Abstract
The incidence of noncommunicable diseases (NCDs) has increased over the last few decades, and one of the major contributors to this is lifestyle, especially diet. High intake of saturated fatty acids and low intake of dietary fiber is linked to an increase in NCDs. Conversely, a low intake of saturated fatty acids and a high intake of dietary fiber seem to have a protective effect on general health. Several mechanisms have been identified that underlie this phenomenon. In this review, we focus on pharmacological receptors, including the aryl hydrocarbon receptor, binding partners of the retinoid X receptor, G-coupled protein receptors, and toll-like receptors, which can be activated by nutritional components and their metabolites. Depending on the nutritional component and the receptors involved, both proinflammatory and anti-inflammatory effects occur, leading to an altered immune response. These insights may provide opportunities for the prevention and treatment of NCDs and their inherent (sub)chronic inflammation.
Significance Statement This review summarizes the reported effects of nutritional components and their metabolites on the immune system through manipulation of specific (pharmacological) receptors, including the aryl hydrocarbon receptor, binding partners of the retinoid X receptor, G-coupled protein receptors, and toll-like receptors. Nutritional components, such as vitamins, fibers, and unsaturated fatty acids are able to resolve inflammation, whereas saturated fatty acids tend to exhibit proinflammatory effects. This may aid decision makers and scientists in developing strategies to decrease the incidence of noncommunicable diseases.
I. Introduction
The incidence of noncommunicable diseases (NCDs) is growing rapidly, particularly, though not exclusively, in developed countries (Alwan and Maclean, 2009; World Health Organization, 2013). NCDs are not caused by infectious agents and are therefore not transmissible from person to person (Kim and Oh, 2013). The World Health Organization estimates that the major NCDs (cancer, diabetes, cardiovascular diseases, and chronic respiratory diseases) account for 63% of the disease burden in 2008 (World Health Organization, 2013). The World Health Organization’s goal is to reduce mortality by 25% by the year 2025 in those between the ages of 30 and 70 years old (World Health Organization, 2013). To reach this goal, there is a need for better preventive and therapeutic measures to manage these “modern” diseases. A major contributor to the increase in prevalence of NCDs during the last century is lifestyle, particularly diet (World Health Organization, 2013).
Diet is an important contributor to diabetes, cardiovascular disease, chronic respiratory disease, and inflammatory bowel disease (IBD) among others (Castro-Rodriguez et al., 2008; Park et al., 2011; Berthon et al., 2013; Lopez-Garcia et al., 2014; Myles, 2014; Thorburn et al., 2014; Di Daniele, 2019; Phillips et al., 2019). A Western diet, which is characterized by high amounts of saturated fatty acids (SFAs) and low amounts of fiber, fruit, and vegetables, is associated with an increased risk of NCDs (Myles, 2014; Thorburn et al., 2014). On the other hand, a Mediterranean diet rich in fiber, fresh fruit, and vegetables and low in SFAs is linked to a lower risk of these diseases (Castro-Rodriguez et al., 2008; Park et al., 2011; Lopez-Garcia et al., 2014; Thorburn et al., 2014; Di Daniele, 2019). These studies emphasize the potential of certain food components being used in therapeutic prevention or treatment of NCDs. A particularly promising route is the influence of nutritional components on the inflammatory response of the immune system (Phillips et al., 2019). In recent decades, it has become clear that nutrition is more than just a source of nourishment or energy, and the Hippocrates quote (400 BC), “Let food be thy medicine and medicine be thy food,” remains highly relevant nowadays; however, this may be a historical misquotation. It has been observed that drugs and food can exhibit overlapping mechanisms, show therapeutic synergy (not necessarily using the same primary target), and reduce side effects (MacDonald et al., 2009; Eussen et al., 2010; Georgiou et al., 2011).
Nutraceuticals have received much attention in the last few years from the scientific community, consumers, and food manufacturers. Nutraceuticals are foods or food ingredients (e.g., natural bioactive and chemical compounds) that provide medical or health benefits, including the prevention and treatment of a disease, which blurs the line between food and drugs (da Costa, 2017). The term “nutraceutical,” a hybrid of “nutrition” and “pharmaceutical,” was coined by Stephen DeFelice, founder and chairman of the Foundation for Innovation in Medicine located in Cranford, New Jersey in 1979 (DeFelice, 1995). In this review, we make use of the broader term “nutritional components,” since not all described components are beneficial for health or have not yet been proven to prevent and treat a disease.
Three main modes of actions of nutritional components have been identified. First, nutritional components can interact with intestinal bacteria and change the gut microbiota composition, leading to physiologic and immunologic changes in the body. In addition, during the digestion of food, an abundance of metabolites can be produced, such as amino-acid derivatives and short-chain fatty acids (SCFAs), which can affect human physiology in both positive and negative ways (Kolodziejczyk et al., 2019). The second mechanism by which diet can alter the immunologic response is via epigenetic modifications (Thorburn et al., 2014; Zhang and Kutateladze, 2018). For example, the activation or inhibition of specific histone deacetylases by nutritional components can alter the transcription of proteins and, therefore, the activity of the immune system (Thorburn et al., 2014). The third mechanism is the involvement of pharmacological receptors on the surface of cells to which nutritional molecules can bind and either activate or inhibit the pathway downstream of the receptor (Kau et al., 2011; Witkamp and van Norren, 2018). This third mechanism is the main focus of this review.
Nutritional biology can significantly contribute to the discovery of new molecular targets in both health and diseases. To progress the therapeutic use of nutritional components, a detailed understanding of the underlying mechanism by which nutritional components can alter the immune system is required. The aim of this paper is to provide an overview of the current literature regarding the direct and indirect effects of nutrition via the modulation of pharmacological receptors. We will discuss the receptor structure and mechanism of action, nutritional components able to interact with the receptor, and effects on immunologic status.
II. Aryl Hydrocarbon Receptor
A. The Receptor
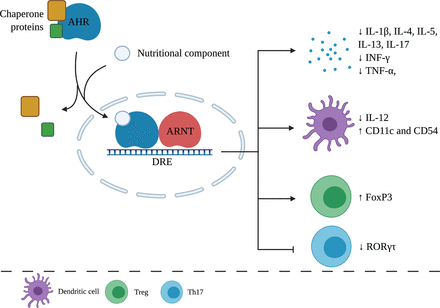
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor expressed by several immune cells, which can be activated by small environmental, dietary, microbial, and metabolic molecules, which subsequently trigger molecular immune response pathways. Research has shown that AHR signaling plays relevant roles in immune responses during health and disease, including modulation of biologic processes important for tissue homeostasis and development of pathologic conditions related to autoimmune, neoplastic, metabolic, degenerative, inflammatory diseases, and tumorigenesis (Schulte et al., 2017; Gutiérrez-Vázquez and Quintana, 2018; Rothhammer and Quintana, 2019). AHR is a part of a family of transcription factors containing a basic helix-loop-helix-PER-ARNT-SIM with a C-terminus transcription activation domain (Xue et al., 2017). Inactive AHR resides in the cytoplasm where the nuclear localization sequence is shielded by chaperone proteins. When an AHR agonist binds to the receptor, a conformational change leads to the uncovering of the nuclear localization sequence. The AHR complex is then translocated toward the nucleus where the chaperone proteins dissociate. The AHR nuclear translocator then forms a heterodimer with AHR. The complex subsequently binds to the dioxin responsive element in the DNA, inducing the transcription of target genes, including cytochrome P450–dependent monooxygenases and AHR repressors, as is shown in Fig. 1 (Schulte et al., 2017).
Effects of nutritional components via AHR on the immune system. After AHR activation by a nutritional component, chaperone proteins dissociate and form a heterodimer with an aryl hydrocarbon receptor nuclear translocator. Target genes are transcribed and lead to anti-inflammatory effects on the immune system. ARNT, aryl hydrocarbon receptor nuclear translocator; DRE, dioxin responsive element. Created with BioRender.com.
B. Nutritional Activators
AHR has a broad range of endogenous activators in addition to dietary components, among which the toxin high-affinity 2,3,7,8-tetrachlorodibenzo-p-dioxin is the best-known ligand (Schulte et al., 2017). The first class of AHR dietary activators includes indoles, which are highly present in cruciferous (brassica) vegetables (Stockinger et al., 2014; Hubbard et al., 2015). The indoles comprise indole-3-acetonitrile and indole-3-carbinol (I3C), both of which can be further converted into indolo-[3,2-b]-carbazole and 3-3′-diindolylmethane (DIM) under acidic conditions in the stomach (Hubbard et al., 2015). In addition, AHR is activated by the flavonoids quercetin and resveratrol, also known as polyphenols, which are found in fruits and vegetables (Xue et al., 2017). The immunomodulatory effects of these nutritional components related to AHR signaling have been described in detail below and summarized in Table 1.
Literature overview nutritional components and their effect on the immune system via AHR
C. Immune Modulators
The best-described effect of dietary components on the immune system via AHR activation is in context of T-cell differentiation. The balance between anti-inflammatory T regulatory cells (Treg) and proinflammatory T helper (Th) 17 cells is crucial in maintaining homeostasis. In vitro studies showed that the Treg-related gene Foxp3 is upregulated after DIM administration, whereas the Th17-related gene retinoic acid receptor-related orphan receptor γτ was downregulated in anti-CD3/CD28 monoclonal antibody-stimulated naïve Th cells (Huang et al., 2013). This indicates a shift in the homeostasis toward an anti-inflammatory state induced by DIM, which is abolished by pretreatment with short hairpin RNA directed at AHR, indicating direct involvement of the AHR in the observed in vitro effects (Huang et al., 2013). Although the involvement of AHR was not proven in vivo, Huang et al. (2013) pointed out that DIM supplementation reduced mucosal inflammation and reduced colonic Th17 infiltration while expanding the Treg population in a murine oxazolone-induced colitis model.
Imbalance of the Treg and Th17 populations is the underlying problem of multiple autoimmune diseases. Pretreatment with DIM and I3C in a murine model of multiple sclerosis [experimental autoimmune encephalomyelitis (EAE)] showed reduced numbers of inflammatory cells in the spinal cord and decreased levels of the proinflammatory cytokines, tumor necrosis factor α (TNF-α), interleukin (IL)‐17, and IL‐6 in serum (Rouse et al., 2013). Additionally, post-treatment with the indoles caused a growing population of Treg cells in the inguinal lymph nodes and brain of mice suffering from EAE, possibly providing an explanation for the reduced inflammation. An AHR-specific antagonist CH223191 was found to reduce the clinical scores as well as growing Treg populations induced by DIM and I3C (Rouse et al., 2013). The growing Treg population as a result of DIM and I3C treatment was found in vitro as well (Rouse et al., 2013). A recent study also using a murine EAE model displayed similar results after DIM administration (Yang et al., 2020). DIM alleviated the severity of EAE by a reduction in proinflammatory cytokines and decrease in Th1 and Th17 cells in serum, which was abolished in the presence of the AHR antagonist CH223191. The effects of DIM and I3C treatment on delayed-type hypersensitivity to methylated bovine serum albumin were measured in a murine study performed by Singh et al. (2016). The indoles DIM and I3C attenuated the delayed-type hypersensitivity and promoted Treg proliferation in lymph nodes. Since the increased Treg proliferation was notably absent in AHR−/− mice, this effect is dependent on AHR activation (Singh et al., 2016). The benefits of increased Treg development were shown as well in a mouse model of Clostridium difficile–associated disease (Julliard et al., 2017). Dietary I3C resulted in greater survival from the bacterial infection caused by an increased Treg population but also by enhanced group 3 innate lymphoid cells and γδ T cell populations in the gut. Mice not treated with I3C developed severe infections, had a mortality rate of 87.5%, and showed increased translocation of Clostridium difficile toward liver and lungs. Additionally, AHR−/− mice fed with I3C lost this observed protection against Clostridium difficile infection (Julliard et al., 2017).
Dendritic cells (DCs), the link between innate and adaptive immunity, are also affected by cruciferous vegetables via AHR. Benson and Shepherd (2011) hypothesized that I3C is able to suppress the immune response and inflammation in DCs. To investigate this hypothesis, the researchers performed a study in which BMDCs from both AHR+/+ and AHR−/− mice were activated with lipopolysaccharide (LPS) with or without the presence of I3C (Benson and Shepherd, 2011). I3C appeared to reverse the LPS-induced CD surface marker CD11c and suppressed the LPS-induced production of IL-12 in an AHR-dependent manner. This underlines the anti-inflammatory effects of I3C.
I3C induced oral tolerance against ovalbumin and reduced peanut allergy symptoms in mice, which might be due an increased number of tolerogenic DCs in the lamina propria as investigated by Hammerschmidt-Kamper et al. (2017). However, I3C-fed AHR-deficient mice (AHR lost in intestinal epithelial cells or in CD11c+ DCs) also supported tolerance, suggesting presence of AHR in one of these cell types is not sufficient for the I3C-induced positive effect. Mohammadi et al. (2018) observed that monocyte-derived macrophages from patients with systemic lupus erythematosus treated with I3C expressed an anti-inflammatory cytokine profile compared with untreated cells from these patients. Since other genes related to AHR activation are also altered, AHR is likely to be involved in this process (Mohammadi et al., 2018). Furthermore, Ociepa-Zawal et al. (2007) showed that I3C is able to increase AHR mRNA expression in a breast cancer cell line [monocyte chemoattractant protein (MCP) 7].
Taken together these studies show that a shift from a proinflammatory environment toward an anti-inflammatory environment involves AHR-mediated pathways and has been observed in different NCDs (Fig. 1). Therefore, a dietary intervention targeting AHR could be beneficial particularly for autoimmune disorders wherein an immune system imbalance is observed.
D. Clinical Application
The effects of I3C and DIM have been studied in clinical trials for the treatment of several cancers, including prostate cancer, cervical dysplasia, and breast cancer (Del Priore et al., 2010; Li et al., 2014; Thomson et al., 2017). The beneficial effects of I3C and DIM on prostate cancer observed in clinical research studies have been reviewed by Li et al. (2014), and potent I3C- and DIM-induced effects are mainly related to tumor growth inhibition. Oral treatment with DIM in patients with cervical dysplasia improved clinical scores, although no significant differences were identified between the control and experimental group (Del Priore et al., 2010). DIM in combination with tamoxifen—an anti–breast cancer drug—has been observed to be associated with lower risk of breast cancer in a phase I clinical trial (Thomson et al., 2017). To the best of our knowledge, there have been no clinical trials investigating the immunomodulatory effects of DIM or I3C, and therefore further research is needed to confirm the observed in vitro and in vivo findings as described above.
III. Retinoid X Receptor
The retinoid X receptor (RXR) plays a pivotal role in the control of multiple intracellular receptor signaling pathways and is part of the nuclear receptor (NR) superfamily. It features a ligand-binding transactivation domain at the C terminus and a DNA-binding domain in the center connected by a hinge region (Kojetin et al., 2015). The N-terminus transactivation domain has two activation components, one dependent and one independent of the ligand. This structure is similar across all NRs. RXR forms a homodimer with one other RXR or a heterodimer with other NRs, such as the retinoic acid receptor (RAR), vitamin D receptor (VDR), liver X receptor (LXR), or peroxisome proliferator-activated receptor (PPAR) (Dawson and Xia, 2012). The thyroid hormone receptor and farnesoid X receptor can also form a heterodimer but are not known to be activated by nutritional components and are therefore not discussed further in this article. The binding partner of RXR determines the genes transcribed upon activation (Dawson and Xia, 2012). The immunomodulatory effects of different nutritional components related to RAR, VDR, LXR, and PPAR signaling have been described in detail below and summarized in Table 2.
Literature overview nutritional components and their effect on the immune system via RXR binding partners: RAR, VDR, LXR, and PPAR
A. Retinoic Acid Receptor
1. The Receptor
One of the RXR binding partners is RAR (Dawson and Xia, 2012). There are three RAR isoforms, RARα, β, and γ (Cunningham and Duester, 2015). RAR has the ability to modify the expression of numerous genes in multiple immune-cell types, such as T cells, B cells, macrophages, and dendritic cells, via transcriptional activity and downstream signaling pathway activation (Larange, 2017). The RAR/RXR receptor is involved in wound healing, development of the nervous and skeletal systems, myeloid development, and embryonic development (Nagy et al., 2012).
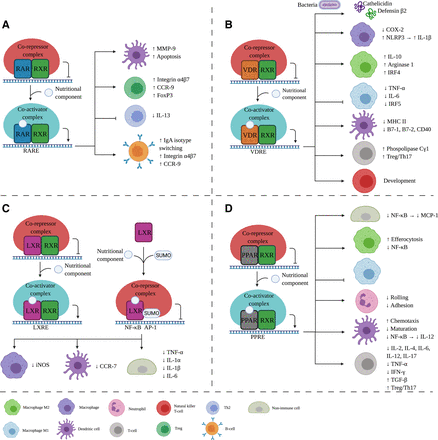
In an inactive state, the RAR/RXR complex is bound to a retinoic acid response element with corepressors 1 and 2, histone deacetylase, and Polycomb repressive complex 2. This causes trimethylation of H3K9 and H3K27 and ubiquitination of H2AK119, which leads to gene silencing. After the binding of the ligand, the corepressors dissociate and nuclear receptor coactivators 1, 2, or 3 can associate with intrinsic histone acetyltransferase because of a conformational change (Fig. 2A). This dissociation-association impacts genes controlling cell proliferation and differentiation (Dawson and Xia, 2012).
Effects of nutritional components via RXR binding partners on the immune system. After nutritional component binding to the RXR binding partner, a corepressor complex is replaced by a coactivator complex leading to gene transcription. In this figure, the immunologic effects are shown for each binding partner, namely RAR (A), VDR (B), LXR (C), and PPAR (D). Aside from RXR as a binding partner, LXR (C) can be SUMOylated where corepressor complex can bind, inhibiting transcription. IL, interleukin; iNOS, inducible NOS; LXRE, LXR responsive element; PPRE, PPAR responsive element; RARE, retinoic acid responsive element; VDRE, vitamin D responsive element. Created with BioRender.com.
2. Nutritional Activators
Vertebrates are not able to synthesize vitamin A de novo and must obtain this vitamin via their diet—mainly from carotenoids and meat (Larange and Cheroutre, 2016). Vitamin A is metabolized into active forms by cells that express alcohol dehydrogenases and retinal dehydrogenases, of which two active forms, all-trans and 9-cis retinoic acid (RA), are known to activate RAR (Mora et al., 2008).
3. Immune Modulators
RA is involved in a broad spectrum of functions that influence the immune system via the RXR/RAR heterodimer (Fig. 2A). Within DCs, the phenotype and migration are altered upon RA supplementation. In porcine-derived DC monocytes pretreated with all-trans RA and cocultured with autologous lymphocytes, the lymphocytes show higher mRNA expression of the integrin β7 and C-C chemokine receptor type (CCR) 9, which is important for mucosal homing of T cells (Saurer et al., 2007). Therefore, all-trans RA possibly plays an important role in the mucosal immunity by targeting DCs. Moreover, matrix metalloproteinase (MMP)-9, which is important for DC migration, was upregulated in bone marrow–derived DCs from BALB/cJ mice, whereas this upregulation was absent in the presence of the RARα-specific antagonist AGN 194301 (Lackey et al., 2008). Furthermore, RAs exhibit the ability to induce apoptosis of human DCs, which is inhibited by inflammatory cytokines, such as TNF-α. In this experiment, antagonists selective for a specific subtype or a pan-RAR antagonist were used, outlining the involvement of the RAR receptor on DCs (Geissmann et al., 2003). RA also influences the movement of T cells toward the intestine and Peyer’s Patches via the expression of Itg-α4 in CCR9-deficient mice, whereas T-cell migration through the vascular cell adhesion molecule 1–coated transwell membranes is defective after in vitro treatment with an RARα antagonist (RO41-5253) (Kang et al., 2011). Iwata et al. (2004) showed that murine DCs are unable to stimulate T cells to express α4β7 integrins when treated with an RAR-antagonist LE135. This indicates that specific gut homing molecules are not adequately expressed when RAR is blocked.
In vitro studies found that supplementation of all-trans RA to CD4+CD25- T cells from cord blood or to CD4+ T cells from Balb/c mice leads to an increase in the Foxp3+ Treg cell population, even in Th17-favorable environments, and RAR-specific antagonists and retroviral overexpression indicated RAR involvement (Kang et al., 2007; Schambach et al., 2007). In addition, the RARα expression in CD4+CD25- T cells from cord blood incubated with all-trans RA was increased (Kang et al., 2007). Also, in healthy mice RA has been shown to favor Treg induction (Mucida et al., 2007; Nolting et al., 2009), whereas the RAR antagonist LE135 (Mucida et al., 2007) and RAR-deficient mice (Nolting et al., 2009) showed no Treg favorability. This is confirmed by Takaki et al. (2008), who displayed that disruption of the RAR/RXR responsive element within the Foxp3 gene stopped the increase in Treg cell numbers in vitro, implying a role for RA and RAR/RXR in this process. Thus, these studies show promotion of a more anti-inflammatory phenotype due to the increase in the number of Treg cells in the presence of RA.
Vitamin A or all-trans RA are also involved in the modulation of the ratio of Th2/Th1 cells. The effects of RA on Th1 and Th2 cell populations were dependent on the timing of RA supplementation in vitro (Iwata et al., 2003). Supplementation of RA to naïve T cells after initial stimulation with anti-CD3 and anti-CD28 in the presence of IL-12 or IL-4 enhanced the Th2 development, whereas supplementation of RA at the beginning of the naïve T cell culture before the initial stimulation suppressed this development (Iwata et al., 2003). Yokota-Nakatsuma et al. (2014) showed that all-trans RA prevented the mesenteric lymph node/DCs from T cell–induced IL-13, IL-17A, TNF-α, and interferon (IFN)-γ production in vitamin A–deficient mice, thus inhibiting an allergic response to oral antigens. Especially the mesenteric lymph node/DC–induced, IL-13–producing inflammatory Th2 cells were inhibited by all-trans RA. Wild-type (WT) mice without vitamin A deficiency did not exhibit Th2 development, indicating that vitamin A is responsible for the suppression of the proinflammatory Th2 phenotype (Yokota-Nakatsuma et al., 2014).
In murine B cells, it was found that RA could induce isotype switching in B cells, leading to more neutralizing immunoglobulin (Ig) A antibodies. RAR antagonist LE540 or silencing of RA signaling abolished the observed effect on neutralizing IgA antibodies (Seo et al., 2013, 2014; Pantazi et al., 2015). Furthermore, expression of homing receptors CCR-9 and α4β7 on murine B cells is upregulated in response to RA alone or RA in combination with transforming growth factor β (TGF-β), although the involvement of RAR is yet to be investigated (Seo et al., 2013). These findings together indicate that RA is important for the (intestinal) humoral response and maintaining mucosal homeostasis.
4. Clinical Application
There are multiple clinical trials investigating the effects of vitamin A or RA alone or in combination with existing therapy. The positive effects of vitamin A in clinical cancer studies have been reviewed before, including halting cancer progression, induction of differentiation, and reduction in proliferation of tumors in different types of cancer, such as pancreatic cancer, skin cancer, and renal cancer (Tang and Gudas, 2011; Davis-Yadley and Malafa, 2015). However, some studies do not report significant improvements of vitamin A in the treatment of cancer (Tang and Gudas, 2011; Davis-Yadley and Malafa, 2015). Important to note is that the beneficial response to RA supplementation is dependent on the combination of vitamin A (or all-trans RA) and existing therapy and that limited effects in patients with cancer were partly due to vitamin A resistance. Furthermore, clinical evidence shows that RARβ expression is often inversely correlated with the tumor grade (Tang and Gudas, 2011).
The role of vitamin A has also been investigated in allergies, including asthma, eczema, rhinitis, and atopy, as reviewed by Hufnagl and Jensen-Jarolim (2019). Results with maternal or neonatal vitamin A supplementation have reported contradicting results (Hufnagl and Jensen-Jarolim, 2019). There are studies reporting a decreased risk of wheezing, eczema, asthma, and allergic rhinitis via maternal intake of vitamin A, although other studies failed to observe this correlation. In adults with chronic airway inflammation and asthma, vitamin A in combination with other supplements, such as antioxidants, other vitamins, and fish oil, and healthy diet exhibits the capacity to alleviate allergic symptoms, including improvement of clinical outcomes and reduction in the occurrence of exacerbations (Nurmatov et al., 2011; Hufnagl and Jensen-Jarolim, 2019). In obese women, vitamin A supplementation leads to reduced IL-1β serum levels. In addition, the ratio IL-1β/IL4 in serum related to the Th1/Th2 ratio is reduced as a result of vitamin A supplementation in women with obesity, promoting an anti-inflammatory phenotype (Farhangi et al., 2013). Multiple sclerosis and atherosclerosis are both associated with dysregulation of Treg cells, whereas vitamin A or RA supplementation increased the expression of TGF-β and FoxP3 in peripheral blood mononuclear cells of these patients, indicating an upregulation of the anti-inflammatory Treg phenotype (Mottaghi et al., 2012; Saboor-Yaraghi et al., 2015).
B. Vitamin D Receptor
1. The Receptor
The VDR is expressed by a majority of immune cells, such as T cells, B cells, monocytes, macrophages, and dendritic cells. Various immunomodulating activities of vitamin D–VDR signaling have been described, including suppression of autoimmunity and inflammation, modulation of differentiation of dendritic cells and B/T cells, and inhibition of inflammatory cytokine secretion (Sassi et al., 2018). Additionally, the VDR is involved in several physiologic functions, including cell cycle progression, apoptosis, and calcium homeostasis (Khammissa et al., 2018). The VDR encloses the ligand after binding, resulting in the linking of coactivators and the binding partner RXR (Bikle, 2014). The VDR then binds to the Vitamin D Responsive Element and recruits coregulatory complexes, including histone acetyltransferases or deacetylases, methyltransferases, and demethylases (Fig. 2B). The genes that are transcribed differ for each vitamin D–VDR complex and are cell-specific.
2. Nutritional Activators
The ligand of the VDR, vitamin D, can be obtained via different sources (Kulie et al., 2009; Khammissa et al., 2018). The precursor of the vitamin is formed in the skin after exposure to UV B radiation or obtained from vitamin D–rich foods, such as oily fish, eggs, and plants. Vitamin D from the sun or fish is pre–vitamin D3, whereas other nutritional components contain mostly pre–vitamin D2 (Bikle, 2014). Both precursors are metabolized in three main steps: 25-hydroxylation, 1α-hydroxylation, and 24-hydroxylation. This is performed by the cytochrome P450 oxidases (Bikle, 2014). The final product is 1,25(OH)2D, the most active form of vitamin D.
3. Immune Modulators
Functions of vitamin D have been described in different immune cells, including macrophages, dendritic cells, and T cells as summarized in Fig. 2A. 1,25(OH)2D3 is responsible for the stabilization and inhibits the degradation of VDR (Costa and Feldman, 1987). Primarily, 1,25(OH)2D3 exhibits the capacity to counter bacterial infections. Airway epithelial cell lines SCC25 or Calu-3 incubated with Escherichia coli and Pseudomonas aeruginosa showed an increase in the expression and protein levels of antibacterial peptides, cathelicidin and defensin β2, in response to 1,25(OH)2D3 treatment (Wang et al., 2004). The production of these antibacterial peptides was mediated via the vitamin D–responsive element in the DNA sequence of the peptides (Gombart et al., 2005). Similarly, the production of the antibacterial peptide cathelicidin increased after vitamin D induced VDR activation in monocytes and macrophages infected with Mycobacterium tuberculosis, compromising the bacterial viability (Liu et al., 2006). A study of Wang et al. (2014) pointed out that the basal expression and LPS-induced cyclooxygenase (COX)-2 in WT murine monocytes and macrophages were inhibited by vitamin D supplementation. This was mediated via VDR, since COX-2 expression was increased in monocytes and macrophages obtained from VDR knockout (KO) mice.
1,25(OH)2D3 also has the ability to activate NOD-like receptor protein (NLRP) 3 inflammasome and subsequently triggers IL-1β release, as observed in mouse primary peritoneal macrophages stimulated with LPS (Cao et al., 2020). The 1,25(OH)2D3-induced NLRP3 activation was dependent on VDR, since there was no difference in NLRP3 activation in macrophages transfected with siRNA . Additionally, attenuation of dextran sulfate sodium (DSS)-induced colitis was observed after 1,25(OH)2D3 supplementation by changing the M1/M2 ratio and inhibiting Th1 and Th17 cell populations in blood, spleen, and lymph nodes. Although it is likely that VDR is involved, this was not proven in this DSS colitis model (Cao et al., 2020).
Another study showed that supplementation of 1,25(OH)2D3 to LPS-stimulated human peripheral mononuclear cells displayed a transformation toward the M2 phenotype with upregulation of IL-10, arginase-1, and interferon regulatory factor 4 (IRF4) expression dependent on VDR, since macrophages transfected with siRNA-VDR and microRNA-125b mimic impaired 1,25(OH)2D3 activity. In addition, 1,25(OH)2D3 supplementation led to an upregulation of VDR in these human peripheral mononuclear cells (Zhu et al., 2019). In a murine DSS-induced colitis model, 1,25(OH)2D3 increased the phosphorylation and expression of IRF4 in lamina propria mononuclear cells and converted M1 macrophages to the M2 subtype, subsequently ameliorating the disease activity index, whereas microRNA-125b agomir injections reversed the action of 1,25(OH)2D3 (Zhu et al., 2019). Although no KO model was used in the in vivo part of this study, the results suggest involvement of VDR in the amelioration of DSS-induced colitis after 1,25(OH)2D3 treatment, which might be a promising target for future IBD treatment.
In murine DCs, vitamin D induces a more tolerogenic phenotype with a reduction of major histocompatibility complex (MHC) II and costimulatory ligands B7-1, B7-2, and CD40, whereas this was not observed in DCs from VDR KO mice (Griffin et al., 2000). T-cell populations have also been shown to be altered by vitamin D, specifically shifting Treg/Th17 populations toward an anti-inflammatory phenotype as reviewed before by Bikle (2014) and Medrano et al. (2018). Phospholipase Cγ1 is a crucial protein for T-cell receptor signaling and T-cell activation, whereas activation of VDR via 1,25(OH)2D3 leads to accumulation of phospholipase Cγ1 and, subsequently, T-cell growth and proliferation (von Essen et al., 2010). The absence of vitamin D has a great impact on CD8+ T cell effector differentiation, as observed by lower granzyme B expression and reduction in B-cell lymphoma 2 expression. Additionally, an inhibition in antigen-specific and memory CD8+ T cells in VDR KO models was observed (Yuzefpolskiy et al., 2014). This shows that VDR is important for CD8+ T-cell survival and immunity to fight against viral and bacterial infections. Finally, vitamin D/VDR signaling is important for the development of natural killer T cells, which are involved in fighting infections, cancer, and autoimmune diseases (Yu and Cantorna, 2008, 2011).
4. Clinical Application
The role of vitamin D in allergies is under discussion, and multiple systematic reviews observed contrasting results with vitamin D supplementation (Bozzetto et al., 2012; Jolliffe et al., 2013; Yepes-Nuñez et al., 2018). A possible explanation for these contrasting results could be the occurrence of VDR polymorphisms or the dosages of vitamin D that are used (Trombetta et al., 2018; Martens et al., 2020). It is remarkable that the dosages in in vivo studies described in this review are generally higher compared with dosages used in clinical studies (Martens et al., 2020). Low serum vitamin D concentrations in observational studies, however, are correlated with higher risk of autoimmune diseases, multiple sclerosis, and IBD (Trombetta et al., 2018). Sharifi et al. (2019) observed that patients with ulcerative colitis showed decreased TNF-α, IFN-γ, and IL12p70 serum levels 3 months after a single muscular dose of vitamin D3 compared with saline as placebo. Vitamin D has the ability to inhibit the Th1 response related to the observed decreased in Th1-related cytokines observed in their clinical study and previously performed in vitro studies (Sharifi et al., 2019). In contrary, vitamin D supplementation had clinical benefit in patients with both IBD and hypovitaminosis D, since the release of proinflammatory cytokines IL-2, IL-12, IL-13, and IL-17 were downregulated, suggesting suppression of the Th17 phenotype, whereas TNF-α levels were decreased, in contrast to the results of Sharifi et al. (2019) (El Amrousy et al., 2020). Furthermore, vitamin D supplementation lowered the incidence of upper airway infections caused by the influenza virus investigated in patients with IBD (Arihiro et al., 2019). The protective effects of vitamin D against airway infection, which is proposed to be linked to VDR activation, can be observed in other airway viruses as well. Several clinical studies have investigated the effects of vitamin D on coronavirus disease 2019 infection as additional supplement to the standard of care during hospitalization, hydroxychloroquine, and azithromycin treatment (Annweiler et al., 2020a, 2020b; Entrenas Castillo et al., 2020) . These studies all highlight better survival rate and less intensive care unit admissions with the inclusion of vitamin D in the treatment. One possible mechanism that is proposed is indeed VDR activation, leading to less severe acute respiratory distress syndrome (Entrenas Castillo et al., 2020).
C. Liver X Receptor
1. The Receptor
LXR is originally known for its role in cholesterol homeostasis but has been shown to be involved in cellular differentiation, apoptosis, lipid and carbohydrate metabolism, and immunity and inflammation (Patel et al., 2008). LXR exhibits anti-inflammatory properties, and LXR agonists used in animal models displayed amelioration of inflammatory symptoms (Fessler, 2018). The NR LXR has two known isoforms (LXRα and β), and LXRα is highly expressed in the liver, macrophages, intestine, adipose tissue, macrophages, and kidney, whereas LXRβ is ubiquitously expressed (Fessler, 2018). LXR forms a heterodimer with RXR to perform the transactivation—the increased rate of gene expression—and two different pathways have been described (Fig. 2C). The first pathway involves binding of the LXR/RXR to the LXR response element. The corepressors are replaced with coactivators, resulting in the transcription of genes, including ATP Binding Cassette (ABC) A1 (Fessler, 2018). Another ABC, the ABCG1, is induced by the demethylation of DNA after ligand binding to the LXR/RXR complex. Both ABCs are involved in cholesterol homeostasis. In addition to transactivation, LXR can induce transrepression without the heterodimerization with RXR. In macrophages, the LXR is SUMOylated after ligand and corepressor complex binding. This leads to the inhibition of proinflammatory genes via nuclear factor κ-light chain enhancer of activated B cells (NF-κB) and activator protein 1 (AP-1) (Fessler, 2018).
2. Nutritional Activators
The LXR has an important role in cholesterol metabolism, and the major activators are, intuitively, derivatives of cholesterol: oxysterols and cholesterol biosynthetic intermediates (Fessler, 2018). Different plant-derived flavonoids like cyanidin, naringenin, genistein, and others can also bind to LXR (Komati et al., 2017).
3. Immune Modulators
The effects of LXR are reported in a broad range of physiologic systems, including the inflammatory response, cell apoptosis, and phagocytosis (Fig. 2C). Synthetic ligands, such as GW9365A and T0901317, have been widely used in LXR research; however, we focus on studies that used natural ligands, including hydroxycholesterols and cyanidin-3-O-β-glucoside (Wang and Tontonoz, 2018). As previously mentioned, LXR can transrepress inflammatory genes, such as NF-κB, and suppress genes, such as inducible nitric oxide synthase (NOS), IL-6, COX-1, MMP-9, MCP-1, MCP-3, and IL-1β (Bensinger and Tontonoz, 2008). Moreover, the downstream targets of toll-like receptor (TLR) 4, including IL-1β, and TNF-α, were antagonized by activating LXR (Bensinger and Tontonoz, 2008). In a murine model, allergic contact dermatitis was treated with 22(R)-hydroxycholesterol, an oxysterol. This treatment led to a reduction of allergic symptoms as observed by a decrease in ear thickness accompanied with reduced TNF-α and IL-1α secretion in the dermis and the epidermis (Fowler et al., 2003). The ear thickness and secretion of proinflammatory cytokines TNF-α and IL-1α by keratinocytes in the dermis and the epidermis in this murine model were also reduced after oxysterol administration. These immunomodulatory effects were not observed in LXRβ-deficient mice and were reduced with 50% in LXRα-deficient mice, indicating a role for the LXR receptor in this process (Fowler et al., 2003). After treatment with the flavonoid cyanidin-3-O-β-glucoside, the lungs of mice challenged with LPS showed a significant decrease in their histologic inflammatory profile with reduced cell infiltration, alveoli wall thickening, interstitial edema, and pulmonary congestion (Fu et al., 2014). This was accompanied with fewer proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, released by ex vivo LPS-restimulated alveolar macrophages treated with cyanidin-3-O-β-glucoside. This anti-inflammatory response was reversed by injection of LXRα siRNA, indicating again the involvement of LXR. In addition, cyanidin-3-O-β-glucoside led to increased expression of LXR in these murine alveolar macrophages (Fu et al., 2014). In murine macrophages, an anti-inflammatory response was observed after treatment with different oxysterols, as their actions appeared to repress inducible NOS (Joseph et al., 2003; Ghisletti et al., 2007). This was not observed in macrophages of LXR−/− mice, possibly because of the SUMOylation-dependent transrepression pathway, as described previously (Joseph et al., 2003; Ghisletti et al., 2007).
In murine macrophages, cholesterol and oxysterols are both implicated in phagocytosis of apoptotic debris induced by dexamethasone (A-Gonzalez et al., 2009). Furthermore, depletion of sterols in apoptotic thymocytes resulted in lowered expression of LXR target genes and Mer, a receptor tyrosine kinase involved in the apoptotic clearance (A-Gonzalez et al., 2009). Mice lacking the LXR gene exhibit a defect in phagocytosis of apoptotic cells causing a proinflammatory response leading to a breakdown in self-tolerance and development of autoantibodies (A-Gonzalez et al., 2009). Furthermore, oxysterols can inhibit the migration of DCs via inhibition of the CCR-7 receptor in murine BMDC (Villablanca et al., 2010). This effect is abrogated in the presence of short hairpin LXR, indicating a role for LXR in DC migration.
4. Clinical Application
Clinical studies showing beneficial effects in the clinic of LXR agonists are limited. Olivas-Aguirre et al. (2016) reviewed the effects of anthocyanins in humans, of which cyanidin-3-O-β-glucoside is the most prevalent of this flavonoid family. In this review, anti-inflammatory effects induced by anthocyanins are described, including decrease in serum inflammatory biomarkers IL-6, MCP-1, C-reactive protein, and others, as well as benefits for cardiovascular protection and anticancer properties of anthocyanins (Cassidy et al., 2015; Olivas-Aguirre et al., 2016). Another review described the effects of the flavonoid naringin in different clinical trials (Salehi et al., 2019); also here, anti-inflammatory properties are reported, such as decreased C-reactive protein and fibrinogen levels in blood samples and an inhibitory effect on oxidative stress in patients who are overweight or patients with chronic hepatitis C (Dallas et al., 2014; Gonçalves et al., 2017).
D. Peroxisome Proliferator-Activated Receptor
1. The Receptor
The final binding partner of the RXR that will be discussed is the NR PPAR. PPARs are known to be involved in the regulation of cellular differentiation, glucose and lipid metabolism, and carcinogenesis. PPAR signaling shows the ability to affect different features of inflammation and immunity, thereby contributing to the essential crosstalk between metabolism and immune system (Kidani and Bensinger, 2012).
PPARα, PPARγ, and PPARβ/δ are the three members of the PPAR subfamily (Kiss et al., 2013). After ligand binding, the receptor forms a heterodimer with RXR that binds to the PPAR responsive element in the DNA (Fig. 2D) (Rakhshandehroo et al., 2010). Activation is dependent on the removal of corepressors and the recruitment of coactivators. The activated genes downstream are primarily involved in lipid metabolism and homeostasis. On the other hand, if a corepressor complex is recruited, transrepression of proinflammatory genes will occur, namely NF-κB and AP-1 (Glass and Saijo, 2010).
2. Nutritional Activators
A range of nutritional activators binds to PPAR, namely polyunsaturated fatty acids (PUFAs), polyphenols, and natural carotenoids (Contreras et al., 2013). PUFAs have the highest binding affinity and can be divided into Ω-3 and Ω-6 based on the location of the double bond. PUFAs are lipid macronutrients present in the diet, the most studied of which are marine Ω-3 PUFAs, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Wu et al., 2019). The polyphenols known to activate PPAR are resveratrol, which is in berries, grapes, and peanuts, and genistein, a soy isoflavone (Contreras et al., 2013).
3. Immune Modulators
Natural ligands of PPAR have been reported to exert immune regulatory functions; however, the described functions can be both PPAR-dependent and PPAR-independent. The PPAR-independent functions are beyond the scope of this review. The most studied impact of natural ligands and PPAR is in the context of inflammation, and generally, EPA and DHA stimulated anti-inflammatory cytokines in a PPARγ-dependent manner (Fig. 2D) (Li et al., 2005; Jaudszus et al., 2013). Li et al. (2005) showed in an in vitro study using HK-2 human kidney cells that both EPA and DHA decreased LPS-induced NF-κB activation and MCP-1 expression accompanied by a PPARγ upregulation. With the PPARγ-specific antagonist bisphenol A diglycidyl ether, these EPA- and DHA-induced effects were not observed (Li et al., 2005). Another study pointed out that both EPA and DHA induced a concentration-dependent reduction of intracellular IL-2, IL-4, and TNF-α expression in Th cells (Jaudszus et al., 2013). EPA exhibited the strongest concentration-dependent effect, and again, the inhibitory effect of EPA and DHA on cytokine production in Th cells was reversed upon the addition of a PPARγ-specific antagonist T0070907.
In the innate immune system, PUFAs influence the M1/M2 balance toward anti-inflammatory M2 macrophages. Luo et al. (2017) showed that DHA is able to shift the macrophage phenotype in murine Kupffer cells and RAW264.7 macrophages toward the more anti-inflammatory M2 phenotype, whereas treatment with a specific PPARγ antagonist GW9662 shifts the balance toward the proinflammatory M1 profile in RAW264.7 cells. This PPARγ-specific effect of DHA was seen to be dependent of the NF-κBp65 signaling pathway (Luo et al., 2017). Although PPARγ was undetectable in the control group, a significant increase in PPARγ protein expression was observed in RAW264.7 cells after DHA treatment (Luo et al., 2017). An important role of M2 macrophages is efferocytosis, the process of clearing apoptotic neutrophils during the final stages of an inflammatory response. In murine macrophage-like RAW264.7 cells, DHA enhanced the efferocytosis, which was proven to be PPARγ-dependent with the use of PPARγ siRNA (Chang et al., 2015). DHA not only increased the mRNA expression in RAW264.7 cells but also caused PPARγ nuclear translocation (Chang et al., 2015). Aside from the PUFAs, two flavonoids, apigenin and chrysin, also induce a PPARγ-dependent shift toward an M2 phenotype in murine peritoneal macrophages from obese mice or macrophage cell lines via NF-κB inhibition (Feng et al., 2014, 2016). Oxidized EPA causes inhibition of rolling and adhesion of neutrophils in a model using leukocytes incubated with an HUVEC monolayer; however, PPAR-related effects were not indicated in vitro (Sethi et al., 2002). The neutrophil-related effects were also observed in the mesentery arteries of mice treated with LPS and oxidized EPA; however, this was not observed in PPARα-deficient mice (Sethi et al., 2002).
The anti-inflammatory effects of DHA are also to be found in DCs. DHA supplementation prevents murine BMDC maturation associated with concentration-dependent PPAR binding to the PPAR-responsive element (Kong et al., 2010). Zapata-Gonzalez and colleagues (2008) linked the immature human DC phenotype to downregulation of costimulation and antigen presentation accompanied by increased chemotactic abilities of DCs in the presence of DHA, whereas mature DCs treated with DHA showed decreased IL‐6 expression and IL‐10 and IL‐12 secretion. When these DCs were cultured in the presence of a specific PPARγ inhibitor (GW9662), all DHA-induced changes were blocked (Zapata-Gonzalez et al., 2008). In addition, an inhibition of IL-12 mediated via inhibition of the NF-κBp65 nuclear translocation was observed in DHA-treated murine BMDC (Kong et al., 2010).
The anti-inflammatory properties of EPA and DHA have also been described in T cells, but only a few articles include the role of PPAR in the mechanism of action. Two allograft transplant studies investigated the effect of EPA alone or EPA and DHA on heart transplantations (Iwami et al., 2009; Ye et al., 2012). In both studies the heart of a specific mouse strain was transplanted into another recipient mice strain treated with or without EPA, which resulted in less rejection and a more anti-inflammatory profile. This is represented by an inhibition of cytokine production in splenocytes, including IL-2, IL-6, IL-12, IL-17, IFN-γ, an increase in TGF-β, and changes in the ratio of different T cells in draining lymph nodes and heart. The ratio of Treg/Th17 was increased in mice supplemented with EPA because of the increase in the FoxP3+ Treg cells. In both in vitro and in vivo studies, these EPA-induced changes in cytokine production and T cell ratios were blocked with treatment of a specific PPARγ inhibitor, bisphenol A diglycidyl ether or GW9662 (Iwami et al., 2009; Ye et al., 2012). Interestingly, EPA also upregulated mRNA expression of PPARγ in spleen and cardiac allografts (Iwami et al., 2009; Ye et al., 2012).
4. Clinical Application
Ω-3 PUFAs have been investigated in clinical trials for the exploration of their immunomodulatory effects in cancer and chronic inflammatory diseases. Reviews have described the Ω-3 PUFA–induced reduction of pain and decrease in inflammatory markers in cancer, such as COX-2 expression and TNF-α, IL-1β, IL-6, and IFN- γ in serum, although there is insufficient information to fully confirm the supportive effects of Ω-3 PUFA supplementation in cancer (Aucoin et al., 2017; Freitas and Campos, 2019). Contradictory results have been reported in systemic reviews regarding the effects of Ω-3 PUFAs in patients with IBD, including production of anti-inflammatory cytokines leading to IBD remission, as well as no protective effect (Marton et al., 2019; Mozaffari et al., 2020). These differences could be due to the mode of consumption, type of food, or different formulations (Marton et al., 2019). The same trend has been observed in allergic diseases, including asthma and eczema. There is a clear trend toward positive effects of Ω-3 PUFA supplementation, which is possibly due to lower IgE levels or by inducing Th2 polarization, but this is not supported by all studies (Miles and Calder, 2017). A greater consensus can be found for the use of Ω-3 PUFA supplementation in patients with rheumatoid arthritis and cardiovascular disease. Reduction of inflammation characterized by a decrease in proinflammatory cytokines TNF-α, IL-6, and leukotriene B4, leading to improved clinical outcomes in these ailments, are described extensively in the following studies after Ω-3 PUFA treatment (Yates et al., 2014; Gioxari et al., 2018; Innes and Calder, 2020; Kostoglou-Athanassiou et al., 2020). To confirm the clinical relevance of Ω-3 PUFAs, more clinical studies are needed to further assess the potentials of these dietary components.
IV. G-Protein–Coupled Receptor
G-coupled protein receptors (GPRs) represent the largest class of drug targets in the pharmaceutical world. They can be activated by multiple ligands, such as chemokines, peptides, lipids, and neurotransmitters. GPR signaling is involved in nearly all physiologic processes, and GPR s have been found to play an important role in the immune system (Hauser et al., 2017). GPR, a seven-transmembrane protein, has an associated G-protein in the inactive form (Moreira, 2014). Upon a ligand binding to the GPR, the Gα and Gβ/γ subunits can dissociate, leading to various physiologic events, depending on the protein subfamily. All discussed GPRs are activated by fatty acids of varying lengths: SCFAs contain 2–6 carbon atoms, medium-chain fatty acids contain 7–12 carbon atoms, and long-chain fatty acids contain 13–22 carbon atoms (Ulven and Christiansen, 2015). The immunomodulatory effects of these nutritional components related to GPR signaling have been described in detail below and summarized in Table 3.
Literature overview nutritional components and their effect on the immune system via G-protein–coupled receptors
A. G-Coupled Protein Receptor 41/43
1. The Receptor
Both GPR41 and GPR43 can be activated in a Gαi/o β-gustducin–dependent manner (Sivaprakasam et al., 2016). Binding of the ligand causes a conformational change and inhibition of the second messenger cAMP (Sivaprakasam et al., 2016). GPR43 can also be activated in a Gαq-dependent manner, leading to a decrease in cAMP (Fig. 3B). Other G-coupled proteins, such as β-arrestin, can associate with GPR41 and activate other signal transduction pathways (Fig. 3A). GPR41 is involved in metabolic homeostasis, namely leptin production and sympathetic activation, whereas GPR43 has functions in adipogenesis, energy homeostasis, and the immune response (Sivaprakasam et al., 2016).
Effects of nutritional components via GPR on the immune system. Binding of a nutritional component to one of the GPRs results in dissociation of the Gβ and Gγ from the Gα subunit. Depending on the Gα subtype, signal transduction cascade is activated, leading to immunologic changes. GPR41 (A), GPR 43 (B), GPR109A (C), and GPR120 (D) are shown in this figure. ROS, reactive oxygen species. Created with BioRender.com.
2. Nutritional Activators
Both receptors are activated by SCFAs, fermentation products of dietary fiber by the microbiota in the gut (Sivaprakasam et al., 2016). SCFAs, are also present in grains, fruits, and vegetables and include pentanoate, butyrate, propionate, and acetate (Tan et al., 2017). The affinity for SCFAs differs between the receptors. GPR41 has high affinity for SCFAs, butyrate and propionate, whereas GPR43 had high-affinity acetate and propionate (Sivaprakasam et al., 2016).
3. Immune Modulators
Immune activation via GPR41/43 is mainly described in the gastrointestinal tract, as their dietary ligands, SCFAs, are produced by microbiota in the gut (Fig. 4, A and B).
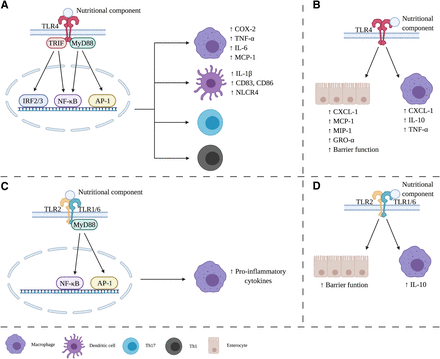
Effects of nutritional components via TLR on the immune system. Dependent on the type of nutrition, TLR can have a proinflammatory or anti-inflammatory effect. Anti-inflammatory effects of TLR4 (A) or TLR2 (C) are mediated through TRIF or MyD88-dependent signal transduction cascade. Other nutritional components binding to TLR4 (B) or TLR2 (D) lead to anti-inflammatory responses. GRO, human growth-related oncogene; MIP, macrophage inflammatory protein; TRIF, TIR-domain-containing adapter-inducing interferon-β. Created with BioRender.com.
Macia et al. (2015) showed that a high-fiber diet increases the butyrate and acetate concentration in the gut, activating GPR43 on intestinal epithelial cells. The cell hyperpolarization via GPR43 second messengers activates the NLRP3 inflammasome and converts pro–IL-18 into IL-18, leading to protection from DSS-induced colitis. This protection was observed as an improvement of clinical scores, histologic scores, and colon length and was not present in GPR43−/− mice (Macia et al., 2015). SCFAs are needed for the production of antibacterial peptides by cells (e.g., RegIIIγ and β-defensins 1, 3, and 4, which were absent in the intestinal epithelial cells of GPR43−/− mice) (Zhao et al., 2018). Furthermore, SFCA acetate promoted the IgA production via induction of Aldh 1a1 in murine splenic dendritic cells, which was also seen to be absent in GPR43−/− mice (Wu et al., 2017). Moreover, Kim et al. (2013) showed that SCFAs induced a rapid production of different chemokines and cytokines, such as IL-6, CXCL-1, and CXCL-10, by murine intestinal epithelial cells, which was abolished after pretreatment with pertussis toxin, a GPR activation blocker, whereas GPR41−/− and GPR43−/− mice exhibited reduced inflammatory responses after ethanol, 2,4,6-trinitrobenzene sulfonic-acid (TNBS), or Citrobacter rodentium administration. In WT mice, SCFAs restore the gut homeostasis in a GPR41- and GPR43-dependent manner by activation of the NLRP3 inflammasome (Macia et al., 2015). Although a majority of research has focused on the effects of SCFAs in the intestine, Trompette et al., (2014) displayed that a high-fiber diet is also able to positively affect the airways. Mice on a low-fiber diet exhibited more proinflammatory cytokines in the lungs, leading to an increased allergic airway responsiveness and subsequent DC activation in lung-draining lymph nodes. In addition, propionate promoted anti-inflammatory effects in the lungs, which was mediated by GPR41 rather than GPR43 (Trompette et al., 2014). An in vitro study indicated that acetate, butyrate, and propionate inhibited phosphorylated NF-κB and MCP-1 expression via the GPR43–β-arrestin-2 pathway in mouse glomerular mesangial cells and SV-40 MES 13 cell line (Huang et al., 2020). The anti-inflammatory effects of SCFAs were more pronounced by the GPR43 overexpression, whereas they were inhibited by siRNA-GPR43. Furthermore, GPR43 mRNA and protein levels were increased in kidney tissue after SCFA incubation (Huang et al., 2020).
It has also been observed that neutrophil recruitment is increased after treatment with acetate, propionate, or butyrate, and this occurs via GPR43 activation, since this is not detected in GPR43-deficient mice (Sina et al., 2009; Vinolo et al., 2011). This chemotactic capacity is promoted via phosphoinositide 3 kinase γ, Rac2, and mitogen-activated protein kinase (Vinolo et al., 2011) and inhibition of the l-selectin concentration (important for adhesion to epithelial cells and migration) via p38 mitogen-activated protein kinase in murine neutrophilic granulocytes (polymorphonuclear leukocytes) (Sina et al., 2009). Acetate exhibited the capacity to stimulate the calcium flux in mouse neutrophils, which was not observed in neutrophils from Gpr43−/− mice. In addition, acetate reduced the expression of other proinflammatory receptors on neutrophils, including C5aR and CXC chemokine receptor 2, but it is not known whether this is GPR43-dependent (Maslowski et al., 2009).
A more recent study investigated SCFA signaling via GPR43 in macrophages in vitro and in vivo and pointed out that SCFA treatment promoted the M2 phenotype, whereas it did not alter the murine M1 phenotype in adipose tissue. This SCFA-induced M2 phenotype expressed more TNF-α compared with untreated M2 macrophages, resulting in the maintenance of adipose tissue homeostasis (Nakajima et al., 2017b).
SCFA activation of GPR41 and GPR43 has been shown to increase the proliferation of the FoxP3+ Treg population in lamina propria, spleen, and thymic lobes without influencing the number of Th17 or Th1 cells in mice (Smith et al., 2013; Nakajima et al., 2017a). Nakajima et al. (2017a) also promoted the importance of butyrate via GPR41 during pregnancy in mice, as it enhanced the Aire expression in fetal thymic Treg cells, which is crucial for the negative selection of T cells and development of immunotolerance in the fetus. In murine colonic T cells, propionate influences the activity of histone deacetylases in a GPR43-dependent manner, leading to an increase in the Treg population (Smith et al., 2013). Finally, GPR41 is critical for the T-cell independent butyrate-induced IgA response and improved gut barrier function in the mouse colon, which is mediated via the upregulation of Aldh1a2 and integrin αvβ8 in DCs (Isobe et al., 2020).
4. Clinical Application
Roduit et al. (2019) suggested that strategies to increase the SCFA concentrations by dietary intervention could be a possible strategy for preventing allergic diseases in children, since higher levels of propionate and butyrate in feces of 1-year-old children are related to decreased incidence of atopic sensitization and asthma (Roduit et al., 2019). The metabolic potential of the gut microbiota responsible for the availability of the SCFAs, plays an important role in this allergy sensitization (Cait et al., 2019). The SCFA composition in feces is changed in patients with IBD, and the link between IBD and intestinal microbiota has been intensely investigated in the recent decades (Hedin et al., 2007; Lavelle and Sokol, 2020). There are changes in the microbiota composition comparing healthy individuals with patients with IBD, leading to changes in SCFA availability (Silva et al., 2018). In patients with ulcerative colitis or Crohn disease, an 8-week treatment with butyrate decreased the NF-κB signaling in the lamina propria or ileum, respectively (Lührs et al., 2002; Di Sabatino et al., 2005). In addition to the downregulation of NF-κB, a butyrate-induced decrease in ileal IL-1β associated with an anti-inflammatory state, was observed in patients suffering from Crohn disease (Di Sabatino et al., 2005). In patients with ulcerative colitis, butyrate treatment also induced a reduction in neutrophil infiltration in gut crypts and surface epithelia (Lührs et al., 2002). In turn, these anti-inflammatory effects correlated with decreased disease activity. Another study showed a decrease in the colonic IL-10/IL-12 ratio, a marker for a more anti-inflammatory phenotype, in patients with ulcerative colitis after butyrate treatment (Hamer et al., 2010). Additionally, a negative association between inflammation, as measured by calprotectin and the antioxidant glutathione levels, and butyrate intervention has been reported in these patients (Hamer et al., 2010). It however has to be noted that not all research found beneficial effects of SCFAs in the treatment of IBD—ulcerative colitis or Crohn—as has been reviewed before (Hedin et al., 2007; Fernández et al., 2016; Sun et al., 2017; Gill et al., 2018). Autoimmune diseases multiple sclerosis and rheumatoid arthritis are characterized by a disbalance in the Treg and Th17 population in tissue, lower proportions of Treg cells, and higher levels of Th17 cells compared with healthy individuals. An elaborate study by Duscha et al. (2020) showed a significant decrease in the number of Th17 cells and increase in Treg cells after treatment with propionate in patients suffering from multiple sclerosis and rheumatoid arthritis. This gives opportunities for future treatment of autoimmune diseases with propionate supplementation.
B. G-Coupled Protein Receptor 109A
1. The Receptor
GPR109 consists of two isoforms in humans: GPR109A and GPR109B, wherein GPR109B is a duplicate of GPR109A (Sivaprakasam et al., 2016). GPR109A is activated in a Gαi/o β-arrestin 1 similar to GPR41/43 (Fig. 3C). GPR109A is involved in intestinal homeostasis, inhibition of lipolysis, high-density lipoprotein biosynthesis, toll-like receptor TLR responsiveness, and the NF-κB pathway (Chai et al., 2013; Sivaprakasam et al., 2016).
2. Nutritional Activators
The receptor is activated by the SCFAs, butyrate, hydroxybutyrate, and niacin—a form of vitamin B3 (Sivaprakasam et al., 2016).
3. Immune Modulators
GPR109A is present on innate immune cells but not on natural killer cells, T cells, and B cells (Singh et al., 2014). Hence, the immunomodulatory effects of GPR109A are restricted to the innate immune system and only indirectly influence the adaptive immune system (Fig. 3C). The expression of epithelial GPR109A in intestinal mucosal tissue in both human and mice is most likely related to the proximity of SCFAs produced by the microbiota (Parada Venegas et al., 2019).
Butyrate induces activation of the NLRP3 inflammasome in murine epithelial cells of the colon via GPR109A in vitro as well as in vivo (Macia et al., 2015). A high amount of dietary fiber resulted in the improvement of clinical and histologic scores in mice with DSS-induced colitis via this NLRP3 inflammasome mechanism dependent on GPR109A and GPR43 signaling (Macia et al., 2015). The importance of GPR109A in gut immunity is furthermore highlighted by research conducted by Singh et al. (2014), who showed that butyrate and niacin treatment promotes the induction of anti-inflammatory IL-10 and Aldh1a1 expression and splenic DCs and macrophages accompanied by a reduction of IL-17, leading to an increase in Treg cells. This was not observed in Niacr1−/− DCs and macrophages. Additionally, mice lacking the GPR109A showed increased pathology after DSS-induced colitis and azoxymethane-induced colon cancer (Singh et al., 2014).
Niacin also demonstrates anti-inflammatory activity in human macrophages by reducing the level of proinflammatory cytokines after TLR2 or 4 stimulation (Digby et al., 2012). Stimulation of TLR2 and 4 with heat-killed Listeria monocytogenes or LPS, respectively, led to an increase in proinflammatory cytokines TNF-α, IL-6, and MCP-1. Niacin reduced this effect, which was mediated via GPR109A and by inhibiting DNA binding of GPR109A to the NF-κB gene. This anti-inflammatory effect of niacin was not observed in knockdown of GPR109A using siRNA (Digby et al., 2012). Activation of GPR109A via β-hydroxybutyrate also leads to fewer brain infarcts due to activation of anti-inflammatory microglia cells, which are absent in GPR109A−/− mice (Rahman et al., 2014).
Less is known about GPR109A function in neutrophils, but niacin acid binding to GPR109A induced a reduction of cAMP, resulting in a reduced proapoptotic factor Bad phosphorylation, and subsequently increased apoptosis via caspase activation in mature blood neutrophils from healthy donors (Kostylina et al., 2008). Blocking the GPR109A signal transduction cascade using pertussis toxin abolished these effects. It appears that only mature neutrophils express GPR109A.
To date, the effect of GPR109A activation in DCs is much less clear. Some studies concluded that GPR109A is not involved in the anti-inflammatory activity of DCs, but HDAC inhibition or retinal dehydrogenase 1 induction is supposed to be responsible (Arpaia et al., 2013; Goverse et al., 2017). In contrast, other studies found that GPR109A (encoded by Niacr1) activation is responsible for the anti-inflammatory action of murine DCs (Singh et al., 2014; Tan et al., 2016; Isobe et al., 2020). Both butyrate and niacin increased the expression of the anti-inflammatory molecules Aldh1 and Il-10 in WT splenic DCs, whereas butyrate and niacin failed to modulate these expression levels in Niacr1−/− splenic DCs. This in turn led to increased capacity of DCs to induce the differentiation of naïve T cells into Treg cells, which are also dependent on GPR109A (Singh et al., 2014). Another study showed that butyrate enhanced the Aldh1a2 expression in DCs in vitro and in vivo and promoted IgA responses in the colon, contributing to the maintenance of the colonic barrier function and the gut immune homeostasis (Isobe et al., 2020). Finally, a high-fiber diet showed increased tolerance and protection against food allergies when GPR43, GPR109A, and RAR signaling were involved (Tan et al., 2016).
4. Clinical Application
The effects of SCFA in human studies have been described before in IV.D.4. Clinical Application.
C. G-Coupled Protein Receptor 120
1. The Receptor
The GPR120, also known as the free fatty acid receptor, has a long and a short isoform in humans (Ulven and Christiansen, 2015). The difference between the two isoforms is the presence or absence of a 16-amino-acid-long segment in the third loop. Only the short isoform can associate with the Gαq/11 protein, but both receptors can associate with the β-arrestin-2 protein (Fig. 3D) (Milligan et al., 2017). This receptor is involved in several physiologic functions, including hormone production in the liver and intestines, perception of fat in the taste buds, and several anti-inflammatory processes (Ulven and Christiansen, 2015).
2. Nutritional Activators
The receptor can be activated by medium-chain fatty acids as well as long-chain fatty acids (Ulven and Christiansen, 2015). The most potent Ω-3 PUFAs activating GPR120 include EPA and DHA in fish and seafood and α-linolenic acid in seeds, nuts, and vegetable oils (Calder, 2013; Ulven and Christiansen, 2015). Lastly, the Ω-6 fatty acid arachidonic acid can activate GPR120 (Ulven and Christiansen, 2015).
3. Immune Modulators
The immunomodulatory effects of GPR120 are mostly related to macrophages, and these effects are summarized in Fig. 3D.
The GPR120-dependent anti-inflammatory effects of Ω-3 PUFAs have also been observed in rat hypothalamic cells and murine in vivo models (Cintra et al., 2012; Wellhauser and Belsham, 2014; Li et al., 2015). Mice with naphthalene-induced airway injury show completely destroyed bronchial epithelium, whereas dietary Ω-3 fatty acids increased clinical epithelial recovery dependent on GPR120 (Lee et al., 2017). In addition, DHA stimulated the proliferation and migration of mouse lung epithelial club cells in an in vitro model, which was also dependent on GPR120 (Lee et al., 2017). The expression of GPR120 is dependent on Ω-3 PUFAs as well. Sprague-Dawley rats fed a diet supplemented with purified fish oil or flaxseed oil, which are both rich in Ω-3 PUFAs, had significantly higher expression of GPR120 in the colon (Cheshmehkani et al., 2015).
Oh et al. (2010) conducted one of the first studies that linked GPR120 signaling to macrophage immunity after supplementation with DHA. In this in vitro study, LPS-induced proinflammatory cytokines, such as TNF-α, IL-6, and MCP-1, are downregulated in response to DHA binding to GPR120 on mouse macrophage RAW264.7 cells, causing ligand binding of β-arrestin 2 with GPR120 and subsequent TAP1 binding to β-arrestin 2. This prevents TAK1 from associating with TAP1 and thus blocks the signaling cascade induced by LPS (Oh et al., 2010). Furthermore, an in vivo study using mice fed control or high-fat diet enriched with Ω-3 PUFAs demonstrated increased macrophage chemotaxis in the adipose stromal vascular fraction, decreased proinflammatory M1 markers, and increased anti-inflammatory M2 markers after Ω-3 PUFA–enriched diet, which was not present in GPR120 KO mice (Oh et al., 2010). Further evidence of anti-inflammatory effects of Ω-3 PUFA treatment on macrophages included inhibition of the inflammasome NLRP3 or proinflammatory NF-κB signaling (Yan et al., 2013; Williams-Bey et al., 2014). siRNA or GPR120 KO models abolished these effects on inflammasome and NF-κB activation. This again emphasizes the anti-inflammatory effects of Ω-3 PUFAs via GPR120 activation (Yan et al., 2013; Williams-Bey et al., 2014). In addition, DHA activation of GPR120 has been shown to inhibit the inflammasome pathway in macrophages in vitro and in vivo, contributing to an anti-inflammatory phenotype (Yan et al., 2013; Williams-Bey et al., 2014; Mo et al., 2020). DHA activation of GPR120 also inhibited the translocation of the nuclear factor p65, inhibiting NF-κB expression in RAW264.7 cells, leading to an anti-inflammatory response after LPS incubation (Liu et al., 2014).
4. Clinical Application
The effects of Ω-3 PUFAs and SCFAs in human studies have been described before in IV.D.4. Clinical Application.
D. Other G-Coupled Protein Receptors
A few GPRs are not discussed in detail because of the limited number of studies investigating the effects of nutritional components at time of writing. Nevertheless, a few results are important to mention to form a complete picture. GPR40 is a GPR that has affinity for certain nutritional components, and the downstream effects have been shown to influence the immunologic activity. It appears that Ω-3 PUFAs suppress the activation of inflammasome NLRP3 in BV-2 microglial cells, murine bone marrow–derived macrophages, and mice and thus reduce inflammation (Yan et al., 2013; Mo et al., 2020), whereas Ω-6 PUFAs, Ω-9 PUFAs, and unsaturated fatty acids increase inflammation as observed by the upregulation of several mediators, including COX-2, CXCL-8, and IL-6 expression on mRNA and protein level, from certain cell types (Mena et al., 2016; Li et al., 2018). Furthermore, 10-hydroxy-cis-12-octadecenoic acid improves the intestinal epithelial barrier function in vitro via upregulation of zonula occludens (ZO) 1, ZO-2, and claudin-3 mediated through the MEK -ERK pathway (Miyamoto et al., 2015). Lactate binding to GPR81 exerted anti-inflammatory effects in both in vitro and in vivo studies (Hoque et al., 2014; Madaan et al., 2017). Bitter taste receptor GPR T2R activation by quinine led to an increase in nitric oxide production and subsequently accelerates ciliary beating in an air-liquid interface model with human sinonasal tissue from healthy individuals or patients with chronic rhinosinusitis (Workman et al., 2018). In addition, flavones apigenin, chrysin, and wogonin, as T2R agonists, demonstrated anti-inflammatory properties in airway cells in response to inflammatory stimuli (Hariri et al., 2017). For more details regarding these studies, see Table 3.
V. Toll-Like Receptor
A. The Receptor
TLRs are the most well characterized class of pattern-recognition receptors. They play a crucial role in “danger” recognition and are critical for eliciting a protective immune response against pathogens via the regulation of host immune effector functions, including cytokine production, inflammasome activation, autophagy, antigen presentation, and B and T cell activation (Kawasaki and Kawai, 2014; Mukherjee et al., 2019). TLRs can be categorized into transmembrane (TLR1, TLR2, TLR4, TLR5, TLR6, and TLR11) or intracellular receptors (TLR3, TLR7, TLR8, and TLR9) (Mukherjee et al., 2019). All TLRs consist of extracellular leucine-rich repeats and an intracellular Toll-IL receptor domain and can recognize pathogen-associated molecular patterns, microbe-associated molecular patterns, and damage-associated molecular pattern molecules and subsequently induce downstream signaling (Kawasaki and Kawai, 2014; Mukherjee et al., 2019). The effects of TLR activation within the cell can be MyD88-dependent or independent (Kawasaki and Kawai, 2014; Rocha et al., 2016; Mukherjee et al., 2019). The MyD88-dependent response involves a complex signal transduction cascade leading to the activation of NF-κB or AP-1 (see Fig. 4, A and C). Toll/IL-1 receptor‐domain‐containing adapter‐inducible IFN‐β recruits the proteins involved in the MyD88-independent pathway, which leads to a type I IFN response via TLR4 activation (Fig. 4A). Both pathways lead to a proinflammatory response and eventual recruitment of the innate immune system.
B. Nutritional Activators
Two different classes of nutritional components are known to activate TLRs. The first class is SFAs, in particular lauric acid (Rocha et al., 2016), and the second type includes the nondigestible oligosaccharides (NDOs) (Ayechu-Muruzabal et al., 2018). In Table 4, an overview of these nutritional components and their effect on the immune system via TLR is presented.
Literature overview nutritional components and their effect on the immune system via TLRs
C. Immune Modulators
TLRs are generally expressed on immune cells, such as monocytes, macrophages, dendritic cells, B lymphocytes, T lymphocytes, mast cells, neutrophils, and eosinophils, but also on nonimmune cells like epithelial cells, endothelium, fibroblasts, and adipocytes. Ligand binding triggers the signaling pathway to initiate immune-effector responses, both adaptive and innate immune responses (Dolasia et al., 2018). SFAs and NDOs have opposite effects on the immune system via TLR activation. Although the immunomodulatory effects of SFAs mainly exhibit a proinflammatory characteristic (Fig. 4, A and C), NDOs possess a primarily anti-inflammatory capacity (Fig. 4, B and D).
In vitro studies have found that the SFAs palmitate, oleate, and lauric acid all induce proinflammatory activities in monocytes and macrophages via TLR2 and TLR4 (Lee et al., 2003; Shi et al., 2006; Nguyen et al., 2007; Suganami et al., 2007; Huang et al., 2012). The fatty acid –induced activation of the TLR signal transduction cascade leads to NF-κB–activated induction of proinflammatory genes and cytokines, including COX-2, TNF-α, IL-6, and MCP-1 in macrophages and BMDC (Lee et al., 2003; Shi et al., 2006; Nguyen et al., 2007; Suganami et al., 2007; Huang et al., 2012). In addition, the mRNA TLR4 expression was higher in adipose tissue of obese mice fed with a high-fat diet compared with mice fed a control diet (Shi et al., 2006). KO models and the use of short hairpin RNA confirmed that TLR4 is necessary for the fatty acid–induced NF-κB activation (Shi et al., 2006; Nguyen et al., 2007; Suganami et al., 2007). An interesting murine study showed that offspring from mothers on a Western diet containing extra fat, cholesterol, and sucrose had skin inflammation and loss of body hair (Du et al., 2012). Crossfostering a normal diet mother with offspring from a Western-like diet mother or weaning recovers these observed effects. This indicates that the negative effects observed in pups from mothers on a Western-like diet may be reversible. A TLR2/TLR4 double-KO model abolished the inflammation and hair loss in the offspring and highlighted that parental nutrition during lactation is also important for offspring health and dependent on TLR2/TLR4 (Du et al., 2012).
Nicholas et al. (2017) investigated the effects of palmitic acid on human DCs and found that the palmitic acid–stimulated DCs are matured and activated as seen by the upregulation of costimulatory factors CD86 and CD83. Furthermore, palmitic acid increased the production of the proinflammatory cytokine, IL-1β, which was inhibited by blocking TLR4 with CLI-095. Therefore, it can be concluded that palmitate can induce a proinflammatory response mediated by TLR4. In addition, palmitic acid caused an increase in NLRC4 inflammasome in DCs, which could be responsible for cleavage of pro–IL-1β, and palmitic acid exhibited the capacity to decrease proteins related to antigen presentation.
Besides their microbiota-dependent effect, various oligosaccharides also exhibit anti-inflammatory properties. In intestinal epithelial cells, fructo-oligosaccharides (FOSs), galacto-oligosaccharides (GOSs), inulins, and goat milk oligosaccharides activate TLR4, increasing the expression of human growth‐related oncogene‐α, MCP-1, and macrophage inflammatory protein 2, and enhance barrier function. These responses were decreased in a MyD88 and TLR4 gene knockdown model (Ortega-González et al., 2014). The barrier function of human intestinal epithelial cells is also improved by fructans. Although the exact mechanism of action is not yet clear, TLR2 might be involved, since TLR2 blocking antibodies reversed these protective effects (Vogt et al., 2014). In a murine model of colitis, the milk oligosaccharide sialyl(α2,3)lactose (present in breast milk) modulated mucosal immunity and aggravated colitis triggering the proinflammatory profile by the expansion of Th1 and Th17 phenotypes, which was absent in TLR4-deficient mice (Kurakevich et al., 2013).
In PBMC, FOS, GOS, and fructans have the capacity to modulate cytokine production, leading to more anti-inflammatory IL-10 production (Vogt et al., 2013; Capitán-Cañadas et al., 2014; Lehmann et al., 2015). This process was dependent on TLR2 (Vogt et al., 2013) or TLR4 (Capitán-Cañadas et al., 2014; Lehmann et al., 2015).
D. Clinical Application
Reviews have reported the beneficial effects of the NDOs, FOS, and GOS, in prevention and treatment of allergic diseases, including atopic dermatitis, eczema, asthma, allergic rhinitis, and food allergies. Because of risk of bias, heterogeneity of clinical trials, and inconsistency of the NDOs used, additional studies are needed (Osborn and Sinn, 2013; Cuello-Garcia et al., 2017; Brosseau et al., 2019). Boyle and colleagues (2016) investigated the effects of partially hydrolyzed whey formula containing oligosaccharides—neutral short-chain GOS, long-chain FOS, pectin, and hydrolysate-derived acidic oligosaccharides—in children with a predisposition to eczema based on family history before the age of 18 weeks. These children had increased levels of FoxP3 Treg cells and lower concentrations of IL-4, IL-13, INF-γ, and TNF-α in peripheral blood mononuclear cells compared with children fed with standard formula. Infants treated with formula containing GOS/FOS resulted in a beneficial antibody profile, since lower plasma levels of IgG1, IgG2, IgG3, and cow’s milk–specific IgE antibodies were observed (van Hoffen et al., 2009). These two studies confirm that GOS/FOS supplementation in children may have beneficial effects on allergies via immune modulation. A review of Davani-Davari et al. (2019) described the effects of NDOs observed in IBD and colorectal cancer, and although some studies report positive results, other studies using NDOs were not effective. The same review also gives an overview of the immunomodulatory of GOS and FOS effects in clinical trials, including improved immune response toward vaccination and decrease in proinflammatory cytokines, such as IL-6, IL-1β, and TNF-α. Another review reported the reduction of the proinflammatory profile in people with obesity after supplementation with inulin type fructans or GOS (Fernandes et al., 2017). From these studies, it is not clear whether these effects are dependent on TLR2 or TLR4 signaling. Different articles emphasized the beneficial effects of these NDOs as a result of changes in the microbiota composition and in turn increased SCFA production, which could have been related to the observed anti-inflammatory and protective effects (Brosseau et al., 2019; Tandon et al., 2019).
VI. Conclusions and Discussion
This review describes in detail the effects of nutritional components and their metabolites on the immune system via specific (pharmacological) receptors, as summarized in Table 1–4. Nutritional components can promote both anti-inflammatory and proinflammatory processes, depending on the nutrient-receptor complex and interactions (and quantity of nutritional components). Generally, the nutritional components, including SFAs, tend to have proinflammatory effects. Conversely, nutritional components, such as vitamins, fibers, and unsaturated fatty acids, are able to resolve inflammation and are generally anti-inflammatory.
In the present review, we collated studies investigating the mechanistic effects of various nutritional components on major receptor types of the immune system, thereby providing a tool to better understand the proinflammatory and anti-inflammatory properties of these nutritional components that may assist efforts to prevent and treat NCDs. Understanding the mechanism of action of these nutritional components may be used to develop informed studies with the aim of developing natural therapeutic options when an imbalance in the immune system is observed. Previous studies have described possible beneficial effects of nutritional components for NCDs, such as cardiovascular diseases, autoimmune diseases, and inflammatory bowel disease, as observed by prevention and alleviation of clinical symptoms (Wędrychowicz et al., 2016; Mijan and Lim, 2018; Constantin et al., 2019; Li et al., 2019; Rivellese et al., 2019). Here, we extended possible descriptions of the pharmacological and physiologic mechanisms underlying these health benefits. Understanding the mode of action of these natural ligands means the manipulation of such receptors to improve the affinity of the natural ligand might be another potential course of action in tackling NCDs.
A. Analytical Discussion (Murine) Experimental Models
Remarkably, very little clinical research involving the immunomodulatory effects of nutritional components via receptor signaling is currently available. In vitro models have provided important tools for focusing on the mechanism behind the immune regulation by dietary components; nevertheless, the physiologic relevance of these monoculture and coculture models needs careful interpretation (Saeidnia et al., 2015). As described in this review, murine models utilizing inbred mouse strains and KO mice are also frequently used to unravel the mechanisms for immune modulation by nutritional components. Mouse models have advantages compared with other species, such as genome similarities between human and mouse and an excellent genetic/molecular toolbox. Balb/C and C57BL/6 backgrounds are the most widely used inbred strains and are considered as golden standard in biologic and biomedical research. However, the genetic background may alter the susceptibility to infection and other diseases, such as allergic diseases (Gueders et al., 2009; Hartmann et al., 2021). It is well known that C57BL/6 mice demonstrate a skewing toward Th1 responses, whereas the Balb/c mice are regarded as a Th2-dominant mouse strain (Yagi et al., 2006). Recently, a study pointed out that Treg subsets take the lead in the regulation of immune responses during infection in Balb/c, whereas various immune regulatory pathways occur in C57BL/6 mice (Hartmann et al., 2021). The use of these strains may mask immunologic pathways that exist in human beings and may even overshadow the consequence of the targeted mutation (Rivera and Tessarollo, 2008).
Therefore, care should be taken when drawing conclusions based on the results from a single genetic background and extrapolating this to the human situation (Rivera and Tessarollo, 2008). Despite the many similarities, differences in the organization of the immune system of man and mouse are highlighted by critics, which makes direct translation of murine data to the human situation difficult (Vandamme, 2014; Zschaler et al., 2014). Outbred mice will show higher levels of genetic variation contributing to individual variation in response to a dietary intervention, and therefore the experimental data may be more applicable to human populations (Tuttle et al., 2018). These outbred mice, but especially humanized mice models (mice engrafted with human cells/tissues or mice that transgenically express human genes), represent promising tools for investigating human immune responses in future preclinical studies (Allen et al., 2019). Of course, clinical studies are needed in the future to confirm the preclinical results and to investigate the effect of nutritional components on the genetic heterogeneity in humans.
The information provided in this review may serve pharmacologists, health care professionals, and researchers that aim to find solutions for NCDs. Increasing evidence shows that particularly during the early stages of disease, lifestyle interventions, such as specific nutritional components, are by no means inferior to medical treatment (Carruba et al., 2016; Hyseni et al., 2017; Witkamp and van Norren, 2018). Pharmacologists as well as nutritionists are increasingly aware that multifactorial diseases, such as NCD, may require a multitarget approach because of their complexity and the involvement of multiple signaling pathways (Witkamp and van Norren, 2018). Therefore, successful concepts to treat, prevent, or delay these diseases might be found in combinations of medicines and nutritional components to increase efficacy and reduce adverse effects. The understanding of the pharmacological modulation of immune responses by nutritional components will move the disciplines of pharmacology and nutrition toward each other in science as well as in the clinic.
There is extreme variation in the production and consumption of food between cultures, regions, and countries and even among individuals depending on multiple factors, such as socioeconomic status or age (Mertens et al., 2019). These factors impact the level of nutritional components and metabolites in the body significantly. Some of the nutritional components mentioned in this review are present in high levels in certain diets, but other diets lack specific foods and these single nutritional components. Sometimes the effective dosage of these nutritional components will be reached, whereas in other populations the optimal dose is not ingested, and in some circumstances, it could even be exceeded. The effective dosage is not only based on intake levels but also depends on the bioavailability of these nutritional components after ingestion. The bioavailability is influenced by 1) bioaccessibility, including the release of nutritional components from food matrices, 2) absorption by the epithelial layer, and 3) chemical and biochemical transformations (Dima et al., 2020). Therefore, it is essential but very difficult to give an unambiguous advice on the efficiency, safety, and toxicity of these nutritional components.
In addition, we must be aware that consumers can respond differently to dietary interventions. Individual differences like age, gender, metabolism, and microbiome composition might contribute to these individual responses. It has even been recognized that genetic variations might affect individual nutritional requirements and bioavailability of nutrients between individuals (Bashiardes et al., 2018). In this regard, personalized nutrition might be a good strategy to improve human health (Adams et al., 2020).
Authorship Contributions
Wrote or contributed to the writing of the manuscript: van Daal, Folkerts, Garssen, Braber.
Footnotes
This paper received no external funding.
No author has an actual or perceived conflict of interest with the contents of this article.
Abbreviations
- ABC
- ATP binding cassette
- AHR
- aryl hydrocarbon receptor
- Aldh
- Aldehyde dehydrogenase
- AP-1
- activator protein 1
- BMDC
- bone marrow–derived dendritic cell
- CCR
- C-C chemokine receptor type
- COX
- cyclooxygenase
- CXCL
- chemokine (C-X-C motif) ligand
- DC
- dendritic cell
- DHA
- docosahexaenoic acid
- DIM
- 3-3′-diindolylmethane
- DSS
- dextran sulfate sodium
- EAE
- experimental autoimmune encephalomyelitis
- EPA
- eicosapentaenoic acid
- ERK
- extracellular signal-regulated kinase
- FOS
- fructo-oligosaccharides
- GATA3
- GATA-binding protein
- GATA3
- GATA-binding protein 3
- GOS
- galacto-oligosaccharides
- GPR
- G-coupled protein receptor
- I3C
- indole-3-carbinol
- IBD
- inflammatory bowel disease
- IFN
- interferon
- Ig
- immunoglobulin
- IL
- interleukin
- IRF
- interferon regulatory factor
- KO
- knockout
- LPS
- lipopolysaccharide
- LXR
- liver X receptor
- MCP
- monocyte chemoattractant protein
- MEK
- mitogen-activated protein kinase kinase
- MHC
- major histocompatibility complex
- MMP
- matrix metalloproteinase
- NCD
- noncommunicable disease
- NDO
- nondigestible oligosaccharide
- NF-κB
- nuclear factor κ-light chain enhancer of activated B cells
- Niacr
- niacin receptor
- NOS
- nitric oxide synthase
- NLRP
- NOD-like receptor protein
- NR
- nuclear receptor
- PBMC
- peripheral blood mononuclear cells
- PPAR
- peroxisome proliferator-activated receptor
- PUFA
- polyunsaturated fatty acid
- 3ROR
- retinoic acid receptor-related orphan receptor
- RA
- retinoic acid
- RAR
- retinoic acid receptor
- ROR
- retinoic acid receptor-related orphan
- RXR
- retinoid X receptor
- SCFA
- short-chain fatty acid
- SFA
- saturated fatty acid
- siRNA
- small interfering RNA
- TGF-β
- transforming growth factor β
- Th
- T helper cell
- TLR
- toll-like receptor
- TNBS
- 2,4,6-trinitrobenzene sulfonic-acid
- TNF-α
- tumor necrosis factor α
- Treg
- T regulatory cell
- VDR
- vitamin D receptor
- WT
- wild type
- ZO
- zonula occludens
- Copyright © 2020 The Author(s)
This is an open access article distributed under the CC BY-NC Attribution 4.0 International license.