posiphen (75 mg/kg/day ×10 days by continuous administration subcutaneous pump). (B) In an equivalent MCI phase I study following 10 days of Posiphen (4×60 mg/day), plasma (ng/ml) and CSF (ng/ml) levels of Posiphen and metabolites were measured, and extrapolated brain levels were determined from steady-state animal data.
We describe, herein, three phase I studies conducted under an active investigational new drug application. Initially, Posiphen's safety was assessed in healthy volunteers in a single ascending dose study and then a multiple ascending dose study (SAD and MAD, respectively). Thereafter, using a well-tolerated dose from the former investigations, an early proof of mechanism (POM) study was conducted in patients with mild cognitive impairment (MCI), wherein time-dependent plasma and cerebrospinal fluid (CSF) samples were obtained prior to and following 10 days of Posiphen administration to permit analysis of drug-induced changes in CSF levels of secreted (s) APPα and APPβ, Aβ42, τ (total (t) and phosphorylated (p)) and inflammatory markers. Additionally, human brain levels of Posiphen and metabolites were estimated from measured plasma and CSF samples of MCI patients in light of steady-state Posiphen plasma–brain–CSF pharmacokinetic studies performed in rats.
Design and methods
Drug substance
Posiphen, (3aR)-1, 3a, 8-trimethyl-1, 2, 3, 3a, 8, 8a-hexahydropyrrolo (2, 3-b) indol-5-yl phenyl-carbamate tartrate (investigational new drug #72 654) was manufactured to good manufacturing process requirements by Rhodia (Boulogne-Billancourt, France) (See online Supplemental information SI-1).
Standards
Posiphen and its metabolites, N1- and N8-norposiphen and N1, N8-bisnorposiphen, were synthesised (National Institute on Aging, Baltimore, Maryland, USA) to >99.5% purity.19 ,20
Animal studies
To aid extrapolation of drug and metabolite concentrations in human brain from those measured in human plasma and CSF in the POM MCI study, Posiphen (75 mg/kg/day continuous infusion) was administered to (male adult Fischer-44) rats under steady-state conditions and samples were simultaneously collected and drug/metabolite concentrations quantified in each compartment (See online Supplemental information SI-2).
Clinical studies
Phase I SAD
A randomised, double blind, placebo-controlled safety, tolerance and pharmacokinetic study (first in human) was performed with six groups of male and female healthy volunteers receiving serially increasing single doses of Posiphen or placebo, followed by monitoring of safety (vital signs, ECGs, clinical laboratory tests, capture of adverse events) and collection of blood and urine samples at regular intervals up to 24 h for preliminary pharmacokinetic analyses. The escalating doses to be studied were 10, 20, 40, 80, 160 and 240 mg (and placebo). Due to the limiting side effects (nausea and vomiting), the highest dose studied was 160 mg of Posiphen; the 240 mg dose was not administered (See online Supplemental information SI-3).
The study was conducted by the PRACS Institute (East Grand Forks, Minnesota, USA) and was fully approved by their Institutional Review Board.
Phase I MAD
A randomised, double blind, placebo-controlled safety, tolerance, pharmacokinetic study was performed with six of eight male and six of eight female healthy volunteers in each of three successive groups being administered one of three serially increasing, multiple dose regimens of Posiphen and two male and two female subjects in each treatment group receiving placebo. Safety (vital signs, ECGs, clinical laboratory tests, capture of adverse events) was monitored throughout the study and blood and urine samples were collected. The escalating regimens were 20, 40 and 60 mg, and placebo four times a day for 7 or 10 days. Plasma obtained from blood samples was analysed as described above (See online Supplemental information SI-4).
For inclusion/exclusion criteria see online Supplemental information SI-5.
The study was likewise conducted by the PRACS Institute and was fully approved by their Institutional Review Board.
Phase I early POM with pharmacokinetics and pharmacodynamics (ClinicalTrials.gov Identifier: NCT01072812)
An open-label study was performed in which five healthy male and female MCI patients (three male and two female patients) received Posiphen at 4×60 mg/day (total 240 mg/day) for 10 days. This dosing regimen was well tolerated in the earlier MAD study. To avoid potential inter-subject variability, subjects were used as their own controls. Specifically, serial plasma and lumbar CSF samples were collected via an indwelling catheter over 12 h (at 0, 1, 1.5, 2, 3, 4, 6, 8 and 12 h) initiated at the same time of day 1 day prior to the start of dosing to obtain time-dependent baseline control data, and then at the exact same times immediately after the last dose was administered.21 ,22 This paradigm was chosen to control for potential circadian alterations in pharmacodynamics markers within each subject. Samples were frozen and then stored at −80°C. CSF and plasma samples were matched and were analysed for (i) pharmacokinetics of Posiphen and metabolites (N1-norposiphen, N8-norposiphen and N1, N8-bisnorposiphen) (figure 2) and (ii) pharmacodynamic studies involving measurement of the following proteins: sAPPα, sAPPβ, Aβ42, t-τ, p-τ, complement 3, factor H, monocyte chemotactic protein-1 (MCP-1), the inflammation marker and chitinase-like protein YKL-40, and soluble cluster of differentiation 14 (sCD14). One MCI subject withdrew from the POM study on day 1 (table 1 legend); hence analyses were undertaken on four subjects in relation to pharmacokinetic and pharmacodynamics measures.
Summary of adverse events (AEs). Treatment-related AEs that occurred in more than one subject in the Posiphen or placebo groups or in the entire cohort are summarised by dose. In all three (AX-PO-101, AX-PO-102, QR-12001) studies, males and female subjects were combined, as there was no apparent difference between the sexes regarding their tolerance to Posiphen. Of note, markers of hepatic and renal function were additionally analysed and were unaltered by drug
Subjects were male or postmenopausal females, between 55 and 80 years of age, with self-reported memory complaints that were corroborated by spouse or companion or caregiver as appropriate, and memory difficulties as measured on neuropsychological tests. MCI was determined according to Petersen's criteria23 with a Mini Mental Status Examination score ≥24, cut-off score on the logical memory II delayed paragraph recall subtest of the Wechsler Memory Scale Revised, Clinical Dementia Rating of 0.5 with a memory box score of 0.5 or 1.0. Inclusion/exclusion criteria were in accordance with those described in online Supplemental information SI-6.
To provide comparative healthy control data, a CSF sample was similarly acquired by spinal tap at 08:00 AM from four healthy volunteers who were not treated with Posiphen. This CSF was analysed for the same key pharmacodynamic factors as those described for the MCI patients prior and following Posiphen treatment.
The study was conducted by the CEDRA/World Wide Clinical Trials (King of Prussia, Pennsylvania, USA) and was fully approved by their Institutional Review Board.
Biochemical pharmacodynamic assays
Four independent laboratories and multiple assay methods were used to quantify the CSF biomarkers analysed in this study.
Meso Scale Discovery (MSD) assays were used for quantification of sAPPα and sAPPβ (human Alzheimer's panel three sAPPα/sAPPβ assay kit (catalogue#: K15120E-1, MSD, Gaithersburg, Maryland, USA)) from 25 μl of CSF. The plates were measured by multiplex analyser (SI2400A) and data evaluated by MSD discovery workbench data analysis toolbox software. The same platform was used to measure MCP-1 (also called CCL2) concentrations in CSF, as previously described.24
AlphaLISA kits from Perkin Elmer were used to quantify sAPPα (catalogue#: AL254C, Perkin Elmer, Waltham, Massachusetts, USA), sAPPβ (catalogue#: AL232C), Aβ42 (catalogue#: AL276C), Aβ40 (catalogue#: AL275C) and t-τ (catalogue#: AL271C) from 5 μl of an undiluted CSF sample which was added to an AlphaPlate-384 (catalogue#: 6005350).
Innogenetics kits were used to measure CSF t-τ and p-τ levels by INNO-BIA AlzBio3 kit (Innogenetics, Gent, Belgium) with CSF samples diluted 1:4 in diluent. Samples were analysed using a LiquiChip Luminex 200 Workstation (Qiagen, Valencia, California, USA).
The proteins C3 and FH in CSF were measured with a kit made by Millipore Corporation (Billerica, Massachusetts, USA), following the manufacturer's instructions with procedures similar to those described for synuclein assay.25
CSF levels of YKL-40 and sCD14 were analysed with commercial ELISAs (R&D Systems, Minneapolis, Minnesota, USA). The CSF was diluted 100 times for the YKL-40 and sCD14 analyses.
Posiphen and metabolite pharmacokinetic assays
Concentrations of Posiphen, N1-norposiphen, N8-norposiphen and N1, N8-bisnorposiphen in human plasma and CSF as well as rat plasma, brain and CSF samples were determined by LC-MS/MS at Absorption Systems (Exton, Pennsylvania, USA) (See online Supplemental information SI-7). Calibration ranges for each analyte ranged from 1000 ng/ml to 1 ng/ml (or ng/g for brain) in plasma, brain and CSF matrices. The detection limit was 0.025 ng/ml.
Statistical analysis
All assay data collected were analysed using a repeated measures mixed model analysis of variance. The model included Day (Day 11/Day 0) as a fixed effect, Time (nine time points per person between 0 and 12 h) as a repeated measure effect and Patient (18 samples per person in total) as a random effect. Compound symmetry was assumed as an appropriate covariance pattern between observations on the same patient, which provided a reasonable model fit. Assumptions of constant variance, normality or residuals and parallelism were used to assess acceptability of the statistical model. Data are presented as means ± SEs, unless otherwise stated. The statistical evaluations were undertaken by Data Magik (Salisbury, UK).
Results
Rat pharmacokinetics of Posiphen/metabolites
The comparative plasma, brain and CSF levels of Posiphen and three primary metabolites in rats following steady-state Posiphen infusion are shown in table 2 and figure 2A. Posiphen was the primary compound in each compartment, with the N1- and N8-metabolites reaching 39.1% and 25.8% of Posiphen levels in plasma, respectively, and N1, N8-bisnorposiphen 20.5%. In accordance with their high lipophilicity (ClogP value, table 2), substantial brain entry of Posiphen and metabolites was evident whereas aqueous CSF levels were low. Specifically, steady-state brain concentrations were greater than concomitant plasma levels, providing high brain to plasma ratios (Posiphen: 6.8, N1-norposiphen: 3.8, N8-norposiphen: 5.8 and N1, N8-bisnorposiphen: 1.3) with CSF levels reaching only approximately 1% of brain levels.
Mean pharmacokinetic parameters for Posiphen and primary metabolites in rat and MCI patients
SAD study (healthy volunteers)
Safety
Posiphen was well tolerated by healthy male and female volunteers at single doses from 10 to 80 mg. A 160 mg dose was associated with an increased incidence of nausea and vomiting (four subjects were nauseous and three vomited). Adverse events were either mild or moderate; none were severe. No higher doses were administered. Posiphen 80 mg was determined as the no observed adverse effect level (table 1).
Pharmacokinetics
Posiphen, at all doses, was absorbed rapidly (mean Tmax: 1.3–1.6 h) and cleared from the circulation biphasically (terminal half life: 3.7–4.3 h), independent of dose. Posiphen systemic availability increased more than linearly with increasing dose, resulting in a disproportionately large increase in Cmax and AUC0–last. Mean Cmax ranged from 1.29 to 288 ng/ml (male subjects) and 6.22 to 480 ng/ml (female subjects) as the dose increased from 10 to 160 mg. Comparable increases were determined for mean AUC0–last, 1.63–998 and 12.1–1530 ng. h/ml, respectively, over the same dose range (data not shown).
MAD study (healthy volunteers)
Safety
Posiphen doses up to 4×60 mg daily ×10 days were well tolerated. This 4×60 mg dose produced a small but statistically insignificant difference from placebo regarding gastrointestinal side effects and dizziness, and hence 4×60 mg four times a day was determined the no observed adverse effect level (table 1).
Pharmacokinetics
Posiphen, at all doses, was absorbed rapidly (Tmax =1.2 to 1.7 h) and cleared from the circulation biphasically (terminal half life of 4.3–4.7 h). As with the SAD study, the systemic availability of Posiphen increased more than linearly with increasing dose, resulting in disproportionately large increases in Cmax and AUC values.
POM study (MCI patients)
Safety
Posiphen (4×60 mg daily ×10 days) in MCI subjects showed a similar safety profile as found in the healthy volunteers (table 1).
Pharmacokinetics
Mean Cmax plasma concentrations of Posiphen and metabolites are shown in Table 2 and figure 2B, and the time-dependent profiles are provided in figure 3. Similar to rat, Posiphen was the primary drug species, with the N1- and N8-metabolites initially accounting for 21.6% and 26.2% of Posiphen's Cmax. Total time-dependent levels (AUC0–last, table 2) were approximately 50% of Posiphen. Contrasting with rat studies, N1, N8-bisnorposiphen represented a minor metabolite in humans, accounting for 3.2% of Cmax and 6.5% total time-dependent Posiphen levels. Calculated pharmacokinetic parameters for Posiphen in plasma of MCI patients were similar to those in healthy volunteers (SAD and MAD). Posiphen mean Tmax was 1.3–1.6 h, mean terminal half life 4.0–5.5 h, apparent volume of distribution: 2171±339 l and total body clearance 310±72 l/h. The four times a day regimen resulted in some accumulation of Posiphen in plasma and accumulation of Posiphen/metabolites in CSF (figure 3).
Time-dependent (A) plasma and (B) CSF Posiphen and metabolite levels following the final dose of 10 day Posiphen administration (4×60 mg). Semi-log plot of serial time-dependent Posiphen and metabolite (N1- and N8-norposiphen and N1, N8-bisnorposiphen) concentrations in plasma and CSF obtained following the final dose of a 10 day Posiphen (4×60 mg) schedule (n=4 MCI subjects, mean ± SD).
Illustrated in figure 2B are Posiphen and metabolite plasma, CSF and estimated brain levels for MCI patients following 10 day Posiphen dosing. Extrapolated brain levels for MCI patients were determined by applying the rat plasma/CSF/brain data (figure 2A) to the human plasma and CSF data (figure 2B). This estimate predicts Posiphen brain levels approaching 1 ug/g or approximately 3.5 μM for a dose of 4×60 mg/day.
Pharmacodynamics
Drug-induced differences in CSF biomarkers for MCI patients are shown in table 3A, determined by comparing the predrug and postdrug biomarker levels at each time point within the same subject to control for both circadian changes and inter-subject variability. The majority of the biomarkers were analysed by two different techniques within two independent institutions to cross-validate the data. In all cases, the direction of change was the same. Specifically, Posiphen lowered sAPPα and sAPPβ levels by −59.9% and −57.7%, respectively, assessed by the AlpaLisa assay, and by −34.1% and −34%, respectively, assessed by the MSD assay, in accordance with Posiphen's proposed mechanism of action to inhibit APP expression.13 ,14 ,18 In line with this, Aβ42 demonstrated a trend to reduction (−45.2%, as assessed by the AlphaLisa assay and −51.4% as assessed by the Innogenetics assay). In addition, Posiphen significantly reduced levels of t-τ (−74.1%, as assessed by the Innogenetics assay and −46.2%, as assessed by the AlphaLisa assay) and p-τ (−61%, as assessed by the Innogenetics assay).
(A) AD biomarkers and (B) inflammatory biomarkers in CSF of MCI subjects after 10 days of Posiphen treatment
Posiphen's actions on CSF inflammation markers are shown in table 3B. A significant lowering of pro-inflammatory, C3 (−86.9%) and microglial activation markers, MCP-1 (−87.5%) and YKL-40 (−72.7%), was evident. By contrast, sCD14, associated with early innate immune response to bacterial and viral infection,26 and the complement control protein, factor H, were unaffected by Posiphen (−26.1% and +23.7%, respectively).
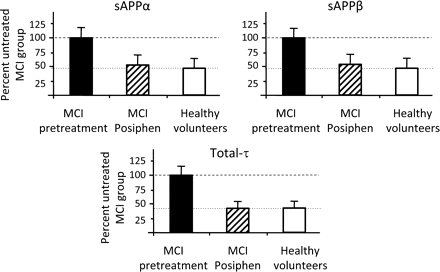
Analysis of CSF samples obtained from four healthy Posiphen naive volunteers under conditions similar to those for the MCI subjects permitted comparison between untreated and treated MCI patients and healthy untreated volunteers. Figure 4 demonstrates that Posiphen administration to MCI patients lowers sAPPα, sAPPβ and t-τ to levels present in healthy volunteers.
Comparison of sAPPα, sAPPβ and t-τ between untreated and treated mild cognitive impairment (MCI) patients and healthy volunteers. Posiphen (4×60 mg/day ×10 days) administered to MCI patients for 10 days lowers their CSF levels of sAPPα, sAPPβ and t-τ to those determined in healthy (Posiphen naive) volunteers. Biomarker concentrations are expressed as a per cent of MCI predrug values (assigned as 100%) and combined all data across assays (AlphaLisa and MSD for sAPPα and sAPPβ, and AlphaLisa and Innogenetics for t-τ) in Table 3. Whereas there were significant Posiphen-induced declines in MCI subjects (see Table 3 for statistical analyses), no dramatic differences were evident between Posiphen-treated MCI subjects and healthy volunteers.
Discussion
Posiphen was shown in 72 healthy volunteers to be safe in SAD and 53 subjects in MAD and POM studies of 7–10 day administration up to levels determined as greater than fivefold the effective dose. This effective dose was determined both by comparing the extrapolated molar concentration in brain with the 50% effective concentration to inhibit APP in neuronal cultures, as well as from animal studies.13 The human pharmacokinetics of Posiphen/metabolites and extrapolation of data determined from rodents suggests that Posiphen readily enters the brain and achieves levels 6.8-fold higher than in plasma at steady-state, in accordance with its high lipophilicity (ClogP value 2.2). Its hydrophobicity and protein binding capacity (96% of Posiphen/ metabolites bind to brain proteins) likely limit levels of Posiphen/metabolites found in CSF of both rodents and MCI patients. Interestingly, Posiphen half life in CSF of MCI patients proved to be longer than its half life in plasma, >12 h versus approximately 5 h, respectively (figure 3). Consistent with its longer half life within the central nervous system, Posiphen's APP, τ and inflammation lowering activity lasted longer than the recorded 12 h of sampling. Recent studies in neuronal cultures indicate that Posiphen's APP lowering actions extend for numerous hours following wash-off and, additionally, are likewise maintained within the brain of transgenic AD mice for over 9 h after cessation of dosing (Sambamurti K, Medical University of S. Carolina, personal communication). The extended duration of Posiphen/metabolites in CSF/brain together with the prolonged inhibition of APP and τ expression may permit once a day dosing and is a focus of current studies.
Our early POM study in MCI patients focused on evaluating target engagement demonstrates that Posiphen lowers both sAPPα and sAPPβ in CSF, consistent with its preclinical actions13 ,18 ,27 and ability to inhibit the translation of APP mRNA via an iron response element within its 5′-untranslated region.14 ,16 ,28 The trend of a Posiphen-induced reduction in CSF Aβ42 in MCI subjects is likewise in line with its known action to inhibit APP synthesis, as Aβ42 is a downstream product and is in accordance with the described decline in CSF sAPPβ in MCI subjects as well as of Aβ42 levels in preclinical studies.13–15 ,18 ,27 ,28 A separate more limited analysis of Aβ40 (AlphaLisa) in CSF collected at 3 and 8 h prior to and following Posiphen administration in MCI subjects provided reduction trends of -32% and -37%, respectively. A caveat of early CNS target engagement investigational studies is the small patient number required to, on one hand, adequately demonstrate pharmacologically driven biological activity in the brain as a result of drug interaction with its intended target to provide proof-of-concept and, on the other hand, protect patients from exposure to potentially inactive or toxic drugs.29 In our Posiphen study, this was undertaken on five MCI subjects, one of whom withdrew, allowing biomarker analyses on four. However, as patients were used as their own controls at 0 day (prior to Posiphen treatment) and as test subjects following 10 days Posiphen treatment, the POM study design limited the potential effect of often large inter-subject variability30 ,31 thereby permitting statistical analyses on data derived from this small patient number. In this regard, individual patient data analyses are shown in figure 5. Clearly evident is the inter-subject difference in biomarker levels under naive (day 0) conditions (determined as the mean value ± SD of the nine timed samples across the 12 h sampling period). Evident also is the sometimes high variance around the mean biomarker value for each individual related to the time-dependent change (consistent with the circadian pattern reported by others31) in biomarker levels over the 12 h study. Consistently across all individuals within figure 5, 10 day Posiphen administration lowered mean levels of sAPPα, sAPPβ, Aβ42, t-τ, p-τ and C3, but not factor H. Importantly, the time-dependent analysis of biomarker levels within the same individual, by matching exact same times predrug versus postdrug (table 3A, B), allowed determination of Posiphen-induced differences in such a small patient number (N=4) in the presence of large inter-subject and time-dependent biomarker differences. Of significance, the pattern of the changes was remarkably alike between the different assays employed blindly to measure the same CSF analyte at different independent institutions (whether AlphaLisa vs MSD in the quantification of sAPPα and sAPPβ, or AlphaLisa vs Innogenetics for Aβ42 and t-τ). Albeit, the percent of the Posiphen-induced inhibition and variance differed between the assay techniques (table 3); similar data deriving from the use of two independent assays provide a valuable level of cross-validation to help guard against unforeseen systematic errors. Clearly, without the potential to match predrug and postdrug time-dependent biomarker levels within the same patient, a far greater number of subjects would have been required to support statistical analyses. Nevertheless, a larger patient number, which is often limiting in early POM studies, would have provided greater statistical power to discriminate drug-induced biomarker actions, as would the inclusion of a placebo group.
Difference in biomarkers between day 0 (naive) and 10 day Posiphen (4×60 mg/day) administration. As the effect of Posiphen on the analysed biomarkers remained present over 12 h, we were able to calculate the mean of the nine samples (collected 0–12 h) on day 0 (naive) and compare this with the mean of the nine samples (collected over the same 0–12 h) after 10 day Posiphen administration. Each bar hence represents the mean of repeat measures over 12 h with SD for each of the four patients (A, B, C, D) who completed the study. Whereas the absolute biomarker level differs between subjects, for each individual subject the post Posiphen values are consistently lower than the predrug (naive) values for all shown markers with the exception of factor H, consistent with its regulatory and not pro-inflammatory role. For this figure, data are derived from the AlphaLisa for sAPPα and sAPPβ, Innogenetics for Aβ42, p-τ and t-τ, and Millipore for C3 and factor H. In general, assay values are in agreement with literature values.37 ,38 MCI, mild cognitive impairment.
Recent reports suggest that CSF elevations in sAPPα and, in particular, sAPPβ may be clinically useful and superior to assessing Aβ42, in the early and differential diagnosis of incipient AD.32 ,33 Hence, as APP represents Posiphen's immediate target, CSF levels of sAPPα and sAPPβ, rather than, simply, Aβ42, were measured and found to be elevated in our MCI patients compared with healthy controls (figure 4), in accordance with others.33 ,34 Posiphen's reduction in CSF sAPPα and sAPPβ in MCI patients brought their values in line with healthy controls. A preliminary analysis of Aβ42, analysed by two techniques (table 3A), suggests reductions in the same order as sAPPα and sAPPβ.
Posiphen treatment led to statistically significant reductions in CSF levels of other key AD biomarkers, in particular t-τ and p-τ. As illustrated in figure 4 and in accordance with others,35 ,36 CSF t-τ levels were elevated in our MCI patients versus healthy controls37 ,38 and were normalised by Posiphen. The relevance of these actions and mechanisms through which they are mediated are a focus of current studies. In this regard, resembling the action of Posiphen to impact the translational regulation of APP mRNA,14 ,15 ,28 τ can also be regulated at the level of its RNA stability,39 ,40 potentially by Posiphen. Alternatively, reductions in τ may be secondary to other actions or a combination of primary effects on translational regulation and secondary actions. Nevertheless, similar Posiphen-induced time-dependent reductions in τ have recently been found in neuronal cell cultures and preclinical AD models (Sambamurti K, personal communication). Posiphen, likewise, induced statistical declines in MCI CSF C3, a pro-inflammatory factor reportedly elevated in both AD transgenic mice41 and the CSF of AD subjects,42 together with key biomarkers (MCP-1, YKL-40) of microglial activation.24 ,26 In contrast, Posiphen treatment did not alter levels of the innate immune response protein sCD14 or factor H. Likewise, the decline in specific inflammatory markers may be a secondary effect to the described reductions of APP and τ.
In synopsis, our pharmacokinetic studies in humans and rodents permitted us to estimate levels of Posiphen/metabolites in human brain after Posiphen (4×60 mg/day, 10 days) to be in the order of 3.5 μM Posiphen, associated with the described biomarker changes. This drug level is greater than the determined 50% effective concentration of Posiphen to lower APP levels in neuronal cultures.13 Recent studies have demonstrated that each Posiphen metabolite, likewise, has APP lowering actions.43 We conclude that Posiphen appears to be a promising experimental drug for MCI and AD as it can effectively lower CSF levels of APP, its primary target in brain, and in addition lower t-τ, p-τ and key inflammatory markers, and may hence impact disease progression at a number of levels.
Acknowledgments
The authors are grateful to the following: (i) Qian-sheng Yu, Intramural Research Program, National Institute on Aging, NIH, for synthesis and chemical characterisation of highly pure samples of Posiphen, N1-norposiphen, N8-norposiphen, and N1, N8-bisnorposiphen that were used as standards for analytical chemistry in pharmacokinetic studies. (ii) Harold W Holloway, Intramural Research Program, National Institute on Aging, NIH, for aid with pharmacokinetic studies. (iii) Karen Poksay and Olivier Descamps, Buck Institute for Research on Aging, for performing AlphaLisa analyses of CSF samples and (iv) David Fleet, Data Magik, for statistical analyses.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online materials
Footnotes
Funding This work was supported in part by QR Pharma, Inc. Henrik Zetterberg was supported by the Swedish Research Council (grant numbers K2010-63P-21562-01-4 and K2011-61X-20401-05-6) and the Swedish State Support for Clinical Research. Nigel H Greig was supported by the Intramural Research Program, National Institute on Aging, NIH.
Competing interests MLM and MYC are employees of QR Pharma, Inc. NHG is an inventor on the original Posiphen patent. Having assigned all rights to the US government, he declares that he has no ownership, financial interest or any other competing interests. All other authors declare no competing interests.
Ethics approval The human studies were conducted at and approved by the IRB and Ethics Committees of CEDRA/World Wide Clinical Trials (King of Prussia, PA) and the PRACS Institute (East Grand Forks, MN).
Provenance and peer review Not commissioned; externally peer reviewed.