Article Text
Abstract
BACKGROUND Neutrophils are likely to play a major role in the inflammatory response seen in chronic obstructive pulmonary disease (COPD). This study sought to address the hypothesis that an enhanced neutrophil response to proinflammatory agents in COPD may contribute to their recruitment and activation in the lungs.
METHODS Circulating neutrophils were obtained from 10 patients with COPD, eight long term smokers with normal lung function, and eight healthy never smoking controls. The in vitro production of reactive oxygen species (ROS) was measured by the NADPH oxidase method (respiratory burst) and the surface expression of several adhesion molecules (Mac-1, LFA-1 andl-selectin) was measured by flow cytometry. Measurements were obtained under basal conditions and after stimulation with phorbol myristate acetate (PMA) and tumour necrosis factor alpha (TNFα). mRNA levels of p22-phox (a subunit of NADPH oxidase) and Mac-1 (CD11b) were also determined by reverse transcriptase polymerase chain reaction (RT-PCR).
RESULTS Patients with COPD showed enhanced respiratory burst compared with smokers with normal lung function, both under basal conditions (mean (SE) fluorescence intensity (MFI) 15.1 (0.5) v 11.6 (0.5); mean difference –3.4 (95% CI of the difference –5.1 to –1.8), p<0.01) and after PMA stimulation (MFI 210 (7) v 133 (10); mean difference –77 (95% CI of the difference –102 to –52), p<0.01). Mac-1 surface expression was also enhanced in patients with COPD, both under basal conditions (MFI 91 (5)v 45 (3); mean difference –46 (95% CI of the difference –61 to –31), p<0.001) and after stimulation with TNFα (MFI 340 (15) v 263 (11); mean difference –77 (95% CI of the difference –119 to –34), p=0.001). These differences were also apparent when patients with COPD were compared with non-smokers (p<0.05). The mRNA levels of p22-phox and Mac-1 (CD11b) were similar in patients with COPD and smokers with normal lung function, suggesting that the observed differences were due to post-transcriptional regulation.
CONCLUSIONS These results demonstrate an enhanced neutrophil response to proinflammatory agents in patients with COPD which may contribute to their enhanced recruitment and activation in the lungs of these patients. These findings support those of other studies which have indicated that the neutrophil is likely to play a major role in the pathogenesis of this disease.
- chronic obstructive pulmonary disease
- neutrophils
- adhesion molecules
- respiratory burst
Statistics from Altmetric.com
Chronic obstructive pulmonary disease (COPD) is characterised by an excessive inflammatory response to (largely) tobacco smoking,1 yet only a fraction of smokers will develop the disease.2 The mechanisms underlying this observation are unknown.3
Neutrophils are key cells in the inflammatory response that characterises COPD.4 Compared with healthy non-smokers, neutrophils in patients with COPD are present in increased numbers, both in sputum and bronchoalveolar lavage fluid,5 exhibit increased chemotactic response and a greater ability to digest connective tissue,6 and show enhanced surface expression of some adhesion molecules.7 Collectively, these observations indicate abnormal neutrophil function in COPD. However, whether this abnormal function represents an intrinsic neutrophil defect that favours the development of COPD or, alternatively, it reflects a state of activation (priming) of these cells due to the disease itself or to tobacco smoking (its main risk factor) is currently unknown.
To investigate these possibilities we studied neutrophil function in three groups of well characterised individuals: (1) patients with COPD; (2) long term smokers (with the same smoking history as the patients with COPD) with normal lung function; and (3) healthy, never smoking, volunteers. In these individuals we determined the production of reactive oxygen species (ROS) by NADPH oxidase (respiratory burst) and the surface expression of several adhesion molecules (Mac-1, LFA-1 andl-selectin). In order to gain insight into the potential genetic mechanisms that regulate the respiratory burst and the expression of adhesion molecules in these cells, we assessed mRNA levels of Mac-1 and the subunit p22-phox of NADPH oxidase.
Methods
SUBJECTS
Ten patients with COPD, eight long term smokers with normal lung function, and eight healthy non-smoking volunteers participated in the study. All were men of a similar age (mean (SD) 67 (2) years, 61 (2) years, and 57 (3) years, respectively, p>0.05). Patients with COPD had moderate airflow obstruction (forced expiratory volume in one second (FEV1) 46.8 (4.2)% predicted value) and mild arterial hypoxaemia (Pao 2 9.7 (0.4) kPa) without hypercapnia (Paco 2 5.3 (0.2) kPa). All patients were clinically stable, as defined by the absence of any change in their regular treatment and/or need to seek medical attention during the previous 4 months. Patients with bronchial asthma, pneumonia, or lung cancer were excluded. All patients were treated with inhaled bronchodilators but none received inhaled or oral steroids. None of the patients were current smokers. Their smoking history (50 (5) pack years) was similar to that of the smokers with normal lung function (53 (3) pack years). By definition, spirometric measurements were normal in the smokers without COPD (FEV1 97.1 (5.4)% predicted value).
All participants gave their written consent, having been fully informed of the nature, risks and potential benefits of the study. The research and ethical review committee of our institution approved the investigation.
STUDY DESIGN
Thirty ml of peripheral venous blood was taken from each participant. Current smokers refrained from smoking for at least 12 hours before blood sampling.8 ,9 From these blood samples neutrophils were harvested (see below) and the respiratory burst and surface expression of adhesion molecules (LFA-1, Mac-1, andl-selectin) in these neutrophils were assessed, both under basal conditions and after stimulation. In addition, in neutrophils obtained from patients with COPD and from long term smokers with normal lung function the mRNA expression of p22-phox and Mac-1 (CD11b) was examined.
LUNG FUNCTION MEASUREMENT
Forced spirometric parameters (GS Warren Collins, USA) were measured according to international guidelines.10Spirometric reference values were those of a Mediterranean population.11 Arterial blood gas tensions (IL BG3, Izasa, Spain) were determined only in patients with COPD.
ISOLATION OF NEUTROPHILS
Neutrophils were isolated from peripheral blood samples according to the methodology previously described in our laboratory.7 Briefly, leucocyte rich plasma was obtained by mixing with an equal volume of endotoxin free Hemoce reagent (Hoechst Iberica, Barcelona, Spain). This was followed by sedimentation during 1 hour at 4°C. Neutrophils were separated from the leucocyte rich plasma by centrifugation on a 15 ml layer of a Ficoll-Paque research grade gradient (Pharmacia Biotech, Uppsala, Sweden) at 900g for 30 minutes at 22°C. Residual erythrocytes were removed by mixing the neutrophil rich pellet with 50 ml of ice cold 0.15 M NH4Cl solution which was gently agitated at 4°C for 10 minutes and then centrifuged at 750g for 10 minutes at 4°C. The neutrophil pellet was washed once with phosphate buffered saline (PBS) and resuspended with 1 ml PBS, counted by Sysmex K-4500 (Toa Medical Electronics Co Ltd) and adjusted to 4 × 106 cells/ml with PBS. Neutrophils harvested by this technique were >97% pure as assessed by Giemsa staining and 99% viable as assessed by trypan blue exclusion.
MEASUREMENT OF THE NEUTROPHIL RESPIRATORY BURST BY FLOW CYTOMETRY
Activation of the neutrophil respiratory burst was determined by the formation of the fluorescent compound rhodamine-123 from dihydrorhodamine-123 (DHR; Molecular Probes, Eugene, OR, USA).12 In brief, two 200 μl samples of the neutrophil suspension (2 × 106 cells/ml) in polypropylene tubes (Falcon no 2052, Beckton-Dickinson, Lincoln Park, NJ, USA) were incubated with 10 μl of a DHR solution (100 μg/ml) for 10 minutes at 37°C. One sample was used to assess spontaneous ROS production. The other was mixed with 20 μl of a 20 μg/ml phorbol myristate acetate solution (PMA; Sigma Chemical Co, St Louis, MO, USA) and incubated for 15 minutes at 37°C. At the end of this second incubation period 500 μl of cold PBS were added to both samples and these were kept on ice until analysed by flow cytometry on a Beckton-Dickinson FACScan (Beckton-Dickinson, Mountain View, CA, USA) with a gate setting for neutrophils on forward and side scatter. Ten thousand cells were analysed; green fluorescence (FL1) was determined and mean cellular fluorescence intensities (MFI) were calculated usinglysis II software.
ADHESION MOLECULE IMMUNOFLUORESCENCE AND FLOW CYTOMETRIC ANALYSIS
One hundred μl samples of neutrophil suspensions (4 × 106 cells/ml) were mixed with 20 μl of FITC labelled monoclonal antibody anti-LFA-1 (CD11a) (25.3.1 clone, Immunotech, Marseille, France), FITC labelled anti-Mac-1 (CD11b) (BEAR 1 clone, Immunotech), FITC labelled anti-l-selectin (CD62L) (DREG56 clone, Immunotech), and FITC labelled IgG1 (2T8–2F5 MsIgG1, Coulter Immunology, Hialeah) that acted as a non-specific control antibody. These were incubated at 4°C for 30 minutes, washed twice in ice cold PBS, resuspended in 1 ml PBS, and kept on ice until analysed. Neutrophils were assessed under resting conditions and after stimulation with 2 ng/ml human tumour necrosis factor α (ΤΝFα; Sigma Chemical Co, St Louis, MO, USA) for 90 minutes at 37°C in 5% CO2. Cells were assessed with a gate setting for neutrophils on forward and side scatter diagrams and fluorescence on the FL1 channel (530 nm) was collected with the Beckton-Dickinson lysis II software. Ten thousand cell counts were accumulated for the analysis. Non-specific binding (IgG1) on resting and stimulated neutrophils was measured at MFI values of 2.59 (0.56) and 3.43 (0.94) units, respectively.
RNA ISOLATION AND REVERSE TRANSCRIPTION-POLYMERASE CHAIN REACTION (RT-PCR)
Total RNA was extracted from the neutrophil sample using a single step isolation system (Trizol Reagent, Gibco BRL, Life Technologies, NY, USA) based on the method of Chomczynski and Sacci.13The RNA was precipitated, washed twice with 70% ethanol, and quantified in a spectrophotometer at 260 nm (Shimadzu UV-Visible Recording Spectrophotometer, Shimadzu Corporation, Kyoto, Japan). Two μg of total RNA was reverse transcripted into cDNA using the oligo(dT)18 primer and AMV reverse transcriptase (Promega, Madison, WI, USA). PCR was performed on the resulting cDNA using specific primers for p22-phox (sense primer 5′-GTTTGTGTGCCTGCTGGAGT-3′ and antisense primer 5′-TGGGCGGCTGCTTG ATGGT-3′),14 for CD11b (5′-CAGAGCGT GGTCCAGGCACCA-3′ and 5′-CCTTCAT CCGCCGAAAGTCA-3′),15 and for β-actin (5′-GTGGGGCGCCCAGGCACCA-3′ and 5′-CTCCTTAATGTCACGCACGATTTC-3′)16 using Taq polymerase according to the manufacturer's instructions. The PCR protocol was as follows. For p22-phox: 3 minutes at 94°C, 1 minute at 55°C, 1 minute at 72°C for one cycle; 1 minute at 94°C, 1 minute at 55°C, 1 minute at 72°C for 35 cycles; 1 minute at 94°C, 10 minutes at 60°C for one cycle. For CD11b: 4 minutes at 94°C for one cycle; 30 seconds at 94°C, 30 seconds at 63°C, 1 minute at 72°C for 30 cycles; 5 minutes at 72°C for one cycle. For β-actin: 1 minute at 94°C, 1 minute at 58°C, 2 minutes at 72°C for 35 cycles; 7 minutes at 72°C for one cycle. Following PCR, products were fractionated by 2% agarose gel electrophoresis and stained with ethidium bromide. Gels were subjected to scanner densitometry and the bands were quantified with the aid of the Sigmagel gel analysis software (Jandel Scientific Corporation, San Rafael, CA, USA). The results are expressed as a ratio of β-actin expression, which served as a housekeeping gene.
STATISTICAL ANALYSIS
The results are shown as mean (SE) together with the mean difference between groups and the 95% confidence interval (CI) of this difference. To assess the statistical significance of differences between the three study groups we used analysis of variance (ANOVA) followed by post hoc analysis (Scheffe test) if appropriate. The paired Student's t test was used to investigate the significance of changes within each group after stimulation. A p value of less than 0.05 was considered significant.
Results
RESPIRATORY BURST
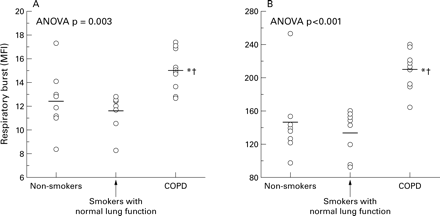
The production of ROS was higher in neutrophils harvested from patients with COPD (MFI 15.1 (0.5) units) than in those from smokers with normal lung function (MFI 11.6 (0.5) units; mean difference –3.4 (95% CI –5.1 to –1.8), p<0.01) or non-smokers (MFI 12.4 (0.9) units; mean difference –2.6 (95% CI –4.8 to –0.5), p=0.03; fig 1). These differences were maintained after PMA stimulation (fig 1). The change induced by PMA on ROS production was higher in patients with COPD (MFI 195 (7) units) than in smokers with normal lung function (MFI 121 (9) units; mean difference –73 (95% CI –98 to –49), p<0.001) or in non-smokers (MFI 133 (16) units; mean difference –61 (95% CI –96 to –27), p=0.001).
Scatter plots showing the DHR fluorescence (MFI, mean fluorescent intensity) of neutrophils studied (A) under basal conditions and (B) after stimulation with PMA. Each dot represents the result from one individual. Horizontal bars represent mean values. *Significant difference v non-smokers; †significant difference v smokers with normal lung function.
EXPRESSION OF ADHESION MOLECULES
Under basal conditions the surface expression of Mac-1 (CD11b) was higher in patients with COPD (MFI 91 (5) units) than in smokers with normal lung function (MFI 45 (3) units; mean difference –46 (95% CI –61 to –31), p<0.001) and non-smokers (MFI 67 (7); mean difference –24 (95% CI –39 to –10), p=0.003; fig 2). After stimulation with TNFα the surface expression of Mac-1 (CD11b) was also higher in patients with COPD (MFI 340 (15) units) than in smokers with normal lung function (MFI 263 (11) units; mean difference –77 (95% CI –119 to –34) units, p=0.001; fig 2); by contrast, there was no significant difference in Mac-1 expression between non-smokers (MFI 310 (9) units; mean difference –30 (95% CI –69 to 9)) and the other groups (fig 2). The surface expression of LFA-1 or l-selectin did not differ between the groups either under basal conditions or after stimulation with TNFα (table 1). However, in agreement with previous studies showing that TNFα induces the shedding ofl-selectin from the neutrophil membrane,17surface expression of L-selectin was significantly reduced after TNFα in all groups (table 1).
Scatter plots showing the surface expression of the adhesion molecule Mac-1 (CD11b) (MFI, mean fluorescent intensity) on neutrophils studied (A) under basal conditions and (B) after stimulation with TNFα. Each dot represents one determination. Horizontal bars represent mean values. *Significant difference v non-smokers; †significant difference v smokers with normal lung function.
Mean (SE) values of LFA-1 andl-selectin expression
GENE EXPRESSION
Figure 3A shows a representative RT-PCR result. The PCR products generated were a 316 bp fragment for p22-phox, a 406 bp fragment for CD11b, and a 540 bp fragment for β-actin. Figure 3B and 3C show the individual and average mRNA values of both Mac-1 and p22-phox in patients with COPD and smokers with normal lung function, neither of which differed significantly between the two groups.
(A) RT-PCR product generated using specific primers for p22-phox, CD11b, and β-actin; individual and mean (bars) values of (B) Mac-1/β-actin and (C) p22-phox/β-actin expression in circulating neutrophils harvested from patients with COPD and long term smokers with normal lung function. Differences between groups were non-significant.
Discussion
This study shows that circulating neutrophils harvested from patients with stable COPD produce more ROS (respiratory burst) and express more adhesion molecules (Mac-1) than those obtained from non-smokers and, importantly, from long term smokers with normal lung function. This enhanced response may be relevant for the pathogenesis of COPD.
Previous studies have shown that, compared with non-smokers, circulating neutrophils in patients with COPD produce more ROS (enhanced respiratory burst)18 ,19 and express abnormal levels of some surface adhesion molecules such as Mac-1.7Our study confirms these observations and extends them to a group of long term smokers with normal lung function. This is important because it allows us to analyse the influence of smoking and COPD independently on neutrophil function. We observed that patients with COPD showed a significant upregulation of respiratory burst and Mac-1 expression, not only with respect to non-smokers (as previously described) but also (and even more markedly) with respect to smokers with normal lung function (figs 1 and 2). Our results therefore suggest that these differences were not caused by smoking but, rather, they indicate that the abnormal neutrophil function described here is characteristic of COPD. Whether it represents a predisposing factor for COPD or is a consequence of the disease is at present unclear.
Our results highlight a potentially relevant pathogenic mechanism in COPD, such as the interaction between the respiratory burst and the expression of some (not all) surface adhesion molecules. Previous studies have established that in vitro phosphorylation of Mac-1 leads to NADPH oxidase activation20 and that cross linking of Mac-1 induces neutrophil respiratory burst.21 ,22 Minamiyaet al 23 showed that neutrophil adhesion to the endothelium in rats activates the respiratory burst. Our results are in agreement with these previous experimental studies and suggest that, in patients with COPD, the enhanced expression of Mac-1 (favouring the recruitment of neutrophils to an inflammatory site) is coupled with an increased respiratory burst, augmenting their potential for lung damage. This combination of events is likely to contribute to the development of COPD.
This study also sought to gain some insight into the genetic regulation of NADPH oxidase and Mac-1 in neutrophils of patients with COPD. To this end, we determined the mRNA levels of p22-phox and Mac-1 in circulating neutrophils harvested from patients with COPD and smokers with normal lung function. NADPH oxidase, the enzyme responsible for the neutrophil respiratory burst, is composed of several subunits. We chose to determine the mRNA levels of one of them (p22-phox) because p22-phox is necessary for superoxide generation24 ,25and several inflammatory stimuli such as TNFα upregulate the expression of the p22-phox gene.26 We found that, in contrast to the significant differences observed in the respiratory burst (fig 1), p22-phox mRNA levels were similar in all the groups we studied (fig 3). The discrepancy between these results may be because NADPH oxidase activity is regulated by subunits other than p22-phox, but this is unlikely in view of the above mentioned arguments; alternatively, it is possible that post-transcriptional regulation occurs. Related to the latter, we propose that mechanisms controlling the mobilisation of secretory vesicles to the membrane in response to a given stimulus may explain our findings.27 This proposal is based on the following previous observations: (1) some NADPH oxidase subunits including p22-phox are stored in secretory vesicles when the enzyme is inactive and fuse to the membrane upon activation28; (2) p22-phox and Mac-1 are stored in the same secretory vesicles28; (3) Mac-1 is important in the activation of the respiratory burst22; and (4) in keeping with the p22-phox mRNA data, we did not find a significant difference in Mac-1 mRNA levels (fig 3). Our observation of increased respiratory burst and surface expression of Mac-1 in patients with COPD in the absence of significant transcriptional differences could therefore be explained by enhanced mobilisation of secretory vesicles to the membrane in response to a given stimulus. This hypothesis will have to be confirmed or refuted in future studies.
In summary, this study shows that circulating neutrophils from patients with stable COPD have increased respiratory burst and express more adhesion molecules than those harvested from non-smokers (as previously described) and, importantly, than those from smokers with normal lung function. This excludes smoking as a significant cause of this abnormal neutrophil function and suggests a link to the disease itself. Future studies are required to investigate whether these abnormalities predispose to the development of COPD or are a consequence of it. In either case, our results suggest a potentially relevant pathogenic mechanism in COPD.
Acknowledgments
The authors thank M Bosch, A Noguera, F Bauzá, and C Santos for their technical collaboration during the study. They also express their gratitude to Dr I Caragol from the Servei de Inmunología (Hospital Vall de Hebron, Barcelona) for her help with the determination of the respiratory burst by flow cytometry.
References
Footnotes
Supported in part by ABEMAR and Fondo de Investigaciones Sanitarias (FIS 99/0511).