I. Introduction
The discovery that the biological actions of endothelium-derived relaxing factor (Furchgott and Zawadzki, 1980) are due to the endogenous release of nitric oxide (NO)c (Palmer et al., 1987; Ignarro et al., 1987; Khan and Furchgott, 1987) revealed the existence of a ubiquitous biochemical pathway (Moncada et al., 1989). NO is formed from the amino acid l-arginine by a family of enzymes, the NO synthases (NOSs), and plays a role in many physiological functions. Its formation in vascular endothelial cells, in response to chemical stimuli and to physical stimuli such as shear stress, maintains a vasodilator tone that is essential for the regulation of blood flow and pressure (Moncada et al., 1989; Vanhoutte, 1989; Furchgott, 1990; Ignarro, 1990; Vane et al., 1990; Luscher, 1991). NO produced by the endothelium and/or platelets also inhibits platelet aggregation and adhesion, inhibits leukocyte adhesion and modulates smooth muscle cell proliferation (Moncada and Higgs, 1993). NO is synthesized in neurons of the central nervous system, where it acts as a neuromediator with many physiological functions, including the formation of memory, coordination between neuronal activity and blood flow, and modulation of pain (Garthwaite, 1991; Snyder and Bredt, 1992). In the peripheral nervous system, NO is now known to be the mediator released by a widespread network of nerves, previously recognized as nonadrenergic and noncholinergic. These nerves mediate some forms of neurogenic vasodilation and regulate certain gastrointestinal, respiratory and genitourinary functions (Gillespie et al., 1990; Rand, 1992; Toda, 1995). These physiological actions of NO are mediated by activation of the soluble guanylate cyclase and consequent increase in the concentration of cyclic guanosine monophosphate in target cells (Murad et al., 1990; Ignarro, 1991).
In addition, NO is generated in large quantities during host defense and immunological reactions (Nathan and Hibbs, 1991; Nussler and Billiar, 1993). Such generation of NO was first observed in activated macrophages (Hibbs et al., 1988; Marletta et al., 1988; Stuehr et al., 1989), where it contributes to their cytotoxicity against tumor cells, bacteria, viruses and other invading microorganisms. The cytostatic/cytotoxic actions of NO result from its inhibitory actions on key enzymes in the respiratory chain and in the synthesis of deoxyribonucleic acid in the target cells (Hibbs et al., 1990; Nguyen et al., 1992). NO may also interact with oxygen-derived radicals to produce other toxic substances (Hibbs, 1992) such as peroxynitrite (Beckman et al., 1990). Peroxynitrite is a powerful oxidant; however, there seem to be very effective mechanisms for its removal and inactivation (Moro et al., 1994). Thus, NO plays a role in immunological host defense and is also involved in the pathogenesis of conditions such as septic shock and inflammation.
NO is a gas at temperatures down to −152°C. It is slightly soluble in many solvents and can diffuse relatively easily across biological membranes. Its solubility in water is low so that it can only occur in dilute solution. Nitrite, rather than nitrite plus nitrate, is believed to be the product of NO in oxygenated water (see Butler et al., 1995;Williams, 1996). Table 1 shows several chemical species related to NO.
NO and related species
II. Nitric Oxide Synthases
NOS is a heme-containing enzyme with a sequence similarity to cytochrome P-450 reductase. Several isoforms of NOS are now known to exist, two of which are constitutive and one of which is inducible by immunological stimuli (for reviews see Knowles and Moncada, 1994;Morris and Billiar, 1994; Nathan and Xie, 1994; Sessa, 1994; Stuehr and Griffith, 1992). The constitutive NOS (cNOS) that was first discovered in the vascular endothelium has been designated as eNOS, whereas that present in the brain, spinal cord and peripheral nervous system is termed nNOS. The form of NOS induced by immunological or inflammatory stimuli is known as iNOS. A comparison of the properties of these three major isoforms of the enzyme is shown in table 2.
Isoforms of NOS
The complementary deoxyribonucleic acids for all three of these isoforms have been cloned from several species, including humans (Charles et al., 1993; Geller et al., 1993; Marsden et al., 1992;Nakane et al., 1993). This has revealed further differences among them, which are shown in table 3. Knockout mice have been generated for each of the three NOS isoforms and have provided useful information concerning the role of each isoform and the effects of its deletion in whole animals (Huang and Fishman, 1996).
Molecular biology of human NOS isoforms3-a
The apparent association of the three NOS isoenzymes with the endothelium, neurons and inducibility (table 2) is an oversimplification. For example, eNOS is located not only in the vascular endothelial cells but also in platelets (Radomski et al., 1990) and in certain neuronal populations in the brain (Dinerman et al., 1994), whereas nNOS has been found in the epithelium of the bronchi and trachea (Kobzik et al., 1993), as well as in skeletal muscle (Kobzik et al., 1994). Furthermore, some differences have been identified between iNOSs obtained from different tissues within the same species (Mohaupt et al., 1994). In addition, the constitutive eNOS can be induced in certain situations such as during chronic exercise (Sessa et al., 1994) or during pregnancy, when both eNOS and iNOS are induced (Weiner et al., 1994), whereas iNOS appears to be present constitutively in some tissues, including human bronchial epithelium (Kobzik et al., 1993), rat kidney (Mohaupt et al., 1994) and some fetal tissues (Baylis et al., 1994).
III. Inhibitors of Nitric Oxide Synthase
The generation of NO from l-arginine proceeds via the formation of Nω-hydroxy-l-arginine (Pufahl et al., 1992). This l-arginine/NO pathway can be inhibited by several analogues of l-arginine, of which the first to be identified was Nω-monomethyl-l-arginine (see Moncada and Higgs, 1993). Before the discovery of NO as a biological mediator, Nω-monomethyl-l-arginine had been found to prevent l-arginine-dependent cytotoxicity in murine macrophages (see Hibbs et al., 1990). It has subsequently been shown to be a competitive inhibitor of the formation of NO in the vasculature and elsewhere (see Moncada et al., 1989), and, as such, it has become a valuable tool for unraveling the biological actions of NO.
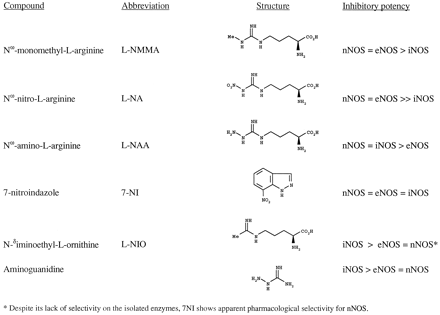
Many other analogues of l-arginine are now known to act as competitive (and in some cases, irreversible) inhibitors of both the constitutive and the inducible NOS. Other non-amino acid compounds that mimic the guanidinium moiety of l-arginine also inhibit the enzyme. Some inhibitors of NOS are shown in figure 1. Interestingly, asymmetric dimethyl-l-arginine (NG-NG-dimethyl-l-arginine,l-ADMA), an inhibitor of NOS, and Nω-monomethyl-l-arginine are present in human plasma and urine (Vallance et al., 1992). Accumulation of these compounds in the plasma may contribute to the pathophysiology of some conditions.
Some inhibitors of NOS.
There are several clinical situations in which it may be desirable to inhibit the production of NO, either by a constitutive NOS (for example, in cerebral ischemia or epilepsy, in which overproduction of NO by nNOS may lead to neurotoxicity) or by iNOS in conditions such as septic shock or certain chronic inflammatory diseases. Although much effort is being put into the search for a selective inhibitor of iNOS and some of the known inhibitors have shown some degree of selectivity in vitro toward one or another NOS isoform (fig. 1), thus far, the drugs available affect both the inducible and the constitutive isoforms (for review, see Griffith and Gross, 1996).
IV. Nitric Oxide Donors
The clinical actions of the nitrovasodilators are now known to result from their ability to liberate NO (Feelisch, 1991); thus, the term “NO donors” has been adopted for this class of drug. Such compounds include the organic nitrates (e.g., glyceryl trinitrate) and nitrites (e.g., amyl nitrite), inorganic nitroso compounds (e.g., sodium nitroprusside), sydnonimines (e.g., molsidomine) and S-nitrosothiols (e.g., S-nitrosoglutathione) (Feelisch and Stamler, 1996 and table 4).
Recommended abbreviations for certain NO donors
Although NO donors have been used traditionally as vasodilators, other clinical applications are emerging as our understanding of the biological actions of NO itself increases. Thus, compounds such as S-nitrosoglutathione, which potently inhibits platelet aggregation at concentrations that do not affect blood pressure (de Belder et al., 1995), may be useful in the treatment of certain thrombotic disorders. Other NO donors have been shown to reduce intimal thickening in injured arteries in an animal model (Lefer and Lefer, 1994). Impaired generation of NO by nitrergic nerves, i.e., those that liberate NO as a neuromodulator, may underlie certain gastrointestinal, genitourinary and respiratory disorders. In such cases, NO donors may mimic nitrergic nerve-mediated responses and have been shown to be effective in the treatment of achalasia and other malfunctions of sphincters in the gastrointestinal tract, as well as in the treatment of impotence in diabetes (see Moncada and Higgs, 1995).
V. Recommended Nomenclature and Abbreviations
In the research literature of the past few years regarding the biological actions of NO, several different names or abbreviations have been used by investigators to identify the same enzyme or the same substance. To avoid confusion arising from nonuniform nomenclature and abbreviations, the following recommendations are made (see sections V.A to V.E.). These reflect current usage and, as such, contain anomalies. In the future, new compounds will be named and abbreviated with greater consistency.
A. Abbreviations for the Isoenzymes of Nitric Oxide Synthase
The abbreviations for the isoenzymes of the NOS are:
-
eNOS for the isoform originally found in endothelial cells;
-
nNOS for that originally found in neuronal tissue; and
-
iNOS for the isoform induced in macrophages and other cell types in response to endotoxin and/or various cytokines.
B. Abbreviations for Nitric Oxide Synthase Substrates
The recommended abbreviations for NOS substrates are as follows:
-
l-arginine: full name or l-Arg;
-
l-homoarginine: full name or l-homoArg; and
-
Nω-hydroxy-l-arginine: l-oharg.
C. Abbreviations for Inhibitors of Nitric Oxide Synthase
The recommended abbreviations for inhibitors of NOS are as follows:
-
Nω-monomethyl-l-arginine:l-NMMA;
-
Nω-nitro-l-arginine: l-NA;
-
Nω-nitro-l-arginine methyl ester:l-NAME;
-
N-iminoethyl-l-ornithine: l-NIO;
-
Nω-amino-l-arginine: l-NAA;
-
Nω-Nω-dimethyl-l-arginine:l-ADMA;
-
Nω-Nω′-dimethyl-l-arginine:l-SDMA;
-
l-canavanine: full name;
-
aminoguanidine: full name; and
-
7-nitroindazole: 7-NI. The use of the l-isomer should be specified.
Other compounds have recently been described as inhibitors of NOS but are not in common use. These include the isothioureas (Garvey et al., 1994; Southan et al., 1995) and l-thiocitrulline (Frey et al., 1994).
D. Abbreviations for Certain Nitric Oxide Donors
The recommended abbreviation for certain NO donors are shown in table 4.
E. Nitrergic Nerves
The term ‘nitrergic’ should be applied to nerves whose transmitter function depends on the release of NO or to transmission mechanisms that are brought about by NO.
VI. Conclusion
Since the identification in 1987 of NO as a biological mediator, more than 14,000 papers have been published in this field. Some basic guidelines for standardization of terminology have been given in this short document. As the amount of work in this area continues to grow, it will be necessary to update and expand these recommendations.
Acknowledgements
The authors thank Timothy R. Billiar, Ian G. Charles, Richard G. Knowles and David J. Madge for their assistance in the preparation of this manuscript.
Footnotes
-
↵FNa Composition of the nitric oxide research subcommittee of the International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification: M. Feelisch, Department of Nitric Oxide Research, Schwarz Pharma AG, Monheim, Germany; R. Furchgott, State University of New York Health Science Center at Brooklyn, Department of Pharmacology, Brooklyn, New York; J. Garthwaite, The Cruciform Project, University College London, London, United Kingdom; J.B. Hibbs, Jr., Veterans Affairs Medical Center and Department of Medicine, Division of Infectious Diseases, University of Utah Medical Center, Salt Lake City, Utah; A. Higgs, The Cruciform Project, University College London, London, United Kingdom; L. Ignarro, University of California, Los Angeles School of Medicine, Department of Molecular Biology, Los Angeles, California; T. Luscher, Division of Cardiology, University Hospital Bern, Bern, Switzerland; M.A. Marletta, College of Pharmacy, School of Medicine, University of Michigan, Ann Arbor, Michigan; W. Martin, University of Glasgow, Clinical Research Initiative, Glasgow, Scotland; S. Moncada, The Cruciform Project, University College London, London, United Kingdom; M. Rand, Royal Melbourne Institute of Technology, Department of Medical Laboratory Science, Melbourne, Australia; M. Spedding, Institut de Recherches Servier, Centre de Recherches de Croissy, Croissy sur Seine, France; S. Snyder, Department of Neuroscience, Johns Hopkins University School of Medicine, Baltimore, Maryland; N. Toda, Department of Pharmacology, Shiga University of Medical Sciences, Seta, Japan; J.R. Vane, The William Harvey Research Institute, St. Bartholomew’s Hospital Medical College, London, United Kingdom; P. Vanhoutte, Institut de Recherches International Servier, Courbevoie, France.
-
↵FNb Address correspondence to: Salvador Moncada, The Cruciform Project, University College London, 140 Tottenham Court Road, London W1P 9LN, United Kingdom.
- Abbreviations:
- NO
- nitric oxide
- NOS
- NO synthase
- The American Society for Pharmacology and Experimental Therapeutics