Abstract
The nitrogen mustards are powerful cytotoxic and lymphoablative agents and have been used for more than 60 years. They are employed in the treatment of cancers, sarcomas, and hematologic malignancies. Cyclophosphamide, the most versatile of the nitrogen mustards, also has a place in stem cell transplantation and the therapy of autoimmune diseases. Adverse effects caused by the nitrogen mustards on the central nervous system, kidney, heart, bladder, and gonads remain important issues. Advances in analytical techniques have facilitated the investigation of the pharmacokinetics of the nitrogen mustards, especially the oxazaphosphorines, which are prodrugs requiring metabolic activation. Enzymes involved in the metabolism of cyclophosphamide and ifosfamide are very polymorphic, but a greater understanding of the pharmacogenomic influences on their activity has not yet translated into a personalized medicine approach. In addition to damaging DNA, the nitrogen mustards can act through other mechanisms, such as antiangiogenesis and immunomodulation. The immunomodulatory properties of cyclophosphamide are an area of current exploration. In particular, cyclophosphamide decreases the number and activity of regulatory T cells, and the interaction between cyclophosphamide and the intestinal microbiome is now recognized as an important factor. New derivatives of the nitrogen mustards continue to be assessed. Oxazaphosphorine analogs have been synthesized in attempts to both improve efficacy and reduce toxicity, with varying degrees of success. Combinations of the nitrogen mustards with monoclonal antibodies and small-molecule targeted agents are being evaluated.
Significance Statement The nitrogen mustards are important, well-established therapeutic agents that are used to treat a variety of diseases. Their role is continuing to evolve.
I. Introduction
The nitrogen mustards are the oldest class of anticancer agents. They originate from sulfur mustard (dichloroethyl sulfide), which was first reliably synthesized and characterized in 1886 by Victor Meyer (Meyer, 1886). Sulfur mustard is a vesicant and was initially used as an agent of chemical warfare in July 1917 near the West Flanders city of Ieper, Belgium, leading to the name mustard gas or “yperite”. The predicted effects on the lungs, eyes, and skin were observed, but in addition bone marrow aplasia, destruction of lymphoid tissue, and gastrointestinal ulceration were noted (Krumbhaar and Krumbhaar, 1919).
After further work in the second world war, Gilman proposed that decreasing the electrophilicity of sulfur mustard would lead to less toxic compounds; by replacing the sulfur atom with a nitrogen atom, nitrogen mustard (now called mechlorethamine) was formed. Experiments with transplanted lymphosarcomas in mice confirmed the cytotoxic action of mechlorethamine on lymphoid tissue, and the first clinical trials in 1943 marked the dawn of modern cancer chemotherapy. Details of its successful use in Hodgkin lymphoma (HL) were subsequently published (Goodman et al., 1946). Mechlorethamine is unstable, but stability is increased by the incorporation of an aromatic ring, producing chlorambucil, which was introduced in 1953. Chlorambucil is less electrophilic and reacts more slowly with DNA. Melphalan was synthesized in the same year by replacing the methyl group on mechlorethamine with L-phenylalanine. Inactivation of the highly reactive mechlorethamine by chemical coupling is necessary for the formation of a well tolerated transport form. The biologic action of the nitrogen mustards is dependent on the reactivity of the two functional chloroethylamino groups, which in turn is related to the basicity of the nitrogen atoms. Substituting electrophilic groups at the amino nitrogen reduces basicity and therefore the reactivity of the functional groups. Conversely, introducing a nucleophilic group increases the reactivity. Arnold and Bourseaux (1958) coupled the mustard group to the electrophilic phosphoryl group, producing cyclophosphamide. Trofosfamide and ifosfamide were introduced in 1972 and 1977 respectively.
The nitrogen mustards formed a cornerstone of anticancer chemotherapy in the second half of the 20th century and remain important today. This article reviews the use of the nitrogen mustards in the treatment of solid tumors and hematologic malignancies, as well as their impact on the management of autoimmune diseases. The immunomodulatory properties of cyclophosphamide continue to be investigated. Advances in analytical techniques have led to an increased understanding of the pharmacokinetics of these agents and the mechanisms underlying their toxic effects. New derivatives of the nitrogen mustards continue to be evaluated in attempts to improve their therapeutic indices. The advent of new approaches to the treatment of cancer, in the form of monoclonal antibodies and small-molecule targeted agents, has provided new opportunities for the development of novel combination regimens.
II. Molecular Chemistry and Pharmacology
A. Molecular Mechanisms of Action
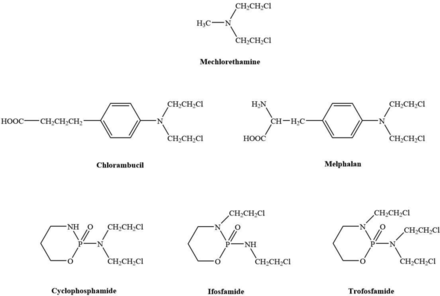
The nitrogen mustards comprise mechlorethamine, chlorambucil, melphalan, and the oxazaphosphorines cyclophosphamide, ifosfamide, and trofosfamide. Ifosfamide is a structural isomer of cyclophosphamide, with one of the 2-chloroethyl groups positioned on the endocyclic nitrogen atom. Trofosfamide is a congener of cyclophosphamide and ifosfamide and has a third chloroethyl group on the position of the cyclic nitrogen. The structures of the nitrogen mustards are given in Fig. 1. The nitrogen mustards are alkylating agents. They are cytotoxic by virtue of their ability to alkylate nucleophilic centers in the DNA molecule via the formation of covalent bonds. Mechlorethamine mainly forms adducts at the guanine-N7 position, melphalan at the guanine-N7 and adenine-N3 positions, cyclophosphamide at the guanine-N7 position, and chlorambucil at the N7 of guanine and the N3 of adenine (Povirk and Shuker, 1994). Cyclophosphamide also produces a phosphoester adduct. The O6 atom on guanine can also be targeted by the oxazaphosphorines. Intra- and interstrand crosslinks are produced within DNA, and interstrand crosslink adducts are the most toxic, as they generate double strand breaks. Nitrogen mustards damage DNA during any phase of the cell cycle, so they are not cell cycle phase specific. Other molecules are damaged by alkylation including RNA, proteins, lipids, and mitochondrial DNA. Binding to cellular proteins also occurs through reaction with carboxyl, mercapto, amino, phosphate, and hydroxyl groups.
The structures of the nitrogen mustards.
Oxazaphosphorines are prodrugs, and their metabolites are therefore essential for their cytotoxicity. Isophosphoramide mustard (IPM) has one 2-chloroethyl group on the exocyclic and one on the endocyclic nitrogen atom, permitting a more effective DNA crosslinking distance between the two independent functional alkylating moieties compared with phosphoramide mustard (PM) (Brock, 1983). The loss of chlorhydric acid from IPM leads to the formation of an aziridine ring, whereas PM produces an aziridinium ion, which is relatively unstable. Acrolein and chloroacetaldehyde (CAA) activate intracellular reactive oxygen species (ROS), producing DNA single strand breaks (Crook et al., 1986; Brüggemann et al., 2006).
Different mechanisms are active in causing cell death after the production of adducts and crosslinks by the nitrogen mustards. The main mechanism is DNA damage, which initiates apoptosis via the mitochondrial pathway by activating caspase 9, which then activates the effector caspases -3 and -7 (Schwartz and Waxman, 2001). Cell cycle modulation also occurs. Furthermore, nitrogen mustards generate ROS, which deplete the cellular antioxidant capacity of cancer cells, also causing apoptosis (Jeelani et al., 2017).
B. Antiangiogenic Activity
The nitrogen mustards were developed with the intention of directly killing cancer cells. However, DNA damage also occurs in endothelial cells during new vessel formation in tumors, and endothelial cells may be more sensitive than cancer cells or normal cells. Browder and colleagues (2000) demonstrated that an antiangiogenic schedule of cyclophosphamide in mice avoided drug resistance and eradicated Lewis lung carcinoma and L1210 leukemia, which did not occur with a conventional schedule. Cyclophosphamide was given at shorter intervals without interruption, and 170 mg/kg [one-third of the maximum tolerated dose (MTD)] every 6 days was the most effective. Long rest periods between conventional doses of cyclophosphamide allowed the recovery of tumor endothelial cells. The antiangiogenic schedule caused apoptosis of endothelial cells within tumors, which occurred before apoptosis of tumor cells.
Cyclophosphamide exerts antiangiogenic effects in a number of different ways (Natale and Bocci, 2018). It decreases the expression of proangiogenic factors, such as serum vascular endothelial growth factor (VEGF), in preclinical tumor models. This is also seen in the clinical arena where lower VEGF and basic fibroblast growth factor (bFGF) levels after 2 months of daily oral metronomic cyclophosphamide are associated with a higher progression-free survival (PFS), and these levels increase when progression occurs. Additionally, metronomic cyclophosphamide induces the expression of thrombospondin-1 (TSP-1), an endothelial cell–specific inhibitor of angiogenesis. Increasing the endothelial expression of TSP-1 causes endothelial apoptosis. Daily metronomic cyclophosphamide increases plasma TSP-1 in responsive human prostate tumor mice xenografts, an effect also seen in murine melanoma, rat prostate tumors, and human ovarian tumors (Natale and Bocci, 2018). Another important mechanism is the effect on circulating endothelial progenitors (CEPs), which stimulate the shift toward the formation of new blood vessels. In a human lymphoma mouse model, metronomic cyclophosphamide reduces CEP levels, which increase when cyclophosphamide is discontinued, with a resultant progression of the lymphoma (Bertolini et al., 2003). Low-dose metronomic trofosfamide, 100 to 150 mg daily, has been shown to significantly reduce CEPs in patients (Stoelting et al., 2008).
C. Mechanisms of Resistance
Intrinsic or acquired resistance can develop to the nitrogen mustards. Various underlying mechanisms have been identified, including an increase in glutathione binding, an increase in DNA repair, and polymorphisms in drug-metabolizing enzymes. Changes in the behavior of amino acid transporters and a decreased entry or increased exit of cytotoxic species from cells can occur. Signaling proteins regulate the downstream processes in apoptosis, and these may be influenced by the nitrogen mustards.
Glutathione S-transferases (GSTs) are a superfamily of phase 2 metabolic enzymes involved in cellular resistance mechanisms. GSTP1 accounts for most of their activity. Cancer cells resistant to the nitrogen mustards can contain higher levels of GSTs, with an increased formation of glutathione conjugates with alkylating species (Zhang et al., 2006). GST isoenzymes are also involved in the transport of cyclophosphamide metabolites, melphalan, and chlorambucil from the cell interior to the exterior. Breast cancer resistance protein (BCRP) and multidrug resistance-associated protein (MRP)1, MRP2, and MRP4 are constituents of the ATP-binding cassette superfamily, which is associated with multidrug resistance. After conjugation by GSTs, nitrogen mustards can be removed by MRP1- and MRP2-facilitated excretion. MRP1 and BCRP may be important in resistance to cyclophosphamide-based chemotherapy. In patients with breast cancer treated with adjuvant cyclophosphamide, methotrexate, and 5-fluorouracil, expression of MRP1 was detrimental. MRP4 may affect the transport of cyclophosphamide and ifosfamide, and the insertion of the MRP4 gene into HepG2 cells induces significant resistance to these agents. Cyclophosphamide is a cell-specific and concentration-dependent MRP4 inducer (Zhang et al., 2010). The glutathione conjugates of chlorambucil and melphalan are substrates for MRP1 (Barnouin et al., 1998), and the chlorambucil conjugate is additionally excreted by MRP2 (Smitherman et al., 2004).
Resistance to cyclophosphamide and ifosfamide is associated with an increase in DNA repair capability, such as nucleotide excision repair (Zhang et al., 2006). Higher concentrations of the DNA repair protein O6-methyl-guanine-DNA methyl transferase (MGMT) are associated with resistance to cyclophosphamide. However, a low MGMT expression in patients with breast cancer treated with cyclophosphamide was unexpectedly associated with poor survival. The mismatch repair gene is important in repairing DNA damage. A reduced mismatch repair gene human mutL homolog 1 (hMLH1) expression in patients with breast cancer treated with cyclophosphamide, methotrexate, and 5-fluorouracil resulted in a poor prognosis.
In mice, at least 26 polymorphisms in the cytochrome P450 (P450) genes, which could influence the activation of cyclophosphamide and ifosfamide, have been identified (Zhang et al., 2006). Activation of cyclophosphamide and ifosfamide by CYP3A4, CYP2C9, and CYP2B6 can be impaired. An intrinsic or induced increase in the activity of aldehyde dehydrogenase (ALDH) can cause resistance to cyclophosphamide and ifosfamide. In patients treated with cyclophosphamide-based chemotherapy, the concentration of ALDH1A1 was significantly greater in nonresponsive metastatic tumors. However, in another study of patients with ovarian cancer treated with cyclophosphamide and carboplatin, neither ALDH, GSTA, nor GSTP were independent factors of resistance.
Defective transport into the cell and also reduced apoptosis have been shown to be factors in the development of resistance to melphalan, and modulation of DNA damage signaling and repair pathways may also be important (Sousa et al., 2013). A common resistance mechanism to chlorambucil in chronic lymphocytic leukemia (CLL) is a mutation in the p53 tumor suppressor gene. Mutations in p53 increase the activity of multidrug resistance protein 1 (MDR1) with a greater efflux of drugs from CLL cells. Resistance to chlorambucil caused by impaired DNA repair and increased expression of antiapoptotic proteins such as B-cell lymphoma 2 (bcl-2) and bcl-xL can be overcome by nucleoside analogs and Bruton’s tyrosine kinase and bcl-2 inhibitors. Work using a p53-null promyelocytic chemotherapy-resistant leukemia cell line showed that the combination of chlorambucil with a histone deacetylase inhibitor is synergistic regarding apoptosis with upregulation of BCL6 and p21 expression (Kwa et al., 2019).
III. Immunomodulatory Activity
Cyclophosphamide is a potent immunosuppressive agent that affects both T and B lymphocytes influencing both humoral and cell-mediated immunity. T lymphocytes have a central role in the control of immunity. Three major subsets of helper T (TH) cells have been identified (TH1, TH2, and TH17). TH1 cells support the cytotoxic T cell responses required for antitumor activity, whereas TH2 cells facilitate the generation of B cell and antibody responses. TH17 cells are important in the pathogenesis of autoimmune and inflammatory disorders, protecting against certain bacterial infections. Regulatory T cells (Tregs), another subset of TH cells, suppress the immune response and are involved in the prevention of autoimmune disorders by controlling self-antigen–reactive T cells. They accumulate in tumors and encourage a microenvironment that reduces the effector function of tumor-infiltrating cytotoxic lymphocytes. Tumors can bypass immune recognition and subsequent killing by modifying immunosuppressive T lymphocytes. This can occur through inhibition of the induction of tumor-associated antigen-specific immunity by promoting Treg trafficking, differentiation, and expansion. The accumulation of Tregs in patients with cancer is generally associated with tumor progression, a poor prognosis, and suppression of antitumor immunity (Curiel, 2008).
Most chemotherapeutic agents cause tumor cell death by apoptosis, historically viewed as immunologically silent. However, cytotoxic agents may partly exert their effects by activating the immune system of the host. Cyclophosphamide was the first chemotherapeutic agent found to have powerful immunomodulatory properties, and in this respect it remains the gold standard. The exact mechanism of cyclophosphamide immunomodulation is unclear, but several effects may be involved. It causes the immunogenic cell death of tumor cells, increases the proliferation and survival of peripheral T lymphocytes, mobilizes bone marrow dendritic cells, and greatly reduces the number and activity of Tregs (Sistigu et al., 2011).
There are a number of ways by which low-dose cyclophosphamide may selectively deplete Tregs: inhibition of the more quickly proliferating Tregs compared with other lymphocyte classes (Ercolini et al., 2005); the precipitation of apoptosis and functional inhibition of Tregs (Lutsiak et al., 2005); the inhibition of inducible nitric oxide (NO) synthase, which controls Treg numbers (Loeffler et al., 2005); an increased sensitivity of Tregs to DNA crosslink formation and differences in the DNA damage response (Heylmann et al., 2013); and the lack of expression on Tregs of the MDR1 transporter, which removes cyclophosphamide metabolites from the cell interior and which is significantly expressed on nonregulatory CD (cluster of differentiation)4+ T cells (Dimeloe et al., 2014). Tregs also have low ATP levels, resulting in the decreased synthesis of glutathione with a reduction in cyclophosphamide detoxification (Zhao et al., 2010).
In rodents, cyclophosphamide is the most potent chemotherapeutic anti-Treg agent (Ghiringhelli et al., 2004). The glucocorticoid-induced tumor necrosis factor (TNF) receptor and Foxp3 genes are involved in the suppressive actions of Tregs, and their expression is downregulated by cyclophosphamide (Lutsiak et al., 2005). Foxp3 is associated with a higher expression of proapoptotic molecules, which in turn may be involved in the greater sensitivity of Tregs to low-dose cyclophosphamide. Cyclophosphamide reduces the number of Tregs in the blood and lymphoid organs of tumor-bearing animals. It also diminishes the number of Tregs infiltrating tumor beds. Furthermore, tumor immune lymphocytes only migrate specifically to the tumor when pretreated with cyclophosphamide (Bracci et al., 2007). Cyclophosphamide-induced Treg depletion is temporary, with a maximum effect after 4 days, and it usually disappears within 10 days. Although it is generally held that cyclophosphamide decreases both the number and activity of Tregs, some reports state the opposite but still describe appropriate antitumor effects (Radojcic et al., 2010).
Cyclophosphamide also inhibits tumor-associated macrophage or myeloid-derived suppressor cell suppressive functions (Radojcic et al., 2010) and stimulates myeloid cell inflammatory activity. During the recovery phase after cyclophosphamide-mediated myelolymphoid depletion, there is homeostatic proliferation (Bracci et al., 2007). The polarization of CD4+ T cells from TH2 into TH1 and TH17 lymphocytes is facilitated by cyclophosphamide (Viaud et al., 2011), and the resulting change of a proangiogenic and immunosuppressive phenotype to an antiangiogenic and immunostimulatory phenotype is advantageous. Cytokines mediate many cyclophosphamide-induced effects. In tumor-bearing rats receiving low-dose cyclophosphamide, the TH2 to TH1 shift in cytokine production is evident in their splenic cells, in which the concentrations of interferon (IFN)-γ and interleukin (IL)-2 are significantly increased (Matar et al., 2002). The opposite is seen with the suppressive type 2 cytokines IL-10 and transforming growth factor (TGF)-β, and NO, which increase in the spleens of tumor-bearing rats and decrease after cyclophosphamide administration (Matar et al., 2000). A cyclophosphamide-induced cytokine storm in the rebound phase after lymphodepletion is important for a therapeutic response.
Melphalan has similar immunomodulatory effects to cyclophosphamide. When administered to tumor-bearing mice, a reduction in Tregs with activation of endogenous CD8+ T cells is seen. Melphalan also causes immunogenic cell death, increases tumor antigen uptake by dendritic cells in the tumor-draining lymph nodes, and promotes a wave of proinflammatory cytokines and chemokines during the cellular recovery phase after myelodepletion and leucodepletion (Lu et al., 2015).
In patients with cancer, Tregs accumulate in the tumor microenvironment and peripheral blood, and their depletion enhances the activity of cancer immunotherapy. Tregs control the natural killer (NK) cell–mediated immunity against cancer cells, and the decrease in circulating Tregs produced by cyclophosphamide restores innate killing activity (Ghiringhelli et al., 2007). Cyclophosphamide changes the Treg/Teffector ratio toward tumor regression, and this ratio in the tumors of patients with advanced cancer may be a better indicator of cyclophosphamide activity than baseline circulating Treg levels. Low doses of cyclophosphamide facilitate the TH2 to TH1 switch and also increase the number of TH17 cells, but whether the latter change is a factor in the antitumor effect of cyclophosphamide remains uncertain. The exact role of TH17 cells in tumor immunosurveillance is not yet clearly defined, as they interact closely with Tregs. However, they form part of the tumor-infiltrating lymphocyte fraction.
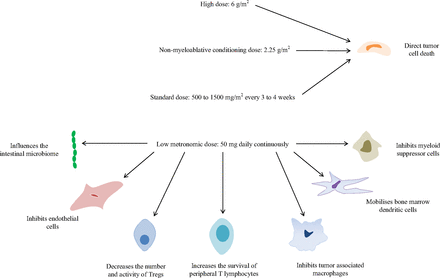
The optimum dose, scheduling, and route of administration of cyclophosphamide required to maximally harness its immunomodulatory properties are only now becoming apparent. In humans, the activity of cyclophosphamide is dose dependent; it is either immunosuppressive or immunopotentiating according to the dose and length of administration (Brodsky et al., 1998). The clinical doses of cyclophosphamide and their predominant therapeutic effects on different cellular targets are illustrated in Fig. 2. High doses are essential for chemotherapeutic treatment, but these also lead to immunosuppression by destroying cytotoxic T cell and helper T cell function and inhibiting the production of neutralizing antibodies. However, low doses can improve immune responses and facilitate antitumor immune-mediated responses by decreasing the number and function of Tregs (Ghiringhelli et al., 2004; Lutsiak et al., 2005). A metronomic schedule has been defined as one characterized by regular and frequent chemotherapeutic drug doses able to maintain a low but active range of drug concentrations during prolonged periods of time without causing important toxicities (Bocci and Kerbel, 2016). Such administration of cyclophosphamide is antiangiogenic, but in addition it selectively restricts Treg expansion and reestablishes immune function in patients with advanced cancer. In a study performed by Ghiringhelli and colleagues (2007), patients were treated with 50 mg oral cyclophosphamide twice daily for 1 week out of 2 for 1 month or longer; the number and function of Tregs decreased without affecting the number and function of other lymphocyte subsets such as cytotoxic CD8+ T cells, and T cell proliferation and NK cell effector function were reestablished. The number of Tregs 2 months after stopping cyclophosphamide returned to pretreatment levels. The selective effect of metronomic cyclophosphamide on the Treg population was a consequence of the low dose used. When 200 mg cyclophosphamide was administered daily, all of the lymphocyte subpopulations were diminished with an absence of Treg specificity. NK cell–dependent cytotoxicity and T cell proliferation capacity were also reduced. Both intravenous and oral cyclophosphamide decrease Treg activity, but metronomic cyclophosphamide may have the most powerful effect. With metronomic dosing, the immunostimulatory and antiangiogenic properties of cyclophosphamide are maximized.
The clinical doses of cyclophosphamide and their predominant therapeutic cellular targets.
The interaction between cyclophosphamide and the intestinal microbiome is an important emerging facet of immunomodulation. The tumor microenvironment is affected by the composition of the intestinal microbiome. Cyclophosphamide disrupts the gut mucosal integrity and causes dysbiosis in the small intestine of patients with cancer (Viaud et al., 2013). It can facilitate the passage of gram-positive bacteria through the intestinal barrier, generating an inflammatory immune response that aids the antitumor activity of the immune system (Viaud et al., 2014). After the administration of cyclophosphamide to tumor-bearing mice, bacterial translocation of the gram-positive genera Lactobacillus, Enterococcus, and Clostridium occurs with the recruitment and accumulation of TH1 and TH17 helper cells. Destroying gram-positive bacteria with vancomycin prior to the administration of cyclophosphamide decreases these effects (Viaud et al., 2013). Two commensals, a gram positive in the small intestine (Enterococcus hirae) and a gram negative in the colon (Barnesiella intestinihominis), have also been shown to potentiate the effect of cyclophosphamide (Daillère et al., 2016). The anticancer activity of cyclophosphamide is dependent on these commensals, which modify the tumor microenvironment, decreasing Tregs and increasing cognate anticancer cytotoxic T cell responses. E. hirae and B. intestinihominis specific memory TH1 cell immune responses are linked to increased PFS in patients with lung and ovarian cancer receiving chemotherapy, the latter group treated with metronomic cyclophosphamide.
IV. Analytical Chemistry
A. The Oxazaphosphorines
The oxazaphosphorines are stable in vitro and relatively easy to quantify in human biofluids. Early analytical methods used gas chromatography (GC) with nitrogen flame ionization detection or electron capture detection to determine cyclophosphamide and ifosfamide concentrations, and “total alkylating activity” was measured by spectroscopic analysis of the blue color produced after reaction with 4-(4′-nitrobenzyl) pyridine (NBP). However, the NBP reaction is nonspecific, and only an approximation of total alkylating activity is obtained. Despite similar oxazaphosphorine doses and amounts of alkylating activity, the metabolic profiles of oxazaphosphorine metabolites can be quite different. The introduction of high-performance liquid chromatography (HPLC) was still not sufficient to separate and measure individual metabolites. 31P nuclear magnetic resonance spectrometry has also been developed (Gilard et al., 1993), and although individual metabolites can be identified, application has been limited to urine samples.
Sensitive and specific analytical methods are needed for the analysis of the oxazaphosphorines and their metabolites in all biologic matrices to facilitate detailed pharmacokinetic investigations. The widespread coupling of separation techniques with mass spectrometry (MS) has permitted such comprehensive studies of individual metabolites, which are separated prior to their introduction into the MS. Gas chromatography-mass spectrometry (GC-MS) generally requires derivatization of metabolites. Cyclophosphamide, nor-nitrogen mustard [(NNM) derived from PM during derivatization], alcophosphamide (AlcP), carboxyethylphosphoramide mustard (CEPM), 4-ketocyclophosphamide (4-keto CY), dechloroethylcyclophosphamide (DCEC), and N-chloroethyl-1,3-oxazolidine-2-one (CNM) have been measured by GC-MS after the formation of methyl and/or trifluoroacetyl derivatives. NNM and CNM were isolated by liquid extraction with ethyl acetate and the other compounds by solid phase extraction. It was possible to measure these compounds in plasma at a concentration of 1 ng/ml (Momerency et al., 1994b). A similar approach has been used to quantify ifosfamide and its metabolites with comparable sensitivity (Momerency et al., 1994a). However, the measurement of the labile primary 4-hydroxy metabolites as separate peaks is not possible using this methodology. These metabolites are particularly unstable in biologic fluids, with a plasma half-life of approximately 4 minutes, and are more difficult to quantify. Immediate derivatization is necessary for stabilization prior to analysis; dansyl hydrazine, phenylhydrazine, semicarbazide, sodium cyanide, and hydroxylamine have been used as derivatizing agents. The number of internal standards that can be used for the hydroxy metabolites is also limited. An alternative approach is to employ a stable isotope dilution technique with the addition of a deuterium-labeled analog to the plasma sample to act as the internal standard. The assumption is made that it decomposes in the same fashion as the nonlabeled metabolite. Cyclophosphamide, PM, AlcP, 4-hydroxy cyclophosphamide (4-OH-CY), and CNM have been analyzed in plasma using GC-MS and stable isotope dilution techniques (Chan et al., 1994). Internal standards labeled with deuterium atoms were employed [CY-D8 (also for AlcP), PM-D8, 4-OH-CY-D4, and CNM-D8]. Cyanohydrin formation was used to stabilize 4-OH-CY. The sensitivity limits for cyclophosphamide and PM were >0.1 μM (26 ng/ml) and >0.2 μM (44 ng/ml) respectively. The detection limit of 4-OH-CY was >0.1 μM (28 ng/ml), and that of AlcP was 0.07 μM (20 ng/ml).
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is selective, sensitive, and fast and is a commonly used technique to analyze small molecules in biologic fluids. Appropriate sample preparation and processing are needed to isolate the analyte, usually with liquid-liquid extraction and solid phase extraction. LC-MS/MS has been used to analyze cyclophosphamide and 4-OH-CY in human plasma (Shu et al., 2011). Ethyl acetate was used as the extractant, ifosfamide as the internal standard for cyclophosphamide, and dansyl hydrazine as a stabilizing agent for 4-OH-CY. The lower limit of quantification (LLOQ) for both cyclophosphamide and 4-OH-CY was 5 ng/ml. Using another LC-MS/MS technique, D4-cyclophosphamide and hexamethylphosphoramide were used as internal standards for cyclophosphamide and 4-OH-CY respectively, and semicarbazide was employed to stabilize 4-OH-CY (Ekhart et al., 2007). The runtime was 6 minutes, and the validated range was 0.2 to 40 μg/ml for cyclophosphamide and 0.05 to 5 μg/ml for 4-OH-CY. The simultaneous analysis of CEPM, 4-OH-CY, cyclophosphamide, 4-keto CY, and DCEC in human plasma using liquid chromatography-mass spectrometry (LC-MS) has been reported (Baumann et al., 1999). Solid phase extraction was used, and 4-OH-CY was stabilized by conversion to the methyloxime with methylhydroxylamine. The runtime was 20 minutes, and the LLOQ was 200 ng/ml for cyclophosphamide and 50 ng/ml for 4-OH-CY. Also using LC-MS, Kalhorn and colleagues (2006) quantified 4-OH-CY and CEPM in plasma with a total assay time from commencing derivatization of under 4 hours. 4-OH-CY was derivatized with phenylhydrazine. The LLOQ was 0.4 μM (117 ng/ml) for CEPM and 0.02 μM (6 ng/ml) for 4-OH-CY. A deuterated internal standard was used for CEPM. More recently, an ultra-performance liquid chromatography (UPLC)-MS/MS assay to determine cyclophosphamide and 4-OH-CY in plasma has been described (Hall et al., 2018). The internal standard for cyclophosphamide was d4-CY and that for 4-OH-CY was AZD7451, a tropomyosin receptor kinase inhibitor. Phenylhydrazine was used as a derivatizing agent. Methyl tert-butyl ether liquid-liquid extraction was employed. The LLOQ for cyclophosphamide and 4-OH-CY was 34.24 and 3.424 ng/ml respectively.
Turbulent-flow liquid chromatography (TFLC) permits the direct online LC-MS injection of untreated plasma, which is injected at a high flow rate on to a large particle, small internal diameter TFLC column. The main advantages of TFLC are reduced sampling handling and preparation time with greater throughput and automation. An online extraction high-turbulence liquid chromatography-electrospray ionization-tandem mass spectrometry method (HTLC-ESI-MS/MS) has been developed to analyze cyclophosphamide and CEPM (Bai et al., 2009). Analytes were detected with positive multiple-reaction monitoring. Mass-to-charge ratio 261/140 was used for cyclophosphamide and 293/221 for CEPM. D6-cyclophosphamide and D8-CEPM were employed as internal standards. The total runtime (extraction, separation, and MS detection) was 6.5 minutes per cycle, and the LLOQ for cyclophosphamide was 0.5 μg/ml and for CEPM 0.05 μg/ml.
Sometimes the determination of the parent oxazaphosphorine alone is still required rather than a detailed metabolite analysis, as in population pharmacokinetic studies or therapeutic drug monitoring (TDM). LC-MS can directly measure plasma cyclophosphamide concentrations without the need for derivatization and is useful in this respect. A specific and sensitive analysis of cyclophosphamide in plasma using solid phase extraction and LC-MS with single ion monitoring at m/z 261 has been reported (Liu et al., 2004). Ifosfamide was employed as an internal standard, and the LLOQ was 0.03 μg/ml. A recent novel approach in bioanalysis is dried blood spot (DBS) analysis. A small drop of whole blood obtained from a finger prick is collected on filter paper and allowed to dry in the air. The spot is then treated with solvent to extract the analytes. This minimizes the trauma and volume of blood required when obtaining blood samples in pediatric patients and has been used to measure ifosfamide in conjunction with UPLC-MS/MS (Torres et al., 2015). Acetonitrile and ethyl acetate were used to extract ifosfamide, and cyclophosphamide was the internal standard. The LLOQ was 100 ng/ml. Cyclophosphamide and 4-OH-CY have also been measured in a DBS (Hasanah et al., 2021). The internal standard was 4-OH-CY-d4, and the DBS card contained dried semicarbazide as a derivatizing agent. Analysis was similarly performed using UPLC-MS/MS, and the LLOQ was 10 and 5 ng/ml for cyclophosphamide and 4-OH-CY respectively. An immunoassay has been developed for cyclophosphamide using an immunizing hapten that exposes the cyclophosphamide moiety (CP2-Cl) (Broto et al., 2019). Antibodies against this hapten are used in an indirect competitive ELISA with CEPM as the competitor hapten. The immunoassay does not require derivatization or an extraction procedure, and a limit of detection of 5.74 ng/ml was reported in serum.
The oxazaphosphorine metabolite CAA has been analyzed in plasma after treatment with thiourea and the production of 2-aminothiazole. 2-aminothiazole was quantified by HPLC after solid phase extraction (Kaijser et al., 1993). Plasma samples required stabilization with formaldehyde to prevent the decomposition of chloroacetaldehyde. The limit of detection was 0.5 nM/ml (39 ng/ml). Trofosfamide has been determined in plasma by nitrogen-phosphorus flame ionization GC, with an LLOQ of 0.3 μmol (0.1 μg/ml). The internal standard was DCEC (Brinker et al., 2002). Trofosfamide has also been quantified in plasma by HPLC-MS, using dimethyl cyclophosphamide as the internal standard, with the same LLOQ of 0.3 μmol (Preiss et al., 2004).
A further complexity when considering the analysis of the oxazaphosphorines is the asymmetry of the phosphorus atom in the oxazaphosphorine ring, with the consequence that cyclophosphamide and ifosfamide are chiral molecules. They exist in the (+)-(R)- and (-)-(S)-forms. The stereochemistry of the oxazaphosphorines has led to the development of techniques to separate the enantiomers of these compounds and their metabolites, which is important as enantiospecific pharmacokinetic differences can occur. The plasma concentrations of both enantiomers of cyclophosphamide and DCEC can be determined employing a chiral GC-MS method (Williams et al., 1999). The enantiomers of cyclophosphamide and CEPM have also been separated on a Chiralpak column after liquid extraction with ethyl acetate (de Castro et al., 2014), whereas the (R)- and (S)- 2-and 3-dechloroethylifosfamide (2- and 3-DCEI) metabolites have been measured using enantioselective LC-MS/MS, with an LLOQ of 1 ng/ml (Aleksa et al., 2009). LC-MS/MS has also been used to determine the enantiomers of 4-OH-CY (de Castro et al., 2016). For the analysis of (R,S) ifosfamide, (R,S)-2-DCEI, and (R,S)-3-DCEI in plasma, an enantioselective LC-MS assay with a teicoplanin-based chiral stationary phase and an isopropanol:methanol (60:40 v/v) mobile phase has been used (Oliveira et al., 2007). Solid phase extraction was employed, and the LLOQ for each ifosfamide enantiomer was 37.5 ng/ml.
Chromatographic methods to determine cyclophosphamide have shown good sensitivity and selectivity with an LLOQ in the nM range. HPLC-UV and GC assays are being replaced by more sensitive methods, such as LC-MS/MS and UPLC. The UPLC method developed by Hall and colleagues (2018) has a more manageable sample preparation and higher sensitivity than previous assays for cyclophosphamide and 4-OH-CY. Only 200 μl of plasma is needed. The sole methyl tert-butyl ether liquid-liquid extraction reduces preparation time, and UPLC produces more well defined peaks. Cyclophosphamide and 4-OH-CY are measured in the same analytical run. DBS sampling is a simple and efficient method. Compared with conventional plasma and serum, DBS samples are easier to store. Transport is also straightforward, more convenient, and can be performed in an ambient environment. However, the inter- and intraindividual variation in the spot size, drying time, and overall homogeneity of DBS samples can be issues. Blood viscosity is instrumental in determining the flux and diffusion of analytes in the DBS technique. The hematocrit affects blood viscosity and is the single most important factor affecting the spread of blood on DBS cards. As oxazaphosphorines and their metabolites partition into erythrocytes (Highley et al., 1997), it is to be expected that variations in hematocrit values may impact their analysis. A strategy for a full validation of DBS sampling is essential before it can routinely replace the analysis of plasma and serum. The specificity and sensitivity of the analytical method used determines the reliability of TDM. Most attention has been given to the TDM of cyclophosphamide, but this is restricted in the hospital setting by the number of samples to be analyzed, the need for qualified personnel, and the use of expensive equipment. Some analytical methods entail prolonged sample preparation with derivatization and are labor intensive and time consuming. Bedside processing of blood samples and the requirement for refrigerated transport are other considerations. A dedicated timely pharmacokinetic analysis is necessary to determine the second dose of cyclophosphamide. The assays developed more recently have advantages. UPLC has a higher chromatographic resolution and is faster and more sensitive than HPLC. The simultaneous analysis of cyclophosphamide and 4-OH-CY employing UPLC-MS/MS with a fast 3-minute runtime permits pharmacokinetic studies using a single blood sample (Hall et al., 2018). The application of TFLC to clean samples reduces preparation time and permits small sample volumes and a high throughput. A rapid turnover is also possible using immunoassays. DBS sampling is an attractive option for the analysis of the nitrogen mustards in a small volume of blood, which may aid TDM.
B. Melphalan and Chlorambucil
HPLC analyses to measure plasma melphalan concentrations with detection limits of between 10 and 100 ng/ml have been described (Shaw et al., 2014). A GC-MS method has been used to measure melphalan and its monohydroxyethylamine and dihydroxyethylamine metabolites in spiked plasma. For melphalan, the LLOQ was 20 μg/ml (De Boeck et al., 1997). LC-tandem MS methods are also available with detection limits below 10 ng/ml (Sparidans et al., 2011). Huang and colleagues (2019) recently developed a rapid UPLC-UV analysis to measure melphalan in low volume (50 μl) plasma samples using a simplified protein precipitation as sample preparation. The internal standard was N-acetyl melphalan, and a photodiode array detector was used. The runtime was 4 minutes per sample, and the LLOQ was 100 ng/ml. The analysis is particularly suited to plasma samples from pediatric patients. TLFC coupled to heated electrospray ionization tandem MS has also been used to quantify melphalan and busulfan simultaneously without derivatization (Schofield et al., 2019). Deuterated melphalan was the internal standard, and the LLOQ of melphalan was 10 ng/ml.
Chlorambucil and its active more toxic metabolite phenylacetic acid mustard have been quantified using HPLC in patients with CLL (Silvennoinen et al., 2000). The internal standard was N, N-diethyl-m-toluamide. Plasma chlorambucil and phenylacetic acid mustard concentrations were determined using an isocratic reversed-phase HPLC process. The LLOQ was 0.01 μg/ml for both compounds.
V. Preclinical Pharmacology
Preclinical work relating to the topically important adverse effects of the nitrogen mustards pertaining to the central nervous system, kidneys, heart, bladder, and ovaries is reviewed.
A. Central Nervous System
Ifosfamide can cause life-threatening encephalopathy, the cause of which has not yet been firmly identified. It is thought to be produced by a metabolite, probably CAA. CAA is metabolized to chloroacetic acid and then to S-carboxymethylcysteine (SCMC) after conjugation with cysteine. SCMC is metabolized in turn to thiodiglycolic acid (TDGA) (Visarius et al., 1998), and both of these compounds are toxic to mouse cortical neurons (Chatton J-Y et al., 2001). SCMC can activate the alpha amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid AMPA/kainate receptor, a possible mechanism of encephalopathy. TDGA disrupts mitochondrial function in rats and is a significant urinary metabolite of ifosfamide in humans, suggesting that this could also be a cause of the encephalopathy. Chloroethylamine (CEA), another metabolite of ifosfamide, may be neurotoxic (Küpfer et al., 1996). CEA is oxidized within mitochondria via monoamine oxidase, producing CAA, and it can also conjugate with cysteine, producing thialysine, which is then metabolized to thialysine ketamine. Both CAA and thialysine ketamine can inhibit the electron binding flavoproteins in the mitochondrial respiratory chain, and thialysine ketamine can also act directly on the central nervous system.
Li and coworkers (2010) identified 18 known and 5 novel metabolites of ifosfamide and cyclophosphamide in the urine of mice using UPLC-linked electrospray ionization quadrupole time-of-flight MS. The five novel metabolites were iminoifosfamide, 4-OH-IF glucuronide, AlcP glucuronide, dechloroethyl keto cyclophosphamide, and dechloroethyl AlcP. The dominant metabolic pathway of ifosfamide was N-dechloroethylation, whereas that of cyclophosphamide was ring opening. Both SCMC and TDGA urinary excretion was increased after ifosfamide. It was postulated that precursors of TDGA may be responsible for ifosfamide-induced encephalopathy and nephropathy and that ifosfamide adverse effects may originate directly from CAA rather than SCMC and TDGA. Cyclophosphamide and ifosfamide metabolism have mainly been studied in rats and humans. The authors suggest that future investigations could be performed in genetically modified mice, allowing more detailed study of enzymes and nuclear receptors that influence their expression. Mice humanized for cyclophosphamide and ifosfamide metabolizing enzyme genes could improve modeling.
In patients, chemotherapy can cause the phenomenon of postchemotherapy cognitive impairment (“chemo-brain”) with neurocognitive deficits such as impaired learning and memory. The hippocampus is important in memory formation and spacial processing. Adult hippocampal neurogenesis is involved in the control of mood and anxiety, and when this is decreased, the likelihood of depression and anxiety increases. Cancer treatment can reduce the proliferation of neural stem cells, thereby reducing neurogenesis (Dias et al., 2014). In rats, cyclophosphamide causes a reduction in adult hippocampal neurogenesis, which may acutely impair the proliferation of cells in the subgranular zone of the adult dendate gyrus. Immediately after intravenous cyclophosphamide administration to rats (30 mg/kg every other day for seven doses), the survival of newly formed hippocampal cells is compromised, but this is not the case 7 days after the final treatment (Lyons et al., 2011). These changes lead to subtle and reversible impaired cognitive and emotional memory, but a long-term effect on spatial working memory or hippocampal proliferation does not occur. However, longer term effects were seen in mice given 200 mg/kg cyclophosphamide weekly for 4 weeks, with a decline in delayed spacial memory that persisted until the last assessment at 3 months after the first cyclophosphamide dose (Janelsins et al., 2016).
B. Kidneys
Nephrotoxicity is an important toxic effect of ifosfamide. Ifosfamide enters renal tubule cells and can be dechloroethylated to CAA. In vitro studies have shown that the minimum toxic concentration of CAA is 500 μM (Springate et al., 1999), compared with circulating levels of 0.5 to 109 μM after ifosfamide administration (Kurowski and Wagner, 1993). Therefore, the production of CAA in the target organ may be important. The local production of CAA and TDGA in human kidney are a significant cause of ifosfamide-induced nephrotoxicity. CAA causes disruption of proximal tubular cells, predominantly the S1 segment, in the kidney (Zamlauski-Tucker et al., 1994). Intracellular CAA also reduces total glutathione and causes oxidative damage. N-acetylcysteine (NAC) is a precursor in the synthesis of glutathione and also detoxifies ROS. It ameliorates ifosfamide nephrotoxicity in cell and rodent models (Hanly et al., 2012). In vitro and in vivo studies support the lack of interference of NAC with the antitumor activity of ifosfamide, and NAC has been assessed as a possible renal protective agent in the clinic.
In mitochondria, CAA inhibits the oxidation of long-chain fatty acids, which is carnitine dependent, but not that of medium-chain fatty acids, which is carnitine independent (Visarius et al., 1999). L-carnitine is a cofactor required for the transport of long-chain fatty acids from the cytoplasm to mitochondria. In isolated rat kidney mitochondria, CAA inhibits nicotinamide adenine dinucleotide (NADH):ubiquinone oxidoreductase. Oxidative phosphorylation is disrupted, with an increase in NADH and a decrease in the pyruvate dehydrogenase reaction and a consequent fall in ATP levels (Nissim et al., 2006). In rats receiving ifosfamide, an increase in serum creatinine, urea, and total nitrate/nitrite production in kidney tissues is observed. mRNA expression of inducible NO synthase is increased, and that of glutathione peroxides and catalase is decreased. The levels of caspase-9 and caspase-3 are increased, and that of bcl-2 is decreased, indicating enhanced apoptosis. L-carnitine reverses these effects (Sayed-Ahmed et al., 2012a). The intracellular concentration of carnitine within myocardial and renal cells is governed by the activity of the novel organic cation/carnitine transporter 2 (OCTN2). Cyclophosphamide and ifosfamide reduce mRNA and protein expression of OCTN2 in both renal tissue and myocardial tissue, and cyclophosphamide-induced (but intriguingly not ifosfamide-induced) expression can also be reversed by carnitine supplementation (Sayed-Ahmed et al., 2012b). A human kidney cell in vitro study has shown that ifosfamide is a substrate for OCTN2, but this is not the case for cyclophosphamide.
C. Heart
High doses of cyclophosphamide and ifosfamide can cause cardiomyopathy, and this remains a problem after the high doses of cyclophosphamide required for autologous stem cell transplantation (ASCT). An increase in ROS and an impairment of antioxidant defense processes in the myocardium are causes of acute cardiotoxicity. The molecular mechanisms of cyclophosphamide-induced cardiotoxicity have been reviewed by Iqubal and colleagues (2019). Cyclophosphamide is thought to cause direct damage to endothelial cells and cardiomyocytes. In cardiomyocytes, it increases proinflammatory and proapoptotic activities, damages the endoplasmic reticulum and mitochondria, and disturbs calcium homeostasis. It also alters the energy pool in cardiomyocytes by disrupting the heart fatty acid binding proteins, which transport long-chain fatty acids from the cytosol to the mitochondria in myocardial cells. Impairments of this system reduce ATP production, causing myocardial damage. Cyclophosphamide and its metabolites can additionally form adducts with α actin and desmin in cardiomyocytes. Cyclophosphamide causes cardiac toxicity by influencing a number of signaling pathways, particularly the toll-like receptor 4, nuclear factor κB (NF-κB), and mitogen-activated protein kinase (MAPK) pathways.
There is a need for agents to protect against cardiotoxicity. L-carnitine and NAC have been investigated as protective agents. In a rat model, ifosfamide and cyclophosphamide reduced the concentration of carnitine in myocardial cells, with a subsequent reduction of intramitochondrial CoA-SH and ATP generation and the development of severe cardiotoxicity (Sayed-Ahmed et al., 2012b). The addition of carnitine to the diet reversed the biochemical and gene expression changes apart from ifosfamide suppression of OCTN2. In other work, a rat myocardial cell line was exposed to cyclophosphamide, 4-OH-CY, CEPM, and acrolein. Pretreatment with NAC led to no differences in 4-OH-CY levels and an increase in CEPM levels but decreased acrolein concentrations. Acrolein appeared to be an important agent in the etiology of cardiotoxicity (Nishikawa et al., 2015).
D. Bladder
An important adverse effect of cyclophosphamide and ifosfamide administration is the development of hemorrhagic cystitis (HC) caused by the metabolite acrolein. This property of cyclophosphamide has led to its use in animal models of cystitis to investigate bladder conditions such as HC, interstitial cystitis, bladder-related pain, and overactive bladder (Lee et al., 2014). Cyclophosphamide reduces the micturition interval and volume and increases basal pressure and postvoid residual urine volume. Acute or chronic models are produced depending on the number and duration of cyclophosphamide exposures. The underlying mechanisms of the pathogenesis of oxazaphosphorine-induced cystitis are unclear, but animal models have provided further information.
In rats, cyclophosphamide causes severe bladder inflammation, vascular congestion, edema, and hemorrhage. The induction of oxidative stress is important. Acrolein enters the uroepithelium, causing injury, with ROS and NO overproduction (Matz and Hsieh, 2017). NF-κB and activator protein 1 are generated, which lead to the overexpression of genes encoding the proinflammatory cytokines TNF-α and IL-1β and an additional increase in ROS and NO. ROS and NO stimulate peroxynitrite synthesis, which damages the bladder further. Chemokines (chemotactic cytokines) act as nociceptive mediators. They induce the migration of leukocytes to inflamed tissues, and chemokine receptors are now implicated in the pathophysiology of cystitis (Dornelles et al., 2014). The expression of chemokines and their receptors are upregulated in rat urothelium in cyclophosphamide-induced HC. The isoenzyme cyclo-oxygenase 2 (COX-2) is also involved in the pathogenesis of acrolein-induced HC, generating prostaglandins, which cause detrusor muscle overactivity (Chuang et al., 2009). Uroplakin expression in rat bladder mucosa is decreased temporarily after cyclophosphamide administration. HC is ameliorated by mesna (sodium-2-mercapto-ethane sulfonate), which can maintain uroplakin protein integrity (Kyung et al., 2011). Cyclophosphamide increases the amount of type I collagen in the bladder of rats by increasing the activity of endogenous nerve growth factor, which in turn stimulates the MAPK and phosphoinositide-3-kinase/protein kinase B pathways. ROS generated by acrolein in the bladder also activates MAPKs. The MAPK and phosphoinositide-3-kinase/protein kinase B pathways are involved in cellular growth (Chung et al., 2010).
The better understanding of the molecular processes underlying cyclophosphamide and ifosfamide-induced HC raises the possibility of identifying novel therapeutic targets (Matz and Hsieh, 2017). The MAPKs promote the proinflammatory effect of cyclophosphamide and ifosfamide. Diallyl disulfide, a natural compound, can downregulate MAPK signaling pathways and NF-κB in rat bladder. It reduces cyclophosphamide-generated HC by inhibiting urine inducible NO synthase and COX-2 protein expression, thereby alleviating the damage caused by ROS. Proinflammatory pathways stimulated by cyclophosphamide and ifosfamide may be targeted to relieve HC. IL-4 inhibits monocyte-derived cytokines and diminishes the induction of COX-2, thereby reducing the release of prostaglandins. Intraperitoneal administration of IL-4 attenuates ifosfamide-induced edema and hemorrhage in the bladders of mice (Macedo et al., 2012). Epinephrine decreases the incidence and severity of cyclophosphamide HC in rats and is more protective than mesna (Lee et al., 2014). Intravesical administration reduces uroplakin expression, submucosal edema, and hemorrhage. Antagonists of chemokine receptor 2 (CXCR2) and of the transient receptor potential vanilloid 1 (TRPV1) channel inhibit cyclophosphamide-induced painlike behavior in rats, and CXCR2 and TRPV1 channels could be potential targets to relieve cyclophosphamide-induced HC (Dornelles et al., 2014). Targeting proinflammatory cascades and oxidation may lead to improved treatments of ifosfamide- and cyclophosphamide-induced HC, but as yet no treatment has shown a definite superiority over mesna in the clinical setting.
E. Ovaries
The nitrogen mustards can cause premature ovarian failure. It is thought that they diminish the pool of primordial follicles and immature oocytes in an agent-specific and dose-dependent fashion. Cyclophosphamide exposure decreases the number of primordial follicles by accelerated activation, as well as by direct effects such as the induction of apoptosis in granulosa cells and follicular atresia (Spears et al., 2019). PUMA, a proapoptotic protein induced by DNA damage, has been shown to have a central role in provoking apoptosis of oocytes in mice after cyclophosphamide administration. Inflammation and vascular damage have also been described. Recently, Di Emidio and coworkers (2019) demonstrated a transgenerational effect of cyclophosphamide. They treated female mice prior to conception, resulting in poor health of the offspring (F1) with postnatal growth retardation. The offspring produced oocytes with modified methylation of three imprinted genes, with a decrease in the methylation of the maternally imprinted genes Igf2r and Peg3 and an increase in the paternal H19 imprinted gene. These changes were also identified in the oocytes of the mice of the next generation (F2). Brayboy and colleagues (2013) showed the expression of MDR transporters in the oocytes of mice and humans at the germinal vesicle, meiosis I, and meiosis II stages. Blocking the MDR1 transporter makes oocytes more sensitive to the toxicity of cyclophosphamide. MDR transport may be central to the protection of oocytes and possibly for the survival of follicles. Further work has demonstrated that MDR1 is present in the oocyte mitochondrial membrane and that female mdr1a mutant mice are more susceptible to cyclophosphamide (Clark et al., 2019). Mouse oocytes can be protected from cyclophosphamide by the prior administration of the gonadotropin-releasing hormone (GnRH) agonist triptorelin (Kishk et al., 2020).
VI. Clinical Pharmacology
A. Pharmacokinetics
1. The Oxazaphosphorines
The pharmacokinetics of cyclophosphamide and ifosfamide have been reviewed by Zhang and colleagues (2006). The oral bioavailability of cyclophosphamide and ifosfamide is over 85%. Trofosfamide is the most lipophilic of the oxazaphosphorines and is only administered orally (Brinker et al., 2002). Cyclophosphamide and ifosfamide are 30% bound to plasma proteins. The majority of metabolites have been identified in the central nervous system (CNS). In one study, the median cerebrospinal fluid (CSF)-plasma ratio of 4-hydroxy ifosfamide (4-OH-IF) was 3.07 (0.62-29.12), indicating that both ifosfamide and 4-OH-IF enter the CSF but that there is a high variation in CSF 4-OH-IF levels compared with those in the plasma (Kiewe et al., 2011).
The oxazaphosphorines possess complex pharmacokinetics, and metabolism is central to their cytotoxic activity. Cyclophosphamide and ifosfamide are prodrugs and are metabolized primarily in the liver by the P450 system via 4-hydroxylation to the active nitrogen mustards. Minor sites of metabolism are the kidneys, erythrocytes, and tumors. CYP2B6 is the main enzyme responsible for the activation of cyclophosphamide, accounting for approximately 45% of the process, with CYP3A4 and CYP2C9 contributing 25% and 12% respectively. CYP2A6, CYP2C8, and CYP2C19 are involved to a lesser extent. The 4-hydroxylation of ifosfamide occurs mainly via CYP3A4, with CYP2B6 also active.
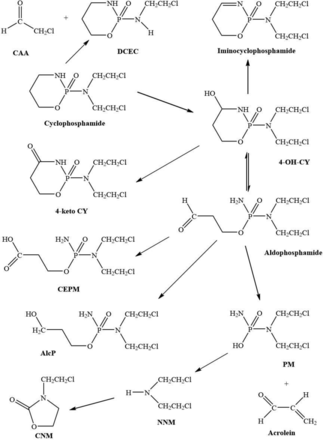
The metabolic pathways of cyclophosphamide are shown in Fig. 3. 4-OH-CY is in equilibrium with its tautomer aldophosphamide, which undergoes beta elimination to form PM, and acrolein. Ifosfamide follows the same metabolic pathways, although these are quantitatively different. The 4-hydroxylation of ifosfamide is slower than that of cyclophosphamide (Kurowski and Wagner, 1993). Activation produces 4-OH-IF, which exists in equilibrium with its tautomeric form aldoifosfamide. Aldoifosfamide can form IPM and acrolein.
The metabolism of cyclophosphamide.
The transport processes of the oxazaphosphorines and their metabolites have not been fully elucidated. The principal location of PM and IPM production and the predominant transport form in the circulation remain uncertain. Cyclophosphamide and ifosfamide are very hydrophilic with low penetration of the lipid cellular bilayer, and their corresponding mustards also cross cell membranes poorly (Zhang et al., 2005). Conversely, in vitro, 4-OH-CY and 4-OH-IF easily enter cells by passive diffusion. The active uptake and efflux of cyclophosphamide, ifosfamide, their mustard derivatives, and conjugates in hepatocytes and cancer cells is regulated by transporters such as BCRP, MRP1, MRP2, and MRP4, but the mechanisms involved are poorly defined. There is a lack of data regarding the impact of the cellular pharmacokinetics of the oxazaphosphorines on transporters and the clinical consequences. The oxazaphosphorines may modify the transporters, changing the pharmacokinetics and pharmacodynamics of their substrates. In addition, how transporters influence the anticancer activity and toxicity of the oxazaphosphorines is still not clear.
It is possible that the 4-hydroxy metabolites of cyclophosphamide and ifosfamide are transported to tumor cells, where they are converted to PM and IPM. Alternatively, PM and IPM, produced mainly in the liver, may be transported in the circulation to tumor cells, which they enter directly. There is evidence supporting both of these scenarios. IPM has been proposed as the major transport form of ifosfamide, as the activity and selectivity of extracellular IPM is comparable to that of 4-OH-IF (Struck et al., 1983). However, a review of the ratios of PM area under the plasma concentration-time curve (AUC) to 4-OH-CY AUC in the plasma of mice, rats, and humans after the administration of cyclophosphamide, as well as the sensitivities of mice and human cell cultures to PM and 4-OH-CY, concluded that 4-OH-CY was the foremost circulating metabolite in plasma, although PM was important too (Sladek, 1988). Red blood cells also have a significant role in the transport of the oxazaphosphorines. The concentrations of cyclophosphamide, ifosfamide, and their active metabolites are higher within the erythrocyte compartment than in plasma. In patients with breast cancer, the 4-OH-CY erythrocyte/plasma AUC ratio after the intravenous or oral administration of cyclophosphamide was 1.6 and 1.8 respectively (Highley et al., 1996). Furthermore, after a 6-hour infusion of ifosfamide, 77% of the whole blood AUC of IPM, calculated from the hematocrit, was found within the erythrocyte compartment (Highley et al., 1997).
The dechloroethylation pathway is important. Ten percent of cyclophosphamide is inactivated by N-dechloroethylation with the production of DCEC and CAA. However, more than 50% of ifosfamide is metabolized by side-chain dechloroethylation, mainly via CYP3A4 and CYP3A5 and to a lesser degree CYP2B6, producing 2- and 3-DCEI and CAA. With high doses of ifosfamide, the generation of these metabolites is increased as 4-hydroxylation becomes saturated (Busse et al., 1997).
Other byproducts of activation are also formed (Zhang et al., 2006). ALDH1A1 is the main enzyme oxidizing 4-OH-CY and 4-OH-IF to the carboxy derivatives. ALDH3A1 and ALDH5A1 are involved to a lesser extent. The 4-hydroxy metabolites of cyclophosphamide and ifosfamide can be metabolized by alcohol dehydrogenase (ADH) to the inactive keto metabolites. Aldophosphamide can also be oxidized by ADH and aldo-keto-reductase to produce alcophosphamide. 4-OH-CY is subject to a dehydration reaction, which is reversible, to produce iminocyclophosphamide. This can then conjugate with intracellular glutathione to produce 4-glutathionylcyclophosphamide. 4-OH-IF is inactivated to 4-thioifosfamide by conjugation with reduced glutathione. PM can be metabolized to NNM, which in the presence of bicarbonate is converted into CNM. The corresponding reactions for IPM yield CEA and 1,3-oxazolidine-2-one respectively.
Trofosfamide is metabolized in the liver via CYP3A4. The main metabolites are 4-hydroxy trofosfamide and ifosfamide, with cyclophosphamide also generated (Preiss et al., 2004). The exocyclic N-dechloroethylation of 4-hydroxy trofosfamide contributes to the production of 4-OH-IF.
Cyclophosphamide and ifosfamide are subjected to autoinduction of metabolism after an increase in CYP3A4 and CYP2B6 expression (Zhang et al., 2006). There is an increase in total clearance, decrease in elimination half-life, and increased production of the 4-hydroxy metabolites after repeated administration every 12 to 24 hours. Autoinduction usually occurs between days 1 and 5 and resolves by day 21. In a study in children, 3-day fractionated high-dose cyclophosphamide resulted in an increase in metabolism, with higher inactive metabolite AUCs on day 3 than on day 1, especially for CEPM, either because of metabolite accumulation or enzyme autoinduction (Chinnaswamy et al., 2011). Cyclophosphamide clearance increased by 88% and 125% on days 2 and 3 respectively. A small systematic difference was seen in clearance between cycles, implying that at the start of the second cyclophosphamide course the autoinduction during course 1 had been reversed. Volume of distribution (Vd) increased by 14% on days 2 and 3. After fixed doses of cyclophosphamide, systemic exposure to cyclophosphamide metabolites may vary 10-fold between patients. The mechanisms of these variations in different populations are still uncertain. Basal metabolizing enzyme activity can be affected by genetic polymorphisms, age, weight, extent of disease, diet, stress, and circadian and seasonal differences (Deeken et al., 2007).
Clinical pharmacology studies of cyclophosphamide in neonates and infants are lacking. In a study of 171 patients aged between 0.07 and 4.9 years with primary brain tumors, where plasma 4-OH-CY and CEPM levels were measured, infants younger than 6 months had higher mean 4-OH-CY concentrations than young children (Campagne et al., 2020). A cyclophosphamide dose of 1.2 g/m2 for young infants and 1.5 g/m2 for children was advised to obtain similar exposure to 4-OH-CY. In a further study of cyclophosphamide in 25 children younger than 2 years at diagnosis, exposure to CEPM, DCEC, and 4-keto CY was similar in patients above or below 10 kg (Barnett et al., 2021). However, a significant decrease in CEPM exposure was seen in patients older than 13 months compared with those aged between 7 and 12 months. The average cyclophosphamide clearance in children younger than 2 years was 46.6 ml/min/m2 compared with 30.5 ml/min/m2 in 49 cancer patients aged between 3 and 19 years reported by the same group.
Elimination of cyclophosphamide and ifosfamide is mainly metabolic, with urinary and fecal excretion of metabolites (Zhang et al., 2006). The elimination half-life of cyclophosphamide is between 3.2 and 7.6 hours, and the total body clearance is between 2.5 and 4.0 l/h/m2. The corresponding values for ifosfamide are 2.1 to 8.6 hours and 2.1 to 5.1 l/h/m2. The elimination half-life of trofosfamide is approximately 1 hour (Brinker et al., 2002). The Vd of both cyclophosphamide and ifosfamide is between 30 and 50 L. The renal clearance of cyclophosphamide is between 15 and 44 ml/min, and that of ifosfamide is 3.5 ml/min. Approximately 10% to 20% of the parent compounds are excreted unchanged in the urine.
Cyclophosphamide and ifosfamide are chiral molecules and are given as racemic mixtures. Stereochemistry is less important in the activity and toxicity of cyclophosphamide than ifosfamide (Zhang et al., 2006). The cyclophosphamide S-enantiomer is eliminated more quickly. After intravenous ifosfamide, urine and plasma contain more R-ifosfamide, suggesting that S-ifosfamide is preferentially metabolized by CYP3A4 and CYP2B6. N-dechloroethylation of S-ifosfamide is mainly catalyzed by CYP2B6, whereas R-ifosfamide dechloroethylation occurs mainly via CYP3A4 and CYP3A5.
There are only a limited number of reports of a personalized approach to cyclophosphamide dosing based on pharmacokinetics. McCune and coworkers (2009) assessed personalized cyclophosphamide doses in 50 patients receiving the cyclophosphamide and total body irradiation (TBI) conditioning regimen and allogeneic stem cell transplantation (allo-SCT). Cyclophosphamide was given on days 1 and 2. The initial dose was 45 mg/kg, and the second was adjusted to a target AUC of CEPM and 4-OH-CY. The mean second dose was 66 mg/kg (0 to 100 mg/kg). In comparison with a control group who received standard cyclophosphamide 60 mg/kg on days 1 and 2, patients treated with personalized dosing had less toxicity and similar survival.
2. Chlorambucil
Chlorambucil is rapidly absorbed from the gastrointestinal tract, with an oral bioavailability of more than 70% (Newell et al., 1983). It is 98% bound to albumin, and the Vd is 0.14 to 0.24 l/kg. It is nearly completely metabolized in the liver by monodichloroethylation and beta oxidation, forming p-N, N-bis (2-chloroethyl) amino phenylacetic acid (PHE), itself an active alkylating agent, as the main metabolite. Degradation of chlorambucil and PHE occurs in vivo, forming monohydroxy and dihydroxy derivatives. Chlorambucil reacts with glutathione to produce mono- and diglutathionyl conjugates. Silvennoinen and coworkers (2000) reported a distribution half-life for chlorambucil of 0.49 hours and an elimination half-life of 2.45 hours. The elimination half-life of PHE was longer than chlorambucil at 2.9 hours.
3. Melphalan
The absorption of oral melphalan is very variable (Samuels and Bitran, 1995). The distribution half-life is between 5 and 15 minutes, and the elimination half-life is approximately 60 minutes. Melphalan is 90% bound to plasma proteins and is transported actively into cells mainly by the L-type amino acid transporter (LAT) system. Values between 8 and 185.7 l/m2 for the Vd of melphalan have been reported. Elimination occurs via spontaneous chemical hydrolysis to inactive monohydroxy and dihydroxy metabolites and renal excretion. Studies have determined melphalan plasma clearance values of between 92 and 961 ml/min/m2, with 5% to 13% of an intravenous dose excreted in the urine. Measurements of melphalan CSF concentrations have also produced variable results. Low levels have been reported using lumbar puncture sampling, suggesting a plasma to CSF ratio of at least 100:1. However, with sampling from an intraventricular CSF reservoir, levels of up to 10% of those in plasma have been found. The clearance of melphalan is affected by creatinine clearance, fat free mass, and the hematocrit (Nath et al., 2010).
B. Pharmacogenomics
1. Influence on Pharmacokinetics
Enzymes involved in the metabolism of cyclophosphamide and ifosfamide are highly polymorphic, possessing variant alleles with diminished or absent metabolic activity. Genetic polymorphisms of genes encoding proteins governing the transport and distribution of cyclophosphamide, ifosfamide, and their metabolites are also important. Individualization of treatment may be improved with a greater understanding of pharmacogenetic factors. Polymorphisms of CYP3A4, CYP2B6, CYP2C9, ALDH1A1, ALDH3A1, GSTT1, GSTM1, GSTP1, and MRP2 are implicated. Those in CYP2B6, CYP2C9, and CYP3A4 influence the pharmacokinetics of cyclophosphamide and ifosfamide (Zhang et al., 2006). 4-OH-CY and PM are converted by GST to 4-glutathionyl cyclophosphamide and diglutathionyl PM respectively. Deletions or mutations in GSTs decrease the detoxification capability and increase exposure to nitrogen mustards. GSTP1 is the main enzyme, providing approximately 90% of the enzymatic activity of the GST family. The GSTP1 polymorphism involves an exchange of isoleucine to valine in position 105 (105Ile/Val), producing decreased enzyme activity in the heterozygous and homozygous states.
CYP2B6 and CYP2C19 are the most important enzymes involved in the 4-hydroxylation of cyclophosphamide and have been studied most widely, as reviewed by Helsby and colleagues (2019). The CYP2C19 genotype influences cyclophosphamide elimination in adult patients treated with doses ≤1000 mg/m2, with CYP2C19*2 carriers having lower elimination rate constants. In a study of 567 patients with non-Hodgkin lymphoma (NHL), there were significant independent associations of both CYP2B6 and CYP2C19*2 genotypes with the AUC of 4-OH-CY. Similarly, a study of 189 patients with systemic lupus erythematosus (SLE) described significant correlations between both CYP2C19 and CYP2B6 variants and 4-OH-CY concentrations, with the CYP2C19*2 genotype responsible for 23.6% of the difference in the 4-OH-CY level. The inactivation pathways have been less frequently studied. The production of 4-keto CY, CEPM, and DCEC have not been found to be associated with CYP2B6 or CYP2C19 variants. However, ALDH3A1 has been reported to be correlated with an increased bioactivation ratio, although in the same patients the CYP2C19 genotype was associated with a lower ratio. Overall, studies have shown evidence of a link between CYP2C19 and CYP2B6 genetic variability and the extent of production of 4-OH-CY. As well as CYP2B6, P450 oxidoreductase (POR) can influence cyclophosphamide metabolism by influencing the metabolic rate according to the P450 allele. In patients receiving intravenous cyclophosphamide as part of their conditioning regimen, the expression of the POR gene in blood is upregulated, and this is positively correlated with the 4-OH-CY to cyclophosphamide concentration ratio (El-Serafi et al., 2015). POR*28 increases P450 activity in vivo. Determining the genotype and expression levels of POR and CYP2B6 may help in personalizing treatment.
In patients with metastatic or inflammatory breast cancer treated with high-dose cyclophosphamide, 1875 mg/m2 per day given on days −6 to −4 with cisplatin and carmustine prior to ASCT, patients with the CYP3A4*1B variant had higher levels of cyclophosphamide after three doses (Petros et al., 2005). Cyclophosphamide levels were also higher with the CYP3A5*1 genotype. Patients with the CYP2C9*2 variant genotype have also been found to have a lower cyclophosphamide elimination rate and higher AUC (Balasubramanian et al., 2012). In a study of Ethiopian patients with breast cancer, those possessing the CYPA5*3 or *6 alleles had a lower elimination rate and longer half-life of cyclophosphamide compared with those with the wild-type alleles (Ahmed et al., 2020). However, in this population the clearance of cyclophosphamide was increased in carriers of CYP2C9*2 or *3. Lupus nephritis patients with the GSTA1*A mutation generate less 4-OH-CY than patients with the wild-type GSTA1 gene (Wang et al., 2015).
There have been fewer pharmacogenetic studies assessing ifosfamide. In three pediatric patients who developed ifosfamide-induced encephalopathy, the presence of CYP2B6 rs4803419 (C>T) was identified in one patient, and the other two had the CYP2B6*6 allelic variant. The CYP3A4 wild-type genotype CYP3A4*1/*1 was present in all patients. An enhanced conversion of S-ifosfamide into neurotoxic metabolites may occur with CYP2B6-deficient alleles (Duflot et al., 2018). A change to CYP2B6 S-ifosfamide activation and CYP3A4 S-ifosfamide deactivation to CAA may be stimulated by the loss of function in CYP2B6 alleles. Eighty percent of the conversion of R-ifosfamide to 4-hydroxy R-ifosfamide is via CYP3A4, and 40% of the conversion of S-ifosfamide to 4-hydroxy S-ifosfamide is via CYP2B6. In a study of 76 children treated with ifosfamide, those with the 105Ile/Val polymorphism in the GSTP1 gene had an increased urinary excretion of 2-DCEI and 3-DCEI compared with those with homozygous wild-type alleles (Zielińska et al., 2005). Those with the GSTT1 deletion had increased amounts of ifosfamide in the urine compared with those with the GSTT1 gene. A correlation was not identified between the GSTM1 or GSTT1 genotype and ifosfamide toxicity and urinary metabolite excretion.
However, despite the findings of the above studies, a comprehensive analysis of 124 Caucasian patients found that variant alleles in the CYP2B6, CYP2C9, CYP2C19, CYP3A4, CYP3A5, GSTA1, GSTP1, ALDH1A1, and ALDH3A1 genes did not account for interindividual variability in cyclophosphamide and 4-OH-CY pharmacokinetics, although carriers of at least one CYP2C19*2 allele tended to have lower inducible clearance of cyclophosphamide (Ekhart et al., 2008a). Similarly, in another study, variant alleles in the CYP2B6, CYP2C19, CYP3A5, GSTA1, GSTM1, GSTP1, and GSTT1 genes did not cause interindividual variability in cyclophosphamide and 4-OH-CY disposition (Kim et al., 2013a).
In a recent study of children aged younger than 5 years, genotypes did not influence cyclophosphamide pharmacokinetics. It was not possible to identify a targeted exposure or therapeutic window for cyclophosphamide, 4-OH-CY, or CEPM to guarantee an optimum treatment outcome and safety profile (Campagne et al., 2020). A second report of children aged younger than 2 years described a significant decrease in cyclophosphamide clearance in children with the CYP2B6*1/*6 genotype (Barnett et al., 2021).
Genetic polymorphisms of MRP2 also occur (Zhang et al., 2006). A population pharmacokinetic analysis of cyclophosphamide and 4-OH-CY in patients receiving alloSCT found that the noninduced clearance of cyclophosphamide was reduced in patients with the MRP2 1249GA heterozygous genotype, in contrast to those with the 1249GG wild-type genotype. Patients with the heterozygous MRP2 1249GA genotype may require lower doses of cyclophosphamide than those with the homozygous MRP2 1249GG wild-type genotype. The variant alleles in the MDR1 gene did not produce interindividual variability in cyclophosphamide and 4-OH-CY disposition (Kim et al., 2013a).
Polymorphisms can also influence melphalan pharmacokinetics. The amino acid transporters LAT 1 and LAT 2 are encoded by the SLC7A5 and SLC7A8 genes respectively. In one study, the rs4240803 SLC7A5 polymorphism was found to be associated with melphalan pharmacokinetic variability and significantly affected melphalan distribution within the peripheral compartment (Cho et al., 2017).
2. Influence on Response and Toxicity
Pharmacogenetic CYP2B6 and CYP2C19 variants have also been linked to response to cyclophosphamide and toxicity (Helsby et al., 2019). In a study of 119 patients with CLL receiving first-line fludarabine and cyclophosphamide, those expressing at least one *6 allele in the CYP2B6 gene were less likely to experience a complete response (CR) or CR plus nodal partial response (PR) than *1/*1 carriers. PFS was also shorter. In a multivariate analysis, the CYP2B6 variant had an independent influence on the likelihood of CR. The *6 allele therefore impaired the activation of cyclophosphamide. The variant alleles CYP2B6*5 and CYP2C19*2 reduce enzymatic activity. In a large study of 567 Chinese patients with NHL, those homozygous for CYP2C19*2 responded poorly to treatment. CYP2B6 516G>T and 785A>G homozygous patients with breast cancer have a diminished survival, whereas patients who are CYP2C19*2 homozygous have an improved survival compared with patients with wild-type alleles. In the report by Petros and coworkers (2005), discussed above, the median overall survival (OS) of patients with breast cancer treated with cyclophosphamide containing combination therapy was 1.3 years in those possessing the CYP3A4*1B variant compared with 2.7 years in those without the variant. The median OS of patients with the CYP3A5*1 polymorphism was also less.
In a study of patients with lupus nephritis, 80% of those homozygous for the wild-type allele CYP2C19*1 developed premature ovarian failure after cyclophosphamide compared with 35% of those who were either heterozygous or homozygous for the variant allele CYP2C19*2 (Takada et al., 2004). The presence of at least one CYP2C19*2 allele was associated with a lower risk. Genotyping may be useful in identifying patients likely to develop premature ovarian failure. Results from the same study also suggested that homozygosity for the variant alleles CYP2B6*5 or CYP2C19*2 may be associated with a greater likelihood of end-stage renal disease. However, in other reports of patients with lupus nephritis, CYP2B6 1459C>T expression was not correlated with a renal response to cyclophosphamide, whereas the CYP2C19*2 genotype was associated with a poor response (Helsby et al., 2019).
Ekhart and colleagues (2008b) found that after high-dose cyclophosphamide combination therapy (1000 to 1500 mg/m2 cyclophosphamide on days 1 to 4 combined with carboplatin and thiotepa), patients heterozygous for the ALDH1A1*2 polymorphism have a higher incidence of liver toxicity compared with those possessing the wild-type alleles (46% vs. 19%) and those heterozygous for the ALDH3A1*2 polymorphism have a higher incidence of HC compared with patients with wild-type alleles (13% vs. 1.4%). The authors postulate that high doses of chemotherapeutic agents may deplete glutathione, rendering the effect of the polymorphisms of GST genes less evident. GST and ALDH enzymes are located mainly intracellularly. Variations in susceptibility to toxicity may therefore occur on account of differences in levels of intracellular detoxification of cyclophosphamide metabolites, rather than through variations in cyclophosphamide plasma pharmacokinetics.
GST polymorphisms have also been formally investigated. In patients with breast cancer treated with chemotherapy, 95% of whom received cyclophosphamide, those with the low activity 105Val/Val GSTP1 variant survived longer (Sweeney et al., 2000). A retrospective study of 70 patients with proliferative lupus nephritis treated with cyclophosphamide showed that the 105Ile/Val GSTP1 genotype was an independent predictor of poor renal outcome and that the GSTM1 null genotype was the only factor to affect the incidence of adverse effects (Audemard-Verger et al., 2016). Conversely, in a study of 33 patients with a variety of autoimmune conditions treated with cyclophosphamide, those with the 105Ile/Val GSTP1 variant had a higher response rate (Hajdinák et al., 2020). In another study, the 105Ile/Val GSTP1 and the 105Val/Val GSTP1 genotypes increased the risk of short-term toxicity with pulsed high-dose cyclophosphamide in patients with SLE (Zhong et al., 2006). Gong and coworkers (2021) performed a meta-analysis incorporating 13 reports assessing the relationship between the GSTP1 105Ile/Val polymorphism and cyclophosphamide toxicity. Just three of these reports used single-agent cyclophosphamide. The study population comprised 1590 patients with malignancies and 332 patients with nonmalignant conditions. Only gastrointestinal toxicity and infection were associated with the GSTP1 105Ile/Val polymorphism in the whole group of patients; a similar finding was seen in patients with SLE and lupus nephritis but not in those with malignancies. The polymorphism was also associated with myelosuppression in patients with SLE and lupus nephritis. Wang and coworkers (2015) reported that lupus nephritis patients with the GSTA1*A mutation have a lower remission rate after cyclophosphamide administration than patients with the wild-type GSTA1 gene (20% vs. 44%). More recently, Attia and colleagues (2021) studied the association between the GSTA1 (-69C>T rs3957356) single nucleotide polymorphism and response rate and toxicity of intravenous cyclophosphamide in 94 patients with lupus nephritis. Those with the wild-type GSTA1 had the highest probability of a complete renal response, whereas those with the homozygous GSTA1 (-69C>T, rs3957356) TT genotype had the lowest chance of response and were more likely to experience toxicity. In children with steroid-sensitive nephrotic syndrome treated with cyclophosphamide, the GSTM1 null polymorphism led to a more prolonged remission compared with the heterozygous or homozygous GSTM1 wild-type (Vester et al., 2005). A decreased rate of sustained remission was seen in patients with the GSTP1 heterozygous or homozygous polymorphisms compared with those with the homozygous wild-type. The GSTT1 genotype did not influence outcome. Those with both the GSTM1 null and GSTP1 wild-type had a 50% relapse rate compared with 94% in those without.
Single nucleotide polymorphisms in drug metabolism, DNA repair, apoptosis, and cell cycle regulation genes are associated with outcome and mucositis after high-dose melphalan (HDM) (Shaw et al., 2014). However, there are only a small number of pharmacogenomic studies of melphalan. In a study of patients with multiple myeloma treated with HDM and ASCT, the single nucleotide polymorphism rs4240803 in SLC7A5 was associated with the need for total parenteral nutrition (Giglia et al., 2014). Variability in melphalan transport appeared to influence the extent of mucosal injury. Conversely, Kühne and colleagues (2007) did not find an association between the genes encoding LAT 1, LAT 2, and 4F2 cell surface antigen heavy chain (4F2hc), which forms a heteromeric amino acid transporter complex with LAT 1 and gastrointestinal toxicity after HDM. The impact of rs4240803 on SLC7A5 expression in 108 patients with multiple myeloma treated with ASCT has been assessed (Poi et al., 2019). Fifty-three patients had the wild-type genotype GG, 46 patients were heterozygous GA carriers, and 9 patients possessed the homozygous AA variant genotype. There was a significant association between rs4240803 and elevated SLC7A5 mRNA expression in peripheral blood mononuclear cells, which were consequently sensitized to melphalan. Rs4240803 was also associated with a better 90-day response. Melphalan is conjugated by GSTs. In 84 patients treated with melphalan doses between 10 and 140 mg/m2, the GSTT1 genotype and the GSTP1 105Ile/Val polymorphism were possibly predictive of diarrhea and mucositis respectively, and the authors suggested that confirmation was needed (Kühne et al., 2008). However, in a study of 35 patients treated with 140 or 200 mg/m2 melphalan and ASCT for multiple myeloma, patients with the wild-type GSTP1 polymorphism had more gastrointestinal toxicity and infections (Nampoothiri et al., 2019).
In summary, pharmacogenomics has a substantial role in the prediction of drug responses, and attempts have been made to develop rational methods to optimize treatment with the nitrogen mustards. Most work has focused mainly on the metabolism of cyclophosphamide, with indications that polymorphisms in CYP2B6 and CYP2C19 influence response and toxicity, but the multiple metabolic pathways of cyclophosphamide have complicated pharmacogenomic studies. The expectation has been that an improved understanding of the pharmacogenetic factors affecting the variation in response and toxicity to the nitrogen mustards would lead to personalized treatment, but inconsistent evidence in the literature currently precludes specific recommendations. The significance of genetic variants within genes coding for DNA repair proteins, including MGMT and transport proteins such as MRP, has not been extensively assessed. Furthermore, most studies have been performed in patients receiving combination chemotherapies, with the result that variants specific to the action of the nitrogen mustards can be obscured.
C. Pharmacodynamics
Myelosuppression is a dose-limiting toxicity (DLT) of both cyclophosphamide and ifosfamide, and cardiac toxicity is dose limiting after the high doses of cyclophosphamide required before SCT. Nausea is common with these agents, and alopecia occurs in almost all patients. HC remains a problem with high-dose cyclophosphamide. Neurotoxicity can be an issue after ifosfamide administration, and nephrotoxicity is a concern in pediatric patients. The oral formulation of trofosfamide is well tolerated when given continuously at a low dose of 150 mg daily. The main side effect is myelotoxicity, and it is less urotoxic than the other oxazaphosphorines. Myelosuppression is the DLT of melphalan, but when administered in conjunction with stem cell support, mucositis is dose limiting (Bayraktar et al., 2013). Other toxic effects of melphalan are nausea, diarrhea, alopecia, transaminitis, interstitial pneumonitis, and cardiac arrhythmias. Myelosuppression, nausea, mouth ulcers, and diarrhea are common toxicities of chlorambucil. The central nervous system toxicity, nephrotoxicity, cardiac toxicity, bladder toxicity, and gonadal toxicity of the nitrogen mustards and the risk of second malignancies need to be highlighted.
1. Central Nervous System Toxicity
Ifosfamide can cause encephalopathy. The incidence after intravenous infusion is 10% but increases to 50% when ifosfamide is given orally (Ajithkumar et al., 2007). Confusion is the prime symptom, but somnolence, seizures, cranial nerve abnormalities, weakness, cerebellar dysfunction, and psychiatric disturbances have also been described, and fatalities have occurred. Encephalopathy develops between 12 and 146 hours after starting an intravenous infusion and is usually self-limiting, resolving within 12 to 72 hours. Accepted risk factors are liver impairment, poor performance status, previous or concomitant cisplatin, CNS disease, and advanced age. A higher total dose of ifosfamide and a shorter infusion time also increase the likelihood of encephalopathy. Methylene blue is used to prevent and treat the encephalopathy. This compound functions as an alternative electron acceptor, replacing the nonfunctioning flavoproteins, with restoration of the mitochondrial respiratory chain. Intravenous thiamine infusions have also been advocated.
2. Nephrotoxicity
Nephrotoxicity is the main adverse effect in children treated with ifosfamide, with an incidence of approximately 30% (Hanly et al., 2013). Renal glomerular and tubular damage occurs, and Fanconi syndrome, which is characterized by type II proximal renal tubular dysfunction, may develop with a loss of phosphate, glucose, amino acids, and low molecular weight proteins from the renal tubules. Fanconi syndrome occurs in 1.4% to 5% of children but much less often in adults, and it can impair growth in children and lead to bone pathology in adults. Glomerular dysfunction is seen less frequently. The risk of ifosfamide-induced nephrotoxicity is associated with higher cumulative doses, a younger age at administration, preexisting renal dysfunction, exposure to other nephrotoxic drugs, and cisplatin treatment. After the in vitro and in vivo work outlined earlier, the use of NAC to treat children in whom renal function deteriorates while receiving ifosfamide has been proposed (Hanly et al., 2013). In a study of adults, a review of 5-year data showed a mean decrease in estimated glomerular filtration rate after the first cycle of ifosfamide of 15 ml/min/1.73 m2. The decline was subsequently slower but progressive (Farry et al., 2012).
3. Cardiac Toxicity
High-dose cyclophosphamide can cause cardiovascular toxicity. In the literature, incidence rates between 7% and 28% have been reported, with mortality rates ranging from 11% to 43% after doses of 170 to 180 mg/kg (Iqubal et al., 2019). In one retrospective analysis of 80 patients receiving cyclophosphamide 50 mg/kg per day for 4 days as a preparation for marrow grafting, 14 developed cardiotoxicity within 10 days of receiving the first dose of cyclophosphamide. Six of these patients died from congestive heart failure. After correcting for body surface area, it was found that cardiotoxicity was significantly more likely to occur with cyclophosphamide doses ≥1.55 g/m2 per day (Goldberg et al., 1986). In another study, 6 of 19 women with metastatic breast cancer receiving a continuous 96-hour infusion of 6 g/m2 cyclophosphamide, thiotepa, and carboplatin developed moderate transient congestive heart failure. These patients had a lower cyclophosphamide AUC than those who did not develop this complication (Ayash et al., 1992). Acute decompensating cardiomyopathy, which is usually irreversible, has also been described after the administration of cyclophosphamide (Lee et al., 1996). Melphalan has also been reported to cause cardiomyopathy. A retrospective review of patients with multiple myeloma or amyloidosis treated with ASCT between 1989 and 2009 found that after HDM, 5.4% of patients with amyloidosis and 1.6% of those with multiple myeloma developed cardiomyopathy (Bleeker et al., 2011). Another retrospective review showed that atrial arrythmias, primarily atrial fibrillation, occurred with 5% of chemotherapy treatments used with SCT; melphalan was the most arrhythmogenic, causing atrial arrythmias in 11% of patients (Feliz et al., 2011).
4. Bladder Toxicity
HC caused by the oxazaphosphorines continues to be an important clinical problem. It is more frequently seen with ifosfamide but is also a particular issue after high-dose cyclophosphamide in conjunction with SCT. Mesna, hyperhydration, and bladder irrigation are most frequently employed to prevent treatment-related HC (Payne et al., 2013). Mesna is a thiol that protects against the urotoxicity by binding acrolein in the bladder. After the concomitant use of mesna, macrohematuria occurs now in fewer than 5% of patients after standard doses of cyclophosphamide and ifosfamide. However, HC can occur days or months after high-dose cyclophosphamide in 20% to 25% of patients. Early-onset HC usually commences 48 to 72 hours after administration, with reported incidences ranging between less than 10% and 35%. Interestingly, in a multivariate analysis, mesna and bladder irrigation actually increased early-onset HC after SCT. The reactivation of viruses, mainly polyomaviruses, following immunosuppression after high-dose cyclophosphamide, is contributory to the hematuria after grafting (Padilla-Fernandez et al., 2014).
5. Gonadal Toxicity
The late effects of cytotoxic treatment are becoming increasingly important as survival rates improve. Infertility is one such issue. Nitrogen mustards can cause gonadal dysfunction in males and females, with the former being more sensitive. There is an inverse correlation between the cumulative dose of cyclophosphamide and gonadal toxicity in males treated in childhood, with a low risk of sterility with doses below 300 mg/kg (Watson et al., 1985). Cyclophosphamide can cause premature ovarian failure and causes a reduction in healthy oocytes (Hickman and Gordon, 2011). Thirty to fifty percent of women with SLE treated with monthly intravenous cyclophosphamide develop ovarian failure. Both the cumulative and absolute doses are important, as is the age of the patient. Females are more likely to remain fertile if cyclophosphamide is given to those younger than 30 years, as a shorter intravenous pulse regimen (≤6 months), at a lower cumulative dose (<7 g), and if amenorrhea is not present before or during the cyclophosphamide treatment. In an analysis of the Wegener’s granulomatosis (now called granulomatosis with polyangiitis) Etanercept trial, most women given a short course of daily 2 mg/kg oral cyclophosphamide for 3 to 6 months had ovarian damage (Clowse et al., 2011). Large randomized controlled trials have shown that GnRH agonists decrease the prevalence of premature ovarian insufficiency in women with breast cancer treated with chemotherapy, although the effect is small (Spears et al., 2019). The use of GnRH agonists during chemotherapy is now a recognized option.
6. Second Malignancies
The nitrogen mustards are the most carcinogenic of the alkylating agents. The level of germ line mutations in the mouse induced by chlorambucil is higher than for any other agent, including X-rays (Povirk and Shuker, 1994). Melphalan is also very toxic in this respect, but cyclophosphamide is less so. The relative risk of a second malignancy increases from 1.6 for cyclophosphamide doses of 1 to 4.3 g/m2 to 7.4 for doses above 13 g/m2 in long-term survivors of childhood cancer (Hawkins et al., 1987). Acute myelogenous leukemia (AML) is the most common malignancy associated with the use of the nitrogen mustards (Povirk and Shuker, 1994). An analysis of 293 granulomatosis with polyangiitis patients treated with cyclophosphamide found a standardized incidence ratio of cancer of 2.1, with a significantly increased standardized incidence ratio for AML, bladder cancer, and nonmelanoma skin cancer (Faurschou et al., 2008). Leukemia and bladder cancers occurred 6.9 to 18.5 years after the commencement of cyclophosphamide. A high risk of developing AML and bladder cancer was seen in patients treated with a cumulative dose exceeding 36 g (100 mg per day for more than 1 year). Another study of 1065 patients with granulomatosis with polyangiitis also found a dose-response relationship, as the risk of bladder cancer doubled for every 10 g increase in cyclophosphamide dose (Knight et al., 2004). Treatment with cyclophosphamide for longer than 1 year was associated with an 8-fold increased risk of developing bladder cancer. Sixteen years after a diagnosis of granulomatosis with polyangiitis, the absolute risk of bladder cancer was 10%. AML is commonly preceded by myelodysplastic syndrome. Deletions of all or part of one copy of chromosomes 5 and 7 are often seen. In a nested case control study of patients treated for NHL, a higher risk of leukemia was observed in patients given a cumulative dose of cyclophosphamide greater than 11.25 g/m2 (Xu et al., 2013). After ASCT with melphalan containing regimens, the risk of secondary leukemia or myelodysplastic syndrome may be as high as 7% (Bayraktar et al., 2013).
VII. The Clinical Use of the Nitrogen Mustards
A. Oncologic Diseases
Cyclophosphamide is used in the treatment of breast cancer, rhabdomyosarcoma (RMS), Ewing sarcoma, thymic tumors, gestational trophoblastic neoplasia, and neuroblastoma, whereas ifosfamide has a place in the management of soft tissue sarcoma (STS), osteosarcoma, germ cell tumors, and thymic tumors. Trofosfamide has efficacy in STS but is not used routinely (Wagner et al., 1997). Melphalan is employed in the treatment of retinoblastoma and in regional therapy. The use of the nitrogen mustards in these diseases has not been superseded by targeted agents or immunotherapies.
1. Breast Cancer
The mortality rate from breast cancer has decreased over recent decades, mainly due to the development and introduction of effective adjuvant therapy. In 1976, Bonadonna and colleagues described the use of cyclophosphamide, methotrexate, and 5-fluorouracil (5-FU) (CMF), which became the standard adjuvant treatment of premenopausal women with node-positive breast cancer. Long-term results, published in 1995, confirmed the increased disease-free survival (DFS) and OS (Bonadonna et al., 1995). Combination chemotherapy including cyclophosphamide is now central to the standard adjuvant therapy of node-positive breast cancer. Systemic chemotherapy containing cyclophosphamide also leads to a significant decrease in the risk of disease recurrence and death in patients with hormone-negative breast cancer. Furthermore, single-agent oral cyclophosphamide reduces the risk of recurrence and mortality in high-risk premenopausal patients with early breast cancer, and the benefits are comparable to those seen with classic CMF (Ejlertsen et al., 2010). Subsequent work therefore focused on adding anthracyclines, and later taxanes, to cyclophosphamide. In the 1980s, trials were performed comparing CMF (for 6 to 12 months) with 6 months of anthracycline-based chemotherapy such as 5-FU, doxorubicin, and cyclophosphamide (FAC) or 5-FU, epirubicin, and cyclophosphamide (FEC). In the 1990s, the standard adjuvant chemotherapy became six cycles of an anthracycline- and cyclophosphamide-containing regimen (Hernandez-Aya and Gonzalez-Angulo, 2013).
A meta-analysis performed by the Early Breast Cancer Trialists Collaborative Group (EBCTCG) of outcomes in 100,000 women with early breast cancer, from more than 100 trials, confirmed the benefit of adjuvant CMF- and anthracycline-containing regimens. Standard CMF and standard four cycles of doxorubicin and cyclophosphamide (AC) are equivalent, with both treatments decreasing the 2-year recurrence rates by half, reducing the recurrence rates over the next 8 years by a third, and lowering breast cancer mortality rates by 20% to 25% (Peto et al., 2012). In a study of 1669 postmenopausal patients with operable lymph node–negative breast cancer randomized to CMF followed by tamoxifen or tamoxifen alone, CMF was beneficial in patients with estrogen-negative disease (13-year DFS of 73% vs. 57%) but was not advantageous in the estrogen-positive group (Aebi et al., 2011). After 7 years of follow-up, docetaxel and cyclophosphamide has been shown to produce a longer DFS than AC (81% vs. 75%) and also a greater OS (87% vs. 82%) (Jones et al., 2009). Two commonly used regimens for the neoadjuvant and adjuvant treatment of patients with breast cancer are docetaxel and cyclophosphamide and sequential AC and paclitaxel. Other cyclophosphamide-containing regimens employed are EC (epirubicin and cyclophosphamide), FEC, FAC, TAC (docetaxel, doxorubicin, and cyclophosphamide), and CMF. Cyclophosphamide-containing combination regimens often used in metastatic disease are FAC, FEC, AC, EC, and CMF.
2. Soft Tissue Sarcoma
Ifosfamide and doxorubicin have been used in the treatment of STS for over 30 years and remain the most active agents in advanced disease despite the introduction of targeted treatments. Single-agent ifosfamide produces response rates of 10% to 30%. Ifosfamide has a dose-response relationship; the response rate is higher using 9 g/m2 fractionated over 3 days than with 5 g/m2 administered over 24 hours (Judson et al., 2014). A randomized phase 3 trial of 75 mg/m2 doxorubicin with or without 10 g/m2 ifosfamide over 4 days, in the first-line treatment of patients with locally advanced, unresectable, or metastatic high-grade STS, detected no significant difference in the median OS, which was 12.8 months in the doxorubicin group and 14.3 months in the doxorubicin and ifosfamide group (Judson et al., 2014). However, the median PFS was significantly longer in the combination group than in the doxorubicin alone group (7.4 vs. 4.6 months). Patients with a poor performance status and high-grade tumors derived the most benefit. The OR was significantly higher at 26% in the combination group compared with 14% in those receiving doxorubicin alone, but the combination was markedly more toxic, with a higher incidence of grade 3 and 4 adverse events, particularly myelosuppression. There was more nausea and vomiting with the combination treatment, which also caused encephalopathy in 6% of patients. The combination is best used in the neoadjuvant setting or to relieve acute symptoms, whereas sequential doxorubicin and ifosfamide can be employed to control asymptomatic metastatic disease. Chemotherapy for STS is evolving according to histologic subtypes. Ifosfamide is particularly active in synovial sarcoma but less active in leiomyosarcoma (Judson et al., 2014). There has been recent interest in the use of trofosfamide. In a randomized phase 2 trial, 120 previously untreated patients with STS, older than 60 years, were treated with either six cycles of 60 mg/m2 doxorubicin every 3 weeks or 300 mg oral trofosfamide daily for 7 days, then 150 mg daily continuously (Hartmann et al., 2020). PFS was 4.3 and 2.8 months in the doxorubicin and trofosfamide arms respectively, with corresponding OS durations of 9.8 and 12.3 months. Trofosfamide may have activity comparable to doxorubicin when given with palliative intent. Less toxicity was observed with trofosfamide, which may be an alternative to doxorubicin in elderly or unfit patients.
Although adjuvant and neoadjuvant chemotherapy is established in pediatric sarcomas, usage in adult sarcomas is still debated. A randomized trial assessing three 3-week cycles of doxorubicin and ifosfamide preoperatively, or surgery alone, in patients with high-risk STS found that the 5-year DFS rates were not significantly different at 56% with chemotherapy compared with 52% without (Gortzak et al., 2001). Respective values for 5-year OS were 65% and 64%. However, a retrospective analysis of neoadjuvant ifosfamide and doxorubicin in 74 patients with high-grade STS of the extremities found a significant improvement in the disease-specific survival rate compared with 282 patients treated with surgery alone (Grobmyer et al., 2004). The benefit of chemotherapy was observed in patients with tumors larger than 10 cm, with a 3-year disease-specific survival of 83%, compared with 62% in patients treated with surgery alone. In a phase 3 trial of neoadjuvant treatment of high-risk STS, three cycles of full-dose epirubicin and ifosfamide were compared with histology-tailored chemotherapy, including high-dose ifosfamide alone for synovial sarcoma and etoposide plus ifosfamide for peripheral nerve sheath tumors (Gronchi et al., 2017). The DFS was lower after histology-tailored chemotherapy in both of these groups, although this may reflect a suboptimal number of chemotherapy cycles.
Adjuvant trials for STS performed in the 1970s and 1980s used single-agent doxorubicin and were mainly negative, but in the late 1980s and 1990s ifosfamide was added to anthracyclines, with an improvement in results. The Italian cooperative group assessed the combination of epirubicin and ifosfamide as adjuvant treatment of high-risk STS of the extremities or girdles and found an estimated 5-year OS of 66.0% in the chemotherapy group and 46.1% in the observation group (Frustaci et al., 2003). In 2008, Pervaiz and coworkers (2008) updated a meta-analysis performed by the Sarcoma Meta-analysis Collaboration in 1997, with the addition of four further trials, all of which included ifosfamide. Chemotherapy provided a marginal improvement in recurrence rates. Doxorubicin as a single agent did not impact on survival, whereas a benefit was identified with the combination of ifosfamide and doxorubicin, which resulted in a risk reduction in death from 41% to 30%. In a further randomized trial of five cycles of adjuvant doxorubicin 75 mg/m2 and ifosfamide 5 g/m2 every 3 weeks compared with observation, OS was the same in both groups (Woll et al., 2012). The 5-year survival rate was 66.5% after chemotherapy and 67.8% in the control group. There was also no difference in DFS. An updated meta-analysis including this study suggested a survival benefit for chemotherapy.
Cyclophosphamide and ifosfamide have been important in extending the survival of children and young adults with STS. RMS occurs mainly, but not exclusively, in children. More than 50% of patients with RMS are of intermediate risk. Systemic therapy for RMS involves intensive chemotherapy regimens containing oxazaphosphorines given over 6 to 9 months. In North America the standard treatment of over 45 years has been vincristine, actinomycin D, and cyclophosphamide (VAC), whereas in Europe it is ifosfamide, vincristine, and actinomycin D (IVA) (Hawkins et al., 2014). VAC is used routinely in patients with intermediate- or high-risk nonmetastatic RMS. Recent work has focused on adjusting the dose of cyclophosphamide in lower risk patients, without affecting efficacy, to reduce toxicity. Toxicity is worse in patients younger than 1 year or older than 10 years. Ifosfamide has similar activity to cyclophosphamide; when used in the first 28 weeks of treatment in place of cyclophosphamide in VAC, outcomes are equivalent (Crist et al., 2001). Cycles of vincristine, doxorubicin, and cyclophosphamide alternating with cycles of etoposide and ifosfamide (VDC/IE) are also active in patients with intermediate-risk RMS (Arndt et al., 2008). Trofosfamide is currently being assessed. NCT00876031 is a randomized study in children, adolescents, and young adults under 21 years of age with localized high-risk RMS and RMS-like STS, comparing 25 weeks of oral maintenance chemotherapy comprising etoposide, idarubicin, and trofosfamide, with no maintenance chemotherapy, after standard IVA therapy. The end points are event-free survival and OS, and the date of expected study completion is July 2024. The combination of cyclophosphamide and topotecan has been used in the treatment of recurrent and refractory RMS, with an overall response (OR) of 66% (Saylors et al., 2001).
3. Osteosarcoma
The introduction of systemic chemotherapy in osteosarcoma has increased cure rates from less than 20% to between 60% and 70% (Link et al., 1986). The combination of high-dose methotrexate, doxorubicin, and cisplatin (MAP) is active in osteosarcoma and is extensively used in the neoadjuvant and adjuvant settings. The European and American Osteosarcoma Study Group (EURAMOS) assessed the addition of high-dose ifosfamide and etoposide to MAP in the adjuvant setting in high-grade osteosarcoma patients with low levels of tumor necrosis after neoadjuvant MAP; event-free survival was not improved, and toxicity was increased (Marina et al., 2016). A randomized comparison of MAP versus MAP plus ifosfamide in the neoadjuvant setting, with adjuvant ifosfamide administered to those given it neoadjuvantly or to those with a poor histologic response, failed to show a difference in outcome (Ferrari et al., 2012). The combination of cisplatin, ifosfamide, and epirubicin has also been used in the perioperative treatment of osteosarcoma, with three cycles given preoperatively and three cycles given postoperatively (Basaran et al., 2007). Histologic responses were seen in 63% of patients at surgery. The 5-year DFS and OS rates were 41.9% and 48.2% respectively. The methotrexate, etoposide, and ifosfamide (M-EI) combination has also been employed in the preoperative setting, with adjuvant M-EI given to patients with a good histologic response and adjuvant MAP given to those with a poor histologic response or residual metastatic or unresectable disease (Gaspar et al., 2018). In a study of 409 patients younger than 25 years, survival rates were comparable to those obtained with the MAP regimen. The 5-year event-free survival was 56%, and the OS was 71%.
In the metastatic setting, single-agent ifosfamide has a response rate of 33% (Anninga et al., 2011). High-dose ifosfamide, with or without etoposide, appears to improve event-free survival. In patients aged between 7 and 22 years given 3.5 g/m2 of ifosfamide daily for 5 days and etoposide 100 mg/m2 daily for 5 days, an OR of 59% was seen (Goorin et al., 2002). The 2-year PFS and survival rates were 43% and 55% respectively. However, myelosuppression and renal toxicity were of concern. In patients with relapsed or refractory osteosarcoma, high-dose ifosfamide (3 g/m2 per day for 4 days) plus etoposide has an OR of 48% (Gentet et al., 1997), whereas two courses of cyclophosphamide plus etoposide have been shown to have a 19% OR (Berger et al., 2009).
Ewing sarcoma is seen predominantly in adolescents and young adults. It is a high-grade malignancy of bones or soft tissues. Micrometastases are common at presentation, and therefore after local treatment chemotherapy is also needed. Prior to the use of systemic chemotherapy, the cure rate of patients with Ewing sarcoma was less than 10%, but a 5-year OS of approximately 70% can now be achieved (Womer et al., 2012). However, in patients presenting with metastatic disease, the estimated event-free survival is 22% to 30% at 5 years, despite the use of combination regimens. Younger patients are usually treated with vincristine, doxorubicin, and cyclophosphamide alternating with etoposide and ifosfamide (VDC/IE), with chemotherapy cycles every 2 weeks. In a study of localized Ewing sarcoma, chemotherapy given every 2 weeks was more effective than every 3 weeks, without an increase in toxicity (Womer et al., 2012). Patients received 14 cycles, and the doses of cyclophosphamide and ifosfamide were 1.2 and 9 g/m2 respectively. The event-free survival was 65% in the 3-week arm and 73% in the intensified arm at a median of 5 years. Vincristine, ifosfamide, doxorubicin, and etoposide (VIDE) are also used, whereas vincristine, ifosfamide, and doxorubicin (VID) are sometimes employed in older patients. In patients who relapse, there is no standard salvage treatment (Van Mater and Wagner, 2019). Nitrogen mustards and topoisomerase I inhibitors are synergistic. Topotecan may prevent the repair of cyclophosphamide-induced DNA damage, and cyclophosphamide combined with topotecan has produced response rates of 23% to 35%, with a median duration of response of approximately 9 months. Single-agent ifosfamide has a response rate of 34%. The rEECur study is being performed by the Euro Ewing Consortium to compare four salvage chemotherapy regimens in patients with recurrent and primary refractory Ewing sarcoma. Patients were initially randomized to topotecan and cyclophosphamide (250 mg/m2 per day for 5 days) (TC), irinotecan and temozolomide (IT), gemcitabine and docetaxel (GD), or high-dose ifosfamide (3 g/m2 per day for 5 days). At the first interim analysis, patients treated with GD had an inferior OR rate and PFS, and recruitment to this arm was discontinued. A second interim evaluation found IT to be inferior to TC and high-dose ifosfamide, and the study is continuing to recruit to the TC and high-dose ifosfamide arms only (McCabe et al., 2020). Cyclophosphamide is also being assessed in combination with nanoliposomal irinotecan in pediatric solid tumors, including osteosarcoma (NCT02013336).
4. Germ Cell Tumors and Gestational Trophoblastic Neoplasia
Paclitaxel, ifosfamide, and cisplatin (TIP) is a well established treatment combination for patients with relapsed germ cell tumors. In the second-line setting, four cycles of TIP produce a durable CR, with a 2-year PFS of 65% (Kondagunta et al., 2005). Vinblastine, ifosfamide, and cisplatin (VeIP) is also used. Ifosfamide is included in the high-dose TI-CE (paclitaxel, ifosfamide, carboplatin, and etoposide) regimen for patients with poor prognostic features (Feldman et al., 2010). The additive nephrotoxicity of ifosfamide and cisplatin restricts its use in young children with mixed germ cell tumors, but the combination of cyclophosphamide, cisplatin, etoposide, and bleomycin is feasible (Malogolowkin et al., 2013). Cyclophosphamide is employed in the treatment of gestational trophoblastic neoplasia as part of the EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, and vincristine) regimen (Alifrangis et al., 2013). It is the most common first-line combination for high-risk patients. Etoposide, ifosfamide, and cisplatin (VIP); ifosfamide, carboplatin, and etoposide (ICE); and TIP are also used.
5. Thymic Tumors
In patients with unresectable advanced thymomas, single-agent chemotherapy regimens and platinum-based combinations are used (Rajan and Giaccone, 2011). Single-agent ifosfamide administered every 3 weeks is associated with a CR of 38.5% and a PR of 7.7% in invasive thymoma, with a median duration of CR of 66+ months and estimated 5-year survival of 57%. Cisplatin, doxorubicin, and cyclophosphamide (PAC) produced an OR of 50% and median OS of 37.7 months in 29 patients with thymoma and one patient with thymic carcinoma. Doxorubicin, cisplatin, vincristine, and cyclophosphamide (ADOC) generated an OR of 92% and a CR of 43% in patients with invasive thymoma. The median duration of response and median OS was 12 and 15 months respectively. The administration of etoposide, ifosfamide, and cisplatin (VIP) to patients with thymoma and thymic carcinoma resulted in a PR of 32%, a median duration of response of 12 months, and a 2-year survival of 70%. Thymic carcinomas do not respond well to chemotherapy, but the ADOC regimen has activity. Single-agent ifosfamide is used as a second-line treatment of both thymoma and thymic carcinoma.
6. Retinoblastoma and Neuroblastoma
Retinoblastoma is a rare retinal tumor of childhood. Melphalan, either alone or in combination with topotecan or carboplatin, is used in intra-arterial chemotherapy as a pulsatile infusion over 30 minutes (Dimaras et al., 2015). The intravitreal injection of melphalan, with or without topotecan, is employed as adjuvant therapy directed at retinoblastoma vitreous seeds once the source of the seeds has been treated. Cyclophosphamide is used in the treatment of neuroblastoma, also a malignancy of childhood, usually in combination with topotecan. A phase 2 randomized study of topotecan plus cyclophosphamide compared with topotecan alone in recurrent or refractory neuroblastoma showed a longer PFS with the combination, but there was no survival advantage (London et al., 2010).
7. Regional Therapy
Regional therapy allows the administration of much higher doses of melphalan. In isolated limb perfusion (ILP), the circulation of the limb is separated from the systemic circulation and connected to an extracorporeal system. Chemotherapy is then administered through the perfusion circuit, sometimes under hyperthermic conditions. A description of ILP, using mechlorethamine or melphalan, to treat melanoma and sarcoma of the extremities, was first published in 1958 (Creech et al., 1958). Melphalan is most commonly used for the ILP of melanoma, often combined with actinomycin D or TNF-α, producing high concentrations in a limb with decreased systemic toxicity. Normal tissues in the extremities can withstand 20 times the concentration of melphalan tolerated by other tissues (Nieweg and Kroon, 2014). Combining melphalan with TNF-α improves response in bulky tumor nodules. In rats, TNF-α increases the uptake of melphalan into tumors by a factor of six (de Wilt et al., 2000). A systematic review of mainly retrospective studies found a median CR of 46.5% with single-agent melphalan, 45% to 65% with melphalan and actinomycin D, and up to 68.9% with melphalan plus TNF-α in patients with unresectable stage IIIB-IIIC metastatic melanoma of the limbs (Moreno-Ramirez et al., 2010).
Initial attempts to use melphalan as ILP for sarcoma were unsuccessful, but the addition of TNF-α to melphalan-based treatments increased activity. In a study of 231 TNF-alpha and melphalan-based isolated limb perfusion (TM-ILP) treatments in 208 patients with locally advanced extremity STS, TM-ILP with 10 to 13 mg/l of limb volume melphalan was performed under mild hyperthermic conditions (Deroose et al., 2011). The CR was 18%, the PR was 53%, and the limb salvage rate was 81%. In systematic reviews, limb preservation rates of 72% to 96% have been described, with aggregate values for OR of 72% to 82.5%, for CR of 22% to 31%, and for PR of 45.8% to 53.5% (Martin-Tellez et al., 2020).
In the early 1990s, isolated limb infusion (ILI) was developed as a simpler technique. This is performed via percutaneously inserted catheters in the axial artery and vein of the disease-bearing limb. Melphalan is commonly used for ILI, often with actinomycin D. An analysis of 576 patients with melanoma treated with the melphalan and actinomycin D combination using ILI showed a CR of 33% and a PR of 40% (Kroon et al., 2014). ILI with melphalan combined with actinomycin D has also been used in STS; in a series of 12 patients, the CR was 17% and the PR was 58% (Turaga et al., 2011).
A phase 2 trial using isolated lung perfusion (ILuP) with 45 mg melphalan at 37°C, followed by resection of all detectable pulmonary metastases and then a systematic lymph node dissection, has been performed in 50 patients with STS or osteosarcoma metastases and 57 patients with colorectal cancer metastases (Beckers et al., 2019). The median time to progression for all patients with sarcoma was 13 months compared with a median of 7 to 8 months after pulmonary metastasectomy reported in the literature. The respective times for patients with colorectal cancer were 14 and 12 to 52 months. Isolated hepatic perfusion of melphalan, and more recently hepatic artery infusion of melphalan, have been used to treat liver metastases from ocular melanoma (Boone et al., 2018). Isolated pelvic perfusion of melphalan can be used for unresectable pelvic melanoma metastases (Guadagni et al., 2017).
B. Hematologic Malignancies
The nitrogen mustards have a key role in the treatment of hematologic malignancies. The MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) regimen is still used in HL, and mechlorethamine is used as a topical treatment of cutaneous T-cell lymphoma (Liner et al., 2018). Chlorambucil was the first successful cytotoxic for CLL, and it also has efficacy in NHL. Cyclophosphamide is also employed in the treatment of CLL and NHL, as well as multiple myeloma and HL. Ifosfamide is incorporated in salvage regimens for HL. Melphalan is used to treat multiple myeloma and lymphoma (Bayraktar et al., 2013). Trofosfamide has activity in NHL, with higher efficacy in indolent compared with aggressive NHL (Wagner et al., 1997). Cyclophosphamide and melphalan have a particular place in ASCT and allo-SCT. Cyclophosphamide is useful in dose escalation strategies, as the DLT is neutropenia, and dose escalation does not significantly increase nonhematologic toxicities.
Bendamustine, which was first described in 1971, having been developed in the former German Democratic Republic, has recently been introduced into routine clinical practice (Cheson et al., 2016). It is a hybrid molecule containing a mechlorethamine group, butyric acid, and a benzimidazole ring. The mechlorethamine moiety confers alkylating activity and the butyric acid side chain increases water solubility, whereas the benzimidazole ring provides similarity to purine analogs and bestows antimetabolite properties. The alkylating activity of bendamustine predominates over the antimetabolite activity. Bendamustine now has an important niche in the management of CLL, NHL, HL, and multiple myeloma.
1. Hematopoietic Stem Cell Transplantation
Hematopoietic stem cell transplantation (HSCT) is a potentially curative procedure for both malignant and nonmalignant hematologic conditions (Gyurkocza and Sandmaier, 2014). Hematopoietic stem cells are stimulated and mobilized from bone marrow using agents such as cyclophosphamide that release proteases with cleavage of adhesion molecules. The stem cells are then harvested from the peripheral circulation and stored. After conditioning using high doses of cyclophosphamide and antithymocyte globulin (ATG) +/− TBI, the stem cells are reinfused.
In malignancies, conditioning treatments cause immunoablation, thereby decreasing graft rejection and tumor volume. High doses of cyclophosphamide are needed for myeloablative conditioning before stem cell transplantation. In high-dose treatment, cyclophosphamide is used at doses of up to 6 g/m2 over 4 days. Conventional doses are in the range of 500 to 1500 mg/m2 every 3 to 4 weeks. Cyclophosphamide is a good agent for dose intensification or high-dose treatment, as it has a steep dose-response curve. High-dose cyclophosphamide (HiCy) causes nearly complete ablation of lymphocytes and profound immunosuppression while sparing hematopoietic stem cells on account of their high ALDH1A1 levels (Brodsky, 2010). T cells, B cells, and NK cells have low levels of ALDH and are therefore very sensitive to HiCy. Conditioning regimens produce space within the marrow by removing hematopoietic cells and malignant cells. Busulfan and cyclophosphamide are the most commonly used alkylating agents in high doses as pretransplant conditioning for HSCT. Melphalan 140 mg/m2 has been combined with busulfan for ASCT and allo-SCT. One of the most frequently employed conditioning regimens for patients with lymphoma is carmustine, etoposide, arabinoside, and melphalan (140 mg/m2) (BEAM) (Bayraktar et al., 2013).
As graft versus tumor effects are an important component of HSCT, reduced intensity and nonmyeloablative conditioning regimens are now also used. High-dose myeloablative regimens comprise alkylating agents with or without TBI and require stem cell support. Nonmyeloablative regimens do not require stem cell support. Reduced-intensity conditioning regimens, where the doses of TBI or alkylating agents are decreased by at least 30% compared with myeloablative regimens, are situated between these two scenarios but still require stem cell support. Melphalan 100 mg/m2 has been combined with fludarabine or cladribine as a reduced-intensity conditioning regimen. Cyclophosphamide (2250 mg/m2) has been used with fludarabine and rituximab, an anti-CD20 monoclonal antibody, as a nonmyeloablative conditioning regimen.
Human leukocyte antigen (HLA) fully matched donors are ideal for allo-SCT but are unavailable for most patients. Using donors who are HLA mismatched causes severe alloreactivity, leading to high rates of graft failure, graft-versus-host disease (GVHD), and mortality. High-dose post-transplantation cyclophosphamide (PTCy) was developed at Johns Hopkins to solve these problems. PTCy reduces the incidence of both severe acute and chronic GVHD and to a lesser extent acute GVHD; it is associated with a low nonrelapse mortality rate and increased survival. Early murine studies, using major histocompatibility complex (MHC)-matched skin allograft models, credited the selective reduction of alloreactive donor T cells required for GVHD, an intrathymic clonal deletion of donor reactive intrathymic host T cells that cause graft rejection, and suppressor T cell induction as the mechanisms of action of PTCy (Nunes and Kanakry, 2019). However, acute GVHD is often seen after PTCy in patients, which is not in keeping with the removal of alloreactive T cells. More recently, using a T cell–replete MHC haploidentical murine allo-SCT model, it has been shown that PTCy does not eliminate alloreactive T cells but inhibits their function and that the thymus is not needed for the efficacy of PTCy. A rapid preferential recovery of Tregs occurs, preventing new donor T cells from causing GVHD. The production of Tregs is the opposite of the finding of reduced Treg survival after cyclophosphamide administration in the treatment of tumors (Kanakry et al., 2013). Human Tregs at equilibrium possess low levels of ALDH, which increase after allogeneic stimulation, possibly contributing to their resistance to cyclophosphamide, and this may be a factor in the effectiveness of PTCy in preventing GVHD. PTCy is now a standard for allo-SCT, with no significant differences in outcomes between the use of partially or fully matched HLA donors. It is very effective and inexpensive. Cyclophosphamide-induced tolerance is currently being investigated in the fields of allo-SCT and kidney transplantation. Renal allograft tolerance induced by allogeneic stem cells and PTCy allows immunosuppressive drugs to be discontinued in two-thirds of patients (Kato et al., 2020).
2. Hodgkin Lymphoma
Soon after their introduction, mechlorethamine, chlorambucil, and cyclophosphamide produced response rates of 50% in HL, but these were not prolonged (Shanbhag and Ambinder, 2018). The combination chemotherapy MOPP followed, with a CR in 81% of patients, but it is associated with sterility (particularly in males), myelodysplasia, acute leukemia, and neuropathy. At the same time as the development of MOPP, the ABVD regimen (doxorubicin, bleomycin, vinblastine, and dacarbazine) was devised, which in a later comparison was found to have a CR of 82% compared with 67% with MOPP in patients with advanced HL. The 5-year OS was 73% after ABVD and 66% subsequent to MOPP. ABVD is now the standard treatment of stages III and IV HL. The cyclophosphamide-containing BEACOPP regimen (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) is also used. However, a randomized comparison of BEACOPP and ABVD in patients with previously untreated and unfavorable HL resulted in no significant difference in the 7-year event-free survivals of 78% and 71% respectively, although BEACOPP produced better initial disease control (Viviani et al., 2011). Toxicity was higher in the BEACOPP group. Eighty-three percent of patients requiring salvage chemotherapy received an ifosfamide-containing combination for reinduction followed by BEAM for consolidation.
Mechlorethamine is used as part of the Stanford V regimen (doxorubicin, vinblastine, mechlorethamine, etoposide, vincristine, bleomycin, and prednisone). The regimen is given over 8 weeks, followed by radiotherapy to involved fields, commencing within 3 weeks of chemotherapy completion. In a study of patients with nonbulky stages I to IIA supradiaphragmatic disease, the freedom from progression, disease-specific survival, and OS rates were 94%, 99%, and 94% respectively at a median follow-up of 10 years (Advani et al., 2013). The Stanford V regimen produces equivalent response rates, PFS, and OS compared with ABVD and is an option when the duration of chemotherapy needs to be limited or when bleomycin or doxorubicin are contraindicated, but this is counterbalanced by the additional toxicity from the radiotherapy.
Ten percent of children and adolescents with HL have the nodular lymphocyte predominant type. A short, reduced intensity regimen of 500 mg/m2 intravenous cyclophosphamide on day 1, 6 mg/m2 vinblastine on days 1 and 8, and 40 mg/m2 prednisone on days 1 to 8 has been developed to treat such patients, minimizing toxicity and long-term sequelae (Shankar et al., 2012). Three cycles were given every 2 to 3 weeks. The CR was 80% after first-line treatment and 100% in patients treated after relapse after surgery.
Up to 30% of patients with HL treated first line will develop primary refractory or relapsed disease. Second-line therapy followed by ASCT cures 50% of relapsed patients. A salvage regimen is first needed to induce a good clinical response and decrease bulky disease prior to high-dose therapy. Ifosfamide-containing regimens such as MINE (mesna, ifosfamide, mitoxantrone, and etoposide) and ICE (ifosfamide, carboplatin, and etoposide) have been used. MINE with 1.33 g/m2 ifosfamide daily for 3 days is associated with an OR of 48% (Rodriguez et al., 1995), and ICE, incorporating 5 g/m2 ifosfamide as a continuous infusion over 24 hours, has resulted in an OR of 88% (Moskowitz et al., 2001). Newer regimens such as IGEV (2 g/m2 ifosfamide on days 1 to 4, gemcitabine, vinorelbine, and prednisone) are effective in relapsed or refractory disease, where an OR of 81.3% and CR of 53.8% have been reported (Santoro et al., 2007). Bendamustine is also active in relapsed or refractory HL, with an OR of 53%, but a short median duration of response of only 5 months (Moskowitz et al., 2013). A 3-year OS rate of 78% can be achieved with ASCT plus BEAM conditioning (Bayraktar et al., 2013). Treatment options for patients in whom ASCT is not successful include MOPP or ChlVPP (chlorambucil, vinblastine, procarbazine, and prednisone).
3. Non-Hodgkin Lymphoma
From the 1960s to the 1990s, chlorambucil became the standard treatment of most patients with indolent (low-grade) NHL, whereas cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) became the standard regimen for patients with aggressive (high-grade) NHL. Chlorambucil produces stability in many symptomatic patients with indolent lymphoma, although it is used less today, and CHOP is capable of curing patients with aggressive NHL. CHOP is associated with 5-year survival rates of 30% to 35%. This combination forms the basis of newer treatments, particularly now in combination with the anti-CD20 monoclonal antibody rituximab (R-CHOP). Six cycles of CHOP-like chemotherapy and rituximab was compared with six cycles of CHOP-like chemotherapy alone in young patients with good prognosis diffuse large B-cell lymphoma (DLBCL). The addition of rituximab to CHOP increased the 3-year OS from 84% to 93% (Pfreundschuh et al., 2006). R-CHOP given on a 21-day cycle is the standard treatment of most patients with newly diagnosed DLBCL.
Ifosfamide is included in second-line regimens for patients with DLBCL who are suitable for ASCT. R-ICE is associated with an OR of 63% before transplantation (Gisselbrecht et al., 2012). The gemcitabine, ifosfamide, dexamethasone, and oxaliplatin (GIDOX) combination has been used in relapsed DLBCL or mantel cell lymphoma, with a CR of 15% and a PR of 37% (Park et al., 2011). A phase 2 study of ifosfamide, etoposide, and oxaliplatin in patients with relapsed or refractory NHL resulted in an OR of 65.2% and a 2-year OS of 56.1% (Kim et al., 2013b). Bendamustine combined with rituximab (BR) has also been used in patients with relapsed or refractory DLBCL, with a CR of 15.3% and a PR of 30.5% (Vacirca et al., 2014). The median duration of response was 17.3 months, and the median PFS was 3.6 months. In a retrospective study, trofosfamide was administered to elderly patients or those with a poor performance status with DLBCL, at a dose of 50 mg twice daily continuously, alone (in 4 patients), or combined with rituximab (in 17 patients) (Witte et al., 2019). The treatment was first line in 10 patients, whereas 11 patients had relapsed or refractory disease. The OR was 90.5% with a 52.4% CR. In patients treated first line, 70% had a CR. The PFS and OS were comparable to those seen with other regimens used in this patient population, and trofosfamide may be less toxic and more tolerable.
Chemoimmunotherapy with CHOP, CVP (cyclophosphamide, vincristine, and prednisone), or bendamustine, plus rituximab or obinutuzumab, is now used as first-line therapy in patients with advanced-stage indolent lymphoma. The addition of obinutuzumab to CHOP, CVP, or bendamustine in the first-line treatment of patients with indolent NHL resulted in a longer PFS compared with the combination of rituximab with these chemotherapeutic agents (Marcus et al., 2017). In a randomized phase 3 study, BR was found to be noninferior to R-CHOP or R-CVP when given first line to patients with indolent or mantel cell lymphoma (Flinn et al., 2019). The 5-year PFS was 65.5% for BR and 55.8% in the R-CHOP/R-CVP group. There was no difference in the 5-year OS rates, but the incidence of hypersensitivity reactions, opportunistic infections, and secondary malignancies was higher after BR.
In patients with indolent NHL who are elderly or who have comorbidities, chlorambucil or cyclophosphamide with or without rituximab has a role. A retrospective analysis of 6 mg/m2 per day of oral chlorambucil for 6 consecutive weeks, with two doses of rituximab given 4 weeks apart, in patients with newly diagnosed indolent follicular lymphoma, demonstrated an OR of 97.3% and a CR of 74.7% (Martinelli et al., 2015). Four further cycles of chlorambucil, 6 mg/m2 per day for 2 weeks every month with monthly rituximab, were given to responding patients. The 5-year event-free survival and the 5-year OS was 71.3% and 98.4% respectively.
Both cyclophosphamide and chlorambucil are active in the treatment of Waldenstrom macroglobulinemia, a rare indolent B-cell neoplasm. A randomized comparison of continuous chlorambucil (0.1 mg/kg per day) with intermittent chlorambucil (0.3 mg/kg per day) for 7 days repeated every 6 weeks for at least 6 months in patients with untreated Waldenstrom macroglobulinemia found that 79% of patients responded to continuous therapy compared with 68% of patients given the intermittent schedule (Kyle et al., 2000). There was no difference in survival. Oral cyclophosphamide is also active when administered for prolonged periods. Rituximab-based therapy is now employed as a standard treatment, often in combination with an alkylating agent, and a common regimen is rituximab, cyclophosphamide, and dexamethasone (Gertz, 2013). In a phase 2 study of this combination, the CR was 7% and the PR was 67%. The 8-year OS was 100%, 55%, and 27% in the low-, intermediate-, and high-risk groups respectively (Kastritis et al., 2015).
4. Multiple Myeloma
Melphalan is the most effective cytotoxic for multiple myeloma. Cyclophosphamide is also active. The combination of melphalan and prednisone (MP) has been used since the 1960s. However, a CR is rare, and the median time to progression is only 15 months. MP is now usually employed with other agents. In newly diagnosed patients with multiple myeloma, the addition of novel agents (such as the antiangiogenic and immunomodulatory drug thalidomide, its derivatives, and proteasome inhibitors such as bortezomib) to melphalan has increased response rates and survival. Patients are usually treated with primary therapy, which is followed in selected cases by high-dose chemotherapy and ASCT. Melphalan is avoided in patients who are potential candidates for ASCT at this stage, as it may compromise stem cell reserve. Repeated low doses can cause severe marrow toxicity. Cyclophosphamide and dexamethasone combined with bortezomib (CyBorD) (Reeder et al., 2009) or lenalidomide (CRD) (Kumar et al., 2011) are often used as a primary therapy regimen in transplant candidates, with approximately 85% of patients achieving at least a PR. The addition of alkylating agents to lenalidomide or thalidomide increases the depth and rate of responses. Oral cyclophosphamide (500 mg per week) combined with either thalidomide and dexamethasone (CTD) or vincristine, doxorubicin, and dexamethasone (CVAD) are also used as induction regimens prior to HDM and ASCT (Morgan et al., 2013).
Multiple myeloma is the most common disease treated with melphalan conditioning in transplantation (Rajkumar, 2011). Melphalan is a prime candidate for high-dose therapy as the incidence of nonhematologic side effects, which are reversible, is low. HDM with ASCT has been used for more than 30 years, with an increase in median OS of more than 1 year. In a comparison of two 4-month cycles of 200 mg/m2 melphalan, followed by ASCT, with six 28-day cycles of 0.18 mg/kg melphalan days 1 to 4, 2 mg/kg prednisone days 1 to 4, and 10 mg lenalidomide days 1 to 21 (MPR) in patients 65 years old or younger with newly diagnosed multiple myeloma, the median PFS after HDM and ASCT was 43 months compared with 22.4 months with MPR (Palumbo et al., 2014). The corresponding 4-year OS rates were 81.6% and 65.3%. HDM with ASCT is now the standard of care in patients with multiple myeloma who are younger than 65 years of age. Standard conditioning prior to ASCT in multiple myeloma uses a melphalan dose of 200 mg/m2. In less fit patients, 140 mg/m2 is often used. Auner and colleagues (2018) performed a retrospective analysis of 1964 patients undergoing ASCT between 2008 and 2012. There were no differences in OS, PFS, cumulative incidence of relapse, nonrelapse mortality rate, hematopoietic recovery, and second primary malignancy rates between patients receiving 200 or 140 mg/m2. In patients transplanted in less than a PR, 200 mg/m2 conferred a significant advantage, whereas in those transplanted in a very good PR or a CR, melphalan 140 mg/m2 was favored for OS. Nath and coworkers (2016) determined the melphalan AUC in 114 patients receiving HDM. The median melphalan dose was 193 mg/m2, and the median melphalan AUC was 12.84 mg/l.hr. An AUC above the median was associated with an increase in OS (8.50 years above; 5.38 years below) and more severe mucositis. The median unbound melphalan AUC was 2.8 mg/l.hr. A 5-fold variation in melphalan total AUC was seen. The optimization of melphalan exposure is important in maximizing response. The authors proposed that a patient-specific pharmacokinetic strategy needs to be formulated, as the therapeutic index of melphalan may be improved by AUC targeted dosing.
Elderly patients or those with significant comorbidities who are unable to receive HDM with ASCT require different management with less toxic therapy. Melphalan and prednisone combined with bortezomib or thalidomide are used in elderly patients, but the dose-limiting hematologic toxicity of melphalan and peripheral neuropathy caused by bortezomib and thalidomide are concerns. Cyclophosphamide does not have the cumulative hematologic toxicity of melphalan, and CyBorD is commonly used, with a PR or better in 79.4% of patients (Chan et al., 2019). Attenuated CTD, with lower doses of thalidomide and dexamethasone, can also be used in newly diagnosed elderly or less fit patients with multiple myeloma. In a randomized comparison of attenuated CTD and MP (7 mg/m2 melphalan per day and 40 mg prednisone per day on days 1 to 4 every 28 days), the median PFS was greater in the attenuated CTD group than in the MP group (13 vs. 12 months) and there was a survival benefit after 18 to 24 months for attenuated CTD (Morgan et al., 2013). Cyclophosphamide, carfilzomib, and dexamethasone are another recommended combination (Bringhen et al., 2014).
Bendamustine is also an option. In the first-line treatment of multiple myeloma, bendamustine combined with prednisone produces a higher CR rate, an increased duration of remission in patients achieving a CR or a PR, and a longer time to treatment failure compared with MP (32% vs. 13%, 18 vs. 12 months, and 14 vs. 10 months respectively). It can therefore be used as first line, either as a single agent or in combination (Cheson et al., 2016).
There are numerous therapeutic strategies for patients who relapse after HDM and ASCT. If there has been a durable response to primary ASCT, a further challenge with HDM can be considered. A retrospective analysis of 66 multiple myeloma patients who relapsed after initial ASCT and who then received intermediate-dose melphalan (100 mg/m2) and stem cell support showed that in patients who have experienced a durable response to ASCT, 100 mg/m2 melphalan may be a good low-toxicity option, with activity similar to those of the newer immunomodulatory drugs (Blimark et al., 2011). The OR was 62%, the median PFS was 8.5 months, and the OS was 24 months. There was no treatment-related mortality. Oral cyclophosphamide (500 mg weekly) combined with alternate-day 100 mg prednisone has been used as a salvage therapy in patients with relapsed multiple myeloma after ASCT with a 61% OR (Trieu et al., 2005). The 1-year PFS was 66% and the median OS was 28.6 months. High activity of the lenalidomide and low-dose cyclophosphamide combination has been demonstrated in patients with heavily pretreated lenalidomide-refractory multiple myeloma (Franssen et al., 2018). Cyclophosphamide was given continuously at a dose of 50 mg daily with 20 mg prednisone daily, and 25 mg lenalidomide was administered on days 1 to 21 of a 28-day cycle. The OR was 67%, and the median PFS and OS were 12.1 and 29.0 months respectively. The combination of cyclophosphamide and lenalidomide appears to be synergistic. The PERSPECTIVE trial assessed the impact of adding cyclophosphamide to pomalidomide and dexamethasone in patients experiencing a suboptimal response after three cycles or primary progression during these cycles (Weisel et al., 2019). A PR was achieved in 36.1% of patients after the addition of cyclophosphamide. The median PFS was 6.4 months.
Patients unresponsive to new biologic agents may not have received an alkylating agent. High-dose single-agent cyclophosphamide in such patients does not require stem cell support and can be used even if renal function is compromised. In patients refractory to bortezomib treated with 3 g/m2 cyclophosphamide, 52.9% had at least a PR, although the benefit did not exceed 3 months (Rivell et al., 2011). Cyclophosphamide used in this way could act as a bridge before more definitive treatment.
5. Chronic Lymphocytic Leukemia
Chlorambucil was the standard first-line treatment of patients with CLL from the 1950s until the 1990s, with cyclophosphamide as an alternative. Chlorambucil has now been superseded in younger patients by newer agents such as purine nucleoside analogs, immunotherapeutic agents, and tyrosine kinase inhibitors. The CLL4 trial compared first-line fludarabine and cyclophosphamide (FC) with fludarabine and chlorambucil as single agents (Catovsky et al., 2007). There was no significant difference in OS between the arms. Respective OR rates were 94%, 80%, and 72%, and respective PFS rates at 5 years were 36%, 10%, and 10%. The CLL8 study compared FC with FC and rituximab (FCR) as first-line therapy, with a median PFS of 56.8 months with FCR and 32.9 months with FC (Fischer et al., 2016). FCR is now the standard treatment of fit patients with untreated CLL. Newer targeted agents such as ibrutinib and idelalisib have less toxicity but are administered indefinitely, whereas FCR is time limited and can produce deep and long remissions without maintenance treatment.
The median age at diagnosis of CLL is 72 years, and the majority of patients are diagnosed after the age of 65. Fludarabine-based chemoimmunotherapies are often too toxic for elderly patients or those with comorbidities, and in these patients chlorambucil continues to have an important first-line role. Several different schedules of chlorambucil are used, such as intermittent dosing (0.4 to 0.8 mg/kg on days 1 and 15 per cycle) or more intense dosing (10 mg/m2 days 1 to 7 per cycle). It is often administered with prednisone. A review of four UK randomized CLL trials and four other published trials where single-agent chlorambucil was included showed that OR rates with chlorambucil were between 57% and 75% if doses of 60 to 70 mg/m2 every 28 days were used; with lower doses this range was 31% to 55% (Catovsky et al., 2011). The administration of six or more cycles was associated with better response rates. Studies incorporating chlorambucil as a comparator showed a lower OR rate with lower doses or with fewer cycles of chlorambucil. Chlorambucil dose and duration are therefore important. Six cycles of chlorambucil are probably not enough, and response assessments prior to the completion of 12 months of treatment are likely to underestimate activity as chlorambucil is slow acting.
An analysis of older patients in frontline Cancer and Leukemia Group B (CALGB) trials showed that chemoimmunotherapy with fludarabine does not improve outcomes in these patients compared with chlorambucil (Woyach et al., 2013). Chlorambucil, with or without an anti-CD20 monoclonal antibody, remains a standard first-line treatment of these patients. Chlorambucil is a useful chemotherapeutic on which to base the development of new combinations in elderly patients. In a randomized trial comparing chlorambucil alone, chlorambucil plus rituximab, and chlorambucil plus obinutuzumab in untreated patients with comorbidities, the median PFS with chlorambucil alone was 11.1 months, with chlorambucil plus rituximab 15.4 months, and with chlorambucil plus obinutuzumab 29.2 months (Goede et al., 2015). Chlorambucil plus obinutuzumab is now a standard first-line treatment of CLL. More recently, chlorambucil combined with ofatumumab has been shown to be superior to chlorambucil alone in the first-line treatment of frail patients or patients 65 years or older, with manageable side effects (Offner et al., 2020). After 5 years of follow-up, the median PFS was 23.39 months with the combination compared with 14.72 months with chlorambucil alone. The median OS was not estimable in the chemoimmunotherapy arm and was 84.67 months in the chlorambucil arm. The estimated OS at 5 years was 68.5% after chlorambucil and ofatumumab and 65.7% after treatment with chlorambucil.
Bendamustine is active in CLL and is usually combined with rituximab. The MABLE study compared BR with chlorambucil plus rituximab in the first-line treatment of patients with CLL unable to tolerate fludarabine-based chemoimmunotherapy (Michallet et al., 2018). The median ages in the former and latter treatment groups were 73 and 72 years respectively. BR produced a higher CR (24% vs. 9%) and longer median PFS (40 vs. 30 months) than chlorambucil plus rituximab, but OR and OS did not differ.
Ibrutinib is an orally administered inhibitor of Bruton tyrosine kinase. A randomized comparison of chlorambucil plus obinutuzumab with ibrutinib plus obinutuzumab in patients with previously untreated CLL or small lymphocytic lymphoma showed that the estimated 30-month PFS was longer with the latter combination (31% vs. 79%) (Moreno et al., 2019). Ibrutinib plus obinutuzumab is therefore an alternative first-line treatment. However, serious adverse events were reported in 58% of patients receiving ibrutinib plus obinutuzumab compared with 35% of patients receiving chlorambucil plus obinutuzumab. In older patients or those with comorbidities, recommended first-line treatments include single-agent ibrutinib or chlorambucil with an anti-CD20 monoclonal antibody. Chemoimmunotherapy is given for six cycles, and the associated breaks can be beneficial compared with treatment with ibrutinib, which is given continuously.
Of late, the backbone for the treatment of CLL has changed swiftly from chlorambucil to chlorambucil plus anti-CD20 monoclonal antibodies, then to Bruton tyrosine kinase inhibitors or combinations of anti-CD20 monoclonal antibodies with inhibitors of B-cell lymphoma 2 (bcl-2), an antiapoptotic protein. Venetoclax is a selective inhibitor of bcl-2. A randomized phase 3 trial in patients aged 18 years or older with untreated CLL compared oral venetoclax plus obinutuzumab for six cycles with chlorambucil plus obinutuzumab (Al-Sawaf et al., 2020). PFS was not reached in the venetoclax arm and was 35.6 months in the chlorambucil arm. Venetoclax plus obinutuzumab is now established as a fixed-duration regimen for patients with CLL.
C. Autoimmune Diseases
Experience of cyclophosphamide as an oncologic agent has been put to good use in the treatment of autoimmune diseases, but the details of the use of cyclophosphamide in oncology and autoimmune diseases differ. HiCy has been used to treat severe refractory autoimmune diseases such as aplastic anemia, systemic sclerosis, multiple sclerosis, amyloidosis, and SLE, as well as myasthenia gravis, autoimmune hemolytic anemia, and chronic inflammatory polyneuropathy, with durable remissions (Brodsky, 2010). HiCy resets the immune system by ablating the autoreactive B and T cells causing lymphocyte-mediated autoimmune disease, without affecting the pluripotent blood stem cells, and is effective in patients who do not respond to conventional immunosuppression. ASCT is not always needed after HiCy in classic autoimmune diseases, which do not affect hematopoietic stem cells and where hematopoietic recovery is rapid but is ordinarily required in the treatment of severe aplastic anemia (SAA).
1. Aplastic Anemia
An immune-mediated effect on hematopoietic stem cells is responsible for most cases of acquired SAA. For young patients with a matched sibling donor, hematopoietic stem cell transplantation is the optimum treatment (Brodsky, 2010). Cyclophosphamide 50 mg/kg per day for 4 days, with or without ATG, is frequently employed as a conditioning regimen prior to ASCT. HiCy without ASCT can also produce longstanding hematologic remissions. A response rate of 71% and an event-free survival rate of 58% at 10 years have been reported in treatment-naïve SAA patients treated with HiCy. Patients with refractory SAA have shown response rates and 10-year event-free survival rates of 48% and 27% respectively.
2. Systemic Sclerosis
In patients with systemic sclerosis (SSc), interstitial lung disease (ILD) is the major cause of death and develops as a result of immunologic and inflammatory processes that lead to vascular damage. Cyclophosphamide is the treatment of choice for ILD secondary to SSc. A meta-analysis of four studies with a total of 495 patients with connective tissue disease–associated ILD, more than 90% of whom had SSc, found a significant improvement in forced vital capacity, but not pulmonary diffusing capacity, after cyclophosphamide compared with placebo (Barnes et al., 2018). A modest improvement in dyspnea was seen. The usual oral dose of cyclophosphamide is 2 mg/kg per day, and the intravenous doses are between 500 and 1000 mg/m2 every 4 to 6 weeks. Treatment is usually for at least 6 months, followed by a less toxic immunosuppressant such as azathioprine as maintenance.
Three randomized controlled trials of ASCT have been performed in patients with SSc (Walker et al., 2018). The conditioning regimens in the three studies included 120 or 200 mg/kg cyclophosphamide, and the standard cyclophosphamide arms were either 1 g/m2 cyclophosphamide monthly for 6 months, 750 mg/m2 monthly for 12 months, or one dose of 500 mg/m2 followed by 11 monthly doses of 750 mg/m2. All three trials showed an improvement in skin condition, pulmonary function, vasculopathy, and health-related quality of life with ASCT. A survival advantage was also seen. Curiously, the benefits of ASCT over cyclophosphamide were not seen in former and current smokers. ASCT is associated with a high risk of adverse effects and is therefore only used in carefully selected patients with rapidly progressing SSc at risk of organ failure.
3. Multiple Sclerosis
Multiple sclerosis (MS) is the most common inflammatory and demyelinating immune-mediated central nervous system disease in young adults. Cyclophosphamide plus TBI or busulfan are high-intensity regimens used in the treatment of MS. BEAM is the most frequently used intermediate-intensity regimen. In a review of eight case series of patients with progressive MS refractory to conventional medical treatment, a higher rate of PFS was seen with ASCT using intermediate-intensity conditioning regimens (79.4% at a median follow-up of 39 months) compared with high-intensity conditioning regimens (44.6% at a median follow-up of 24 months) (Reston et al., 2011). More recently, a preliminary study randomized 110 patients with relapsing-remitting multiple sclerosis on disease progression to either nonmyeloablative ASCT with 200 mg/kg cyclophosphamide and 6 mg/kg ATG (n = 55) or continuation of disease-modifying therapy of higher efficacy or a different class (n = 55). Treatment with ASCT significantly prolonged the time to further disease progression compared with disease-modifying therapy (hazard ratio 0.07) (Burt et al., 2019).
4. Amyloidosis
Immunoglobulin light chain amyloidosis, the most frequent variant of systemic amyloidosis, is the only form of amyloidosis treated with chemotherapy or ASCT (Gertz, 2018). The combination of melphalan and prednisone was first used in 1972. Treatment with 0.22 mg/kg oral melphalan and 40 mg dexamethasone daily on days 1 to 4 in 28-day cycles is associated with a hematologic response rate of 76% and a median OS of 7.4 years (Palladini et al., 2014). ASCT was initially described in 1996. However, only one-quarter to one-fifth of patients are fit enough for transplantation. ASCT has not been demonstrated to be superior to oral therapy as patient selection affects outcomes. Gertz and colleagues (2016) treated patients with melphalan and dexamethasone or ASCT with 140 or 200 mg/m2 melphalan. The 3-year PFS rates were 29.1% for melphalan and dexamethasone and 51.7% for ASCT. The respective 3-year OS rates were 58.8% and 83.6%. The chance of a complete hematologic response at 12 months was higher for those who chose ASCT. The study was limited in terms of accrual and the number of patients randomized, and the authors concluded that the hematologic response rate and improved survival was similar after ASCT or melphalan plus dexamethasone; an advantage of ASCT over the melphalan and dexamethasone combination could not be established. Cyclophosphamide has been combined with dexamethasone and thalidomide or lenalidomide, and melphalan has been combined with dexamethasone and lenalidomide. However, CyBorD, with a hematologic response rate of 94% and a CR rate of 71%, and the melphalan and dexamethasone combination are recommended for patients ineligible for transplantation (Gertz, 2018).
5. Nephritis
Idiopathic membranous nephropathy (iMN) is a frequent cause of nephrotic syndrome in adults and a very common cause of chronic renal failure in patients with primary glomerular diseases. Autoantibodies are generated against the M-type phospholipase A2 receptor normally expressed on podocytes. Nitrogen mustards in combination with glucocorticoids are effective in treating iMN, increasing remission rates and renal survival through immunosuppression. Studies of patients with iMN and renal impairment receiving either chlorambucil or cyclophosphamide suggest that cyclophosphamide is more effective and that chlorambucil causes more toxicity (Hofstra and Wetzels, 2010). Cyclophosphamide is given at a dose of 2 mg/kg per day for 3 months, and it should only be administered to high-risk patients based on urinary protein analysis.
Idiopathic nephrotic syndrome is the most frequent glomerular disease in children. The majority of patients respond to glucocorticoids, but some relapse or become steroid dependent. Cyclophosphamide has been used since the early 1970s to treat nephrotic syndrome, especially as a steroid-sparing agent. Both cyclophosphamide and chlorambucil decrease the risk of relapse by approximately 50% in children with relapsing steroid-sensitive nephrotic syndrome compared with prednisone alone (Pravitsitthikul et al., 2013). The benefits of cyclophosphamide and chlorambucil treatment over toxicity only exist in children who relapse frequently. The activity of intravenous cyclophosphamide is the same as oral cyclophosphamide, and the optimum duration of treatment is 8 weeks. Oral cyclophosphamide is also used in children with steroid-dependent nephrotic syndrome and is an active second-line treatment. In a study of 90 children, it was most effective in those older than 7.5 years (Azib et al., 2011). Even though new immunosuppressive agents are now employed to treat steroid-dependent nephrotic syndrome, a short course of cyclophosphamide is an effective second-line therapy, as 60% of the 90 patients did not require other immunosuppressives.
SLE is a complex autoimmune disorder involving multiple organs. In patients with organ-threatening lupus (such as renal, cardiac, pulmonary, and neuropsychiatric complications) or refractory disease, cyclophosphamide can be used (Fanouriakis et al., 2019). About 35% of adults with SLE in the US have clinical features of nephritis at diagnosis, and 50% to 60% will develop nephritis within 10 years (Hahn et al., 2012). The use of intravenous cyclophosphamide in lupus nephritis was pioneered in the 1970s and 1980s at the National Institutes of Health. High-dose cyclophosphamide (500 to 1000 mg/m2 intravenously once every month for six doses plus prednisone) has a 53% response rate and has demonstrated a durable benefit. The Euro-Lupus Nephritis Trial compared intravenous low-dose cyclophosphamide (six 500 mg doses, each 2 weeks apart) and intravenous high-dose cyclophosphamide (six monthly doses and two quarterly doses of 500 mg/m2, increasing by 250 mg, according to the white blood cell nadir, to a maximum of 1500 mg), followed by azathioprine maintenance in the treatment of proliferative lupus nephritis. All patients received high-dose glucocorticoids for the initial 4 weeks, after which the dose was decreased to a maintenance level for at least 30 months after the start of treatment. After 10 years of follow-up, the outcome did not differ between the low-dose and high-dose cyclophosphamide groups (Houssiau et al., 2010).
Two intravenous cyclophosphamide regimens are recommended in conjunction with glucocorticoids (Hahn et al., 2012). The low-dose “Euro-Lupus” schedule of 500 mg every 2 weeks for six doses and the high-dose treatment of 500 to 1000 mg/m2 once monthly for six doses, both of which are followed by maintenance daily azathioprine or mycophenolate mofetil. African Americans and Hispanics are less responsive to intravenous cyclophosphamide than white or Asian patients. The low “Euro-Lupus” dose is advised for white patients with a western or southern European racial or ethnic background, whereas in other patients high-dose cyclophosphamide is an option.
6. Vasculitides
Antineutrophil cytoplasm antibody (ANCA)-associated vasculitides affect small blood vessels. They comprise granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA). In the 1970s and 1980s, the addition of cyclophosphamide to glucocorticoid therapy was a significant advance, but the frequency of adverse events was higher. The current treatment of severe ANCA-associated vasculitides has an induction phase of glucocorticoids and an immunosuppressive agent followed by a maintenance phase (Pagnoux and Mendel, 2019). Cyclophosphamide or rituximab, in combination with glucocorticoids, are used to induce remission in patients with severe GPA or MPA and are effective in more than 80% of patients. Cyclophosphamide is given as regular bolus injections of 15 mg/kg (with a maximum of 1200 mg per bolus) every 2 weeks for 1 month and then every 3 weeks for 3 to 6 months, or it is administered orally at 2 mg/kg per day continuously (with a maximum of 200 mg per day) for the same duration; the remission rate is the same for both routes. Patients who achieve a clinical remission with induction cyclophosphamide can receive a less toxic immunosuppressant such as azathioprine or methotrexate as maintenance. The treatment of EGPA is similar to that of GPA and MPA. Less severe manifestations can be managed with glucocorticoids initially, but those with severe EGPA require combined glucocorticoids and cyclophosphamide. Polyarteritis nodosa is a medium-sized vessel vasculitis. Patients with the idiopathic form and major organ involvement are treated with glucocorticoids and cyclophosphamide (Pagnoux and Mendel, 2019). The doses of cyclophosphamide are those used in severe GPA and MPA, followed by the same maintenance agents once remission has been achieved.
7. Other Autoimmune Conditions
Nitrogen mustards have been used in other autoimmune conditions. Behçet’s syndrome is caused by a vasculitis affecting the skin, mucosa, gastrointestinal tract, arteries, veins, eyes, and nervous system. Cyclophosphamide is a recommended treatment of acute deep vein thrombosis and pulmonary artery aneurysms. Patients with arterial involvement are given pulse methylprednisolone (1 g daily for 3 days) and cyclophosphamide (1 g monthly for 6 to 12 months). Azathioprine is then used as maintenance (Esatoglu and Hatemi, 2019). In Behçet’s uveitis, chlorambucil at 2 to 6 mg daily induced durable remissions in 6 of 14 patients (Zaghetto et al., 2010). The median duration of treatment was 28 months. Pemphigus vulgaris is a chronic epidermal immunobullous disease, and the main treatment is glucocorticoids. Cyclophosphamide is used primarily as a third-line therapy after glucocorticoids, azathioprine, rituximab, or mycophenolate mofetil (Harman et al., 2017). However, dexamethasone and cyclophosphamide pulse (DCP) therapy is used extensively in India, with disease-free periods of more than 5 years in 16% of patients (Kanwar and De, 2011). DCP therapy heals lesions more rapidly, produces longer clinical remission, and is less toxic compared with conventional glucocorticoid therapy. There is limited data on the use of chlorambucil in pemphigus vulgaris, but it is considered if more conventional treatments cannot be used (Harman et al., 2017). Polymyositis and dermatomyositis are other rare conditions. Oral glucocorticoids are used as first-line treatment, but 20% to 30% of patients do not respond and a large number of responders ultimately relapse. In a retrospective study of nine patients with a loss of benefit from glucocorticoids, 1.0 to 1.5 g/m2 intravenous cyclophosphamide, fractionated over 4 to 5 days and given monthly for 6 months and every 3 months thereafter for 18 months, was added to the glucocorticoid therapy (Nagappa et al., 2013). All patients experienced an improvement in motor power and a reduction in serum creatine kinase after the addition of cyclophosphamide. The clinical course of type 1 diabetes can be modulated by immune ablation and ASCT. In a study of eight patients with newly diagnosed type 1 diabetes treated with ASCT using conditioning with 200 mg/kg cyclophosphamide and ATG, all patients became independent of exogenous insulin (Snarski et al., 2011).
Paraquat poisoning remains a global problem. An extensive inflammatory response is generated, leading to respiratory failure or multiple organ failure. A combination of glucocorticoids and cyclophosphamide is often used as treatment. A meta-analysis of seven trials containing 426 patients found that the combination given as a pulse therapy reduced mortality to 59.3% from 81.0% in a control group. Cyclophosphamide may act by decreasing the number of CD4+ lymphocytes (Xu and Lu, 2019).
VIII. Metronomic Scheduling
Metronomic chemotherapy has a number of different actions: a direct cytotoxic effect on cancer stem cells, tumor initiating cells, and cancer cells; targeting Tregs; and inhibiting tumor angiogenesis by selective action on endothelial cells. Metronomic cyclophosphamide may act by these different mechanisms depending on the plasma concentration (Bocci and Kerbel, 2016). MTD chemotherapy mainly targets tumor cells, whereas metronomic chemotherapy has predominantly antiangiogenic effects, but other mechanisms have been described. Metronomic cyclophosphamide can reset T and NK cell responses, which are reduced in end-stage cancer patients, specifically decreasing circulating Treg levels and replenishing innate and acquired T cell receptor–driven T cell responses (Ghiringhelli et al., 2007). Doubling the dose of cyclophosphamide, however, results in systemic lymphodepletion.
There is an effective antiangiogenic response and increased innate immunity with low-dose chemotherapy regimens. In animal studies, different schedules of cyclophosphamide administration, which produce different plasma concentrations of 4-OH-CY, generate antitumor effects via different mechanisms; 1 day of cyclophosphamide every 6 days activates the innate immune system, whereas daily administration of cyclophosphamide inhibits angiogenesis. A 6-day schedule may be cytotoxic but may also generate bursts of cytokine and chemokine responses leading to antitumor immunity, and the breaks in treatment may minimize the killing of immune cells recruited into the tumor.
Oral cyclophosphamide at 50 mg daily given to patients with metastatic breast cancer produced a clinical benefit in those with an increased fraction of apoptotic circulating endothelial cells. A reduction in circulating VEGF-A, platelet-derived growth factor (PDGF)-BB, and endothelial cell precursors after 2 months of treatment was also seen in long-term responders. In another cohort of patients with breast cancer, the same regimen of cyclophosphamide initially decreased Tregs, but the reduction reversed during therapy (Bocci and Kerbel, 2016). Oral cyclophosphamide at 100 mg daily on alternate weeks decreases circulating Tregs (Ghiringhelli et al., 2007).
The clinical use of metronomic chemotherapy has been reviewed by Simsek and colleagues (2019). Oral cyclophosphamide has been the most widely studied metronomic regimen. A combination of oral cyclophosphamide at 50 mg daily and oral methotrexate at 2.5 mg twice daily 2 days per week (CM) has been used to treat metastatic breast cancer, producing an OR of 20.9%. CM has also been combined with bevacizumab and trastuzumab with an OS of 13.6 months. The combination of oral cyclophosphamide at 65 mg/m2 daily and low-dose oral capecitabine at 1000 mg/m2 twice daily, both given on days 1 to 14 every 28 days, is effective in the long-term control of patients with metastatic breast cancer, with a clinical benefit rate (CBR) (the sum of patients with a PR, a CR, and stabilization of disease for more than 24 weeks) of 53% (Simsek et al., 2019). The addition of bevacizumab to oral cyclophosphamide (50 mg daily) and capecitabine led to a CBR of 68% in patients with advanced breast cancer (Dellapasqua et al., 2008).
Metronomic cyclophosphamide has been used for over 25 years in the treatment of castrate-resistant and hormone-refractory prostate cancer. Oral cyclophosphamide has activity, even in those patients previously treated with docetaxel. With cyclophosphamide at 50 mg daily, a prostate-specific antigen (PSA) response (a greater than 50% decline of pretreatment value) of 34.5% and response duration of 7.5 months have been reported. Oral cyclophosphamide has been combined with methotrexate and a GnRH agonist, UFT (uracil and tegafur) and dexamethasone, cetuximab and dexamethasone, capecitabine and thalidomide, and lenalidomide only, with PSA response rates of between 25% and 63% (Simsek et al., 2019). However, with the advent of new anti-testosterone drugs such as enzalutamide and abiraterone, the role of oral cyclophosphamide in castrate-resistant prostate cancer has become less important. Oral cyclophosphamide at 50 mg daily has been used as salvage therapy in patients with ovarian cancer, with a median PFS of 4 months and a median OS of 13 months (Simsek et al., 2019); when combined with bevacizumab, a PR of 24% and a median OS of 16.9 months has been reported (Garcia et al., 2008). Oral metronomic cyclophosphamide has also been combined with IFN-α in patients with renal cell cancer. IFN-α was given three times per week on a 4-week cycle and 50 mg oral cyclophosphamide was administered daily from day 2 of each cycle. A CBR of 40% and a median OS of 13.2 months was observed (Tupikowski et al., 2015).
Celecoxib is a selective COX-2 inhibitor, and when combined with cyclophosphamide it is expected to have a synergistic antiangiogenic effect. A fall in PSA was seen in 46% of patients with advanced castrate-resistant prostate cancer receiving cyclophosphamide at 50 mg daily, celecoxib at 200 mg twice daily, and dexamethasone (Simsek et al., 2019). Using the same schedule of oral cyclophosphamide and celecoxib in patients with advanced breast cancer, the CBR was 46.7% and the 1-year OS was also 46.7%. Oral cyclophosphamide at 50 mg daily and celecoxib at 400 mg twice daily given continuously had activity in relapsed and refractory aggressive histology NHL, with an OR of 37.5% and a median PFS of 14.4 months (Buckstein et al., 2006). However, Khan and coworkers (2011) found that oral cyclophosphamide at 50 mg daily and celecoxib at 400 mg twice daily administered continuously, with methotrexate at 2.5 mg twice daily for 2 consecutive days each week, had little activity in patients with advanced cancer, although the combination led to disease stabilization for more than 4 months in 34.3% of patients.
IX. Novel Nitrogen Mustard Derivatives and Targeted Delivery Approaches
A. Oxazaphosphorine Derivatives
New pharmacologic strategies are required to decrease the toxicities associated with the metabolism of the oxazaphosphorines. Oxazaphosphorine analogs have been synthesized in attempts to both improve efficacy and reduce toxicity. IPM has been covalently attached to β-D-glucose to form glufosfamide (Shimizu et al., 2010). Rapidly proliferating cancer cells overexpress glucose transporter proteins, and by being a substrate for these, glufosfamide can target tumor cells with increased uptake. Glufosfamide then decomposes spontaneously or via glucosidase-induced hydrolysis to IPM. It does not generate acrolein and produces less CAA compared with ifosfamide. The clinical development of glufosfamide centered on pancreatic cancer (Chiorean et al., 2010), but significant hematologic and renal toxicity was observed, and glufosfamide has not entered routine clinical practice. Mafosfamide is the 4-thioethane sulfonic acid salt of 4-OH-CY. In phase 1 studies, intravenous administration caused severe pain at the infusion site, precluding further development as a systemic treatment (Mazur et al., 2012). Intrathecal administration of mafosfamide is possible, with activity against neoplastic meningitis, headache being the DLT. However, mafosfamide has not been developed further.
Palifosfamide and evofosfamide have been assessed in large phase 3 trials. Palifosfamide-tris is the tris salt of IPM (Ryan et al., 2016). Preclinical studies and phase 1 and 1/2 clinical trials have shown that palifosfamide possesses antitumor activity that is similar or better than that of ifosfamide, and patients exposed to palifosfamide are less likely to develop neurotoxicity or nephrotoxicity. An improved PFS with doxorubicin combined with palifosfamide was seen compared with doxorubicin alone in a randomized phase 2 trial of patients with STS. However, in the Picasso III trial, where 447 patients with STS were randomized to doxorubicin or doxorubicin plus palifosfamide, there were no significant differences in PFS (5.2 vs. 6.0 months) or OS (16.9 vs. 15.9 months) (Ryan et al., 2016). The objective response rate was higher with the combination compared with doxorubicin alone (CBR 51.8% vs. 41.2%), but toxicity was greater.
Evofosfamide is a prodrug designed to be activated in hypoxic cells to produce the nitrogen mustard bromo-iso-phosphoramide and avoid the ifosfamide-associated hematologic, renal, bladder, and CNS toxicities. However, skin and mucosal toxicities remain significant. A phase 1 study of evofosfamide in patients with heavily pretreated relapsed or refractory leukemia showed limited activity but sufficiently so that further evaluation has been advocated, particularly in combination with other cytotoxics or demethylating agents (Badar et al., 2016). Five patients with advanced human papilloma virus (HPV)-negative head and neck squamous cell carcinoma (HNSCC) were treated with evofosfamide, with two achieving a PR and three showing stable disease (Jamieson et al., 2018). In the MAESTRO trial, the OS in the first-line treatment of patients with advanced pancreatic cancer was not increased by the addition of evofosfamide to gemcitabine, although the PFS was longer and the OR was greater (Van Cutsem et al., 2016). It is possible that the outcome of this study may have been different had a poly-ligand profiling library, developed in parallel, been used in patient stratification (Domenyuk et al., 2018). A further caveat is that the formulation of evofosfamide was changed in this trial, perhaps significantly decreasing the exposure to evofosfamide (Higgins et al., 2018).
The addition of evofosfamide to doxorubicin in the first-line treatment of advanced unresectable or metastatic intermediate- or high-grade STS did not improve median OS (18.4 months with the combination compared with 19.0 months with single-agent doxorubicin) (Tap et al., 2017). No differences were seen in PFS (6.3 months with the combination vs. 6.0 months with the single agent). The OR was higher with the dual treatment than with the single agent (28% vs. 18%), but it was more toxic with increased myelosuppression. A subgroup analysis showed a potential benefit of the combination in patients with synovial sarcoma.
In preliminary in vitro work, ifosfamide has been synthesized with deuterium at the alpha and alpha' carbons to block N-dechloroethylation and increase 4-hydroxylation (Calinski et al., 2015). This “metabolic switching” approach suppressed the production of CAA and increased 4-hydroxylation and could be developed further.
B. Melphalan Derivatives
Melflufen (melphalan flufenamide) is the ethyl ester of a dipeptide of melphalan and para-fluoro-L-phenylalanine, which is more lipophilic than melphalan (Wickström et al., 2017). Aminopeptidases are important in the development of cancer through angiogenesis, and aminopeptidase N (APN) is overexpressed in a number of malignancies. Enzymatic cleavage occurs in aminopeptidase-positive cells, and metabolites that are less lipophilic are trapped intracellularly. By means of a simple peptide bond, melflufen is directed to APN-expressing cells. In vitro and in vivo studies have demonstrated that melflufen is more active than melphalan. In an in vivo assay in mice, melflufen showed antiangiogenic activity, whereas melphalan was inactive in this regard. However, both melflufen and melphalan failed to inhibit the enzymatic activity of APN at antiangiogenic concentrations, suggesting that another mechanism may be active. Melflufen is distributed rapidly from the plasma into cells. At the end of a 30-minute infusion, the plasma half-life is between 1.4 and 4.9 minutes. Plasma melphalan levels are greater than melflufen levels within 15 minutes of a melflufen infusion and continue to rise for up to 10 minutes, indicating a high rate of melphalan formation in peripheral tissues, with redistribution into plasma.
Richardson and coworkers (2020) reported results from a dose escalation and dose expansion phase 1/2 study of intravenous melflufen as a single agent, or combined with oral dexamethasone, in patients with relapsed and refractory multiple myeloma. Forty-five patients received the combination of 40 mg melflufen on day 1 of each cycle (21 or 28 days) and 40 mg dexamethasone once weekly. The OR was 41% and CBR 65% in the efficacy evaluable patients, and the median PFS in all treated patients was 5.7 months. The OR was similar to those described for other agents assessed in relapsed and refractory multiple myeloma, and melflufen combined with dexamethasone was active in patients refractory to alkylating agents. The recommended regimen for further evaluation was 40 mg melflufen, with a cycle length of 28 days, in combination with dexamethasone. Low blood counts are common with melflufen, especially thrombocytopenia, but are manageable. Alopecia is not an issue, and the incidence of mucositis is low.
In the HORIZON study evaluating melflufen plus dexamethasone, patients with multiple myeloma had received at least two previous lines of therapy and were refractory to pomalidomide or daratumumab, a monoclonal antibody, or both (Oriol et al., 2020). In an interim analysis, the OR in 125 efficacy-evaluable patients was 29% and the CBR was 44%. The median duration of response was 4.4 months. ANCHOR is a phase 1/2 study investigating melflufen and dexamethasone as components of triplet combinations in patients with relapsed refractory multiple myeloma. The activity of melflufen and dexamethasone combined with daratumumab or bortezomib has been reported in a preliminary analysis. The OR was 76% in 33 patients receiving melflufen, dexamethasone, and daratumumab and 67% in six patients receiving melflufen, dexamethasone, and bortezomib. The OCEAN study is a phase 3 study comparing melflufen and dexamethasone with pomalidomide and dexamethasone in patients with multiple myeloma treated with two to four prior therapies. Patients were refractory to both their last line of therapy and lenalidomide. In an initial report, the median PFS with melflufen was longer than that with pomalidomide (6.8 vs. 4.9 months) (Schjesvold et al., 2021).
Further studies are in progress (Schjesvold et al., 2020). NCT04115956 is a phase 1/2 dose escalation and dose expansion study of melflufen combined with dexamethasone in patients with immunoglobulin light chain amyloidosis pretreated with a minimum of one line of therapy, and the BRIDGE study (NCT03639610) is evaluating the safety and pharmacokinetics of melflufen in patients with renal impairment. The planned LIGHTHOUSE study aims to compare daratumumab with daratumumab plus melflufen in patients with multiple myeloma refractory to an immunomodulating agent and a proteasome inhibitor (Oriol et al., 2020).
The use of the nitrogen mustards in early multiple myeloma has diminished as novel targeted agents have been introduced. However, most patients with multiple myeloma will relapse within 12 to 18 months of diagnosis, and further intervention will be necessary for disease control. Many patients relapsing at this point have more aggressive and resistant disease. Third-line treatment is usually required after proteosome inhibitors, immunomodulatory drugs, and monoclonal antibodies with such patients often refractory to one or more of these agents. Melflufen, with a novel mode of action and good tolerance, can satisfy this increasing unmet need. The activity of melflufen in triple-class refractory patients is encouraging, as it is effective in patients who are alkylating agent naïve and can also overcome resistance to prior chemotherapy.
C. Chlorambucil Derivatives
In an attempt to increase the penetration of chlorambucil into the CNS, a chlorambucil-scopine prodrug has been synthesized. After intravenous administration to rodents, the AUC and Cmax of the prodrug in the brain were 14.25 and 12.20 times higher respectively compared with the chlorambucil values (Wang et al., 2014). Conjugates of chlorambucil with mitochondrial targeting compounds have been produced, leading to increased preclinical cytotoxicity. The triphenylphosphonium-chlorambucil conjugate acts on mitochondrial DNA, producing an 80-fold increase in cytotoxicity to breast and pancreatic cell lines (Millard et al., 2013). However, despite these promising preclinical data, chlorambucil derivatives have not yet been assessed in clinical trials.
D. Targeted Delivery Approaches
Gene-directed enzyme prodrug therapy involves the transfer of a gene encoding a prodrug-activating enzyme followed by the administration of the inactive prodrug. The intention of gene-directed enzyme prodrug therapy is to induce localized intratumoral prodrug activation and minimize toxicity to the patient. A therapeutic effect is generated in tumor cells and also in surrounding cells via a bystander effect. The administration of CYP3A4, CYP3A5, and CYP2B6 to cause in situ bioactivation of ifosfamide can reduce side effects while maintaining cytotoxicity. In a phase 1/2 clinical trial of 14 patients with advanced pancreatic cancer, human 293 cells genetically modified to overexpress CYP2B1 were encapsulated in cellulose sulfate polymers and delivered angiographically into blood vessels supplying the primary site (Löhr et al., 2003). This was followed by the intravenous administration of ifosfamide, which the cells locally activated to active metabolites while maintaining immunologic and spatial isolation. The PR was 14.3%, and 78.6% of patients had stable disease. The median OS was twice that of a historic control group. A phase 1 study of the direct intratumoral injection of MetXia-P450, a retroviral vector facilitating the delivery and subsequent expression of the CYP2B6 gene, into metastatic cutaneous tumor nodules of patients with breast cancer and melanoma, followed by oral cyclophosphamide at 100 mg/m2 daily between days 8 and 22, yielded one PR and four instances of stable disease for at least 12 weeks in the nine patients with breast cancer (Braybrooke et al., 2005).
Targeting DNA disruptive agents to mitochondrial DNA is a potential route for bypassing nuclear or cytosolic resistance factors. Mitochondria-targeted chlorambucil derivatives are more active against resistant cancer cells compared with chlorambucil, rapidly alkylating mitochondrial proteins. They overcome resistance due to cytosolic GSTM upregulation by rapidly accumulating in mitochondria (Jean et al., 2014). This approach has not yet entered clinical trials.
X. Nitrogen Mustard Combinations with Monoclonal Antibodies and Small-Molecule Targeted Agents
The systemic treatment of cancer has changed immensely over the last 20 years with the routine use of monoclonal antibodies and small-molecule targeted agents, opening the possibility of personalized medicine. This has also raised intriguing questions as to whether these new agents can be combined with established cytotoxic agents such as the nitrogen mustards. The first humanized monoclonal antibody introduced into oncologic practice for the treatment of solid tumors was trastuzumab, which is now routinely included in regimens containing cyclophosphamide to treat breast cancer. Metronomic cyclophosphamide has also been combined with bevacizumab, although the use of this combination is less well established. Combining small-molecule tyrosine kinase inhibitors with traditional chemotherapy, a potentially synergistic maneuver, is a promising approach to the treatment of malignant disease. Tyrosine kinase inhibitors can increase the sensitivity of malignant tumors to nitrogen mustards (Aloyz et al., 2004).
Studies of nitrogen mustards combined with newer small-molecule targeted agents and monoclonal antibodies that act on the immune system are summarized in Table 1. Sunitinib, pazopanib, sorafenib, and vandetanib are small-molecule tyrosine kinase inhibitors, which target VEGF. There is a lack of overlapping toxicities between many VEGF inhibitors and conventional cytotoxics. Imatinib sensitizes CLL lymphocytes to chlorambucil by inhibiting chlorambucil-induced phosphorylation of the non-receptor tyrosine kinase c-Abl. Veliparib and olaparib are poly (ADP-ribose) polymerase (PARP) inhibitors. Small-molecule targeted agents have been studied in combination with cyclophosphamide, ifosfamide, melphalan, and chlorambucil. Everolimus, which is active in renal cell cancer, increases the number of Tregs and has been combined with cyclophosphamide.
Studies of nitrogen mustards combined with small-molecule targeted agents and monoclonal antibodies
Immune checkpoint inhibitors have changed the management of numerous malignancies, such as melanoma and renal, colorectal, and non–small-cell lung cancers, but 60% to 70% of patients fail to respond to single-agent immune checkpoint blockade (Yan et al., 2018). Chemoimmunotherapy combinations are therefore attractive. Ipilimumab, a monoclonal antibody that blocks cytotoxic T lymphocyte antigen 4 (CTLA-4) also has an anti-Treg action. Cyclophosphamide can downregulate CTLA-4 expression on T cells, implying that the combination of cyclophosphamide and ipilimumab may be of interest. However, the combination showed no activity in a small study of 10 patients with metastatic melanoma and was associated with greater than anticipated immunotoxicity, particularly diarrhea. A report of cyclophosphamide and pembrolizumab, a monoclonal antibody that binds to the programmed death-1 (PD-1) receptor, in heavily pretreated patients with STS was also disappointing, although the solitary responder was the only patient with a greater than 10% programmed death-ligand 1 (PD-L1) expression in immune cells, suggesting that response rates may be higher with better patient selection. Cyclophosphamide has also been combined with pembrolizumab to treat osteosarcoma and with cemiplimab (another monoclonal antibody to the PD-1 receptor) as part of a phase 1 trial, although very little data from this latter study are available. Hypoxia can lead to resistance to immunotherapy, and therefore evofosfamide, which reduces hypoxia, has been administered with ipilimumab. Therapeutic efficacy was seen, with responders having an increased peripheral T cell proliferation and an enhanced intratumoral T cell infiltration. The unique role of melphalan among the nitrogen mustards in ILP and ILI is reflected in the investigation of novel combinations in this setting. ADH-1, a pentapeptide that disturbs N-cadherin adhesion complexes, has been combined with melphalan ILI. More recently, a phase 2 trial has been performed in patients with recurrent melanoma, using ILI melphalan and actinomycin D with systemic ipilimumab. Four doses of ipilimumab were given commencing 7 to 21 days after the ILI. The PFS and OR were higher than with ILI or ipilimumab alone. The combination resulted in a shift to an inflammatory microenvironment within the tumor with an influx of T cells, which was associated with a clinical response. During the antitumor response, PD-L1 expression increased, perhaps because of chemotherapy-induced tumor inflammation precipitating rapid cell death and local inflammatory responses at the tumor site. Melphalan-based ILI in combination with CTLA-4 blockade produced a fast and prolonged response in tumors that were not overtly inflamed. A phase 1/2 trial assessing the intratumoral injection of the oncolytic virus T-VEC, followed by ILP with melphalan and TNF-α, in patients with melanoma or sarcoma has completed recruitment (NCT03555032).
Several combination studies have assessed pharmacokinetic interactions, and some conclusions can be made. The mean Cmax of cyclophosphamide is lower after the simultaneous administration of sorafenib and letrozole compared with that seen when cyclophosphamide is given with letrozole alone. The half-life of cyclophosphamide is also decreased. In children and young adults, the ratio of 4-OH-CY AUC to cyclophosphamide AUC is similar to that in adult patients, suggesting that hepatic metabolism of cyclophosphamide is unaffected by sorafenib administration. However, the median sorafenib AUC(0–24h) after the initial dose given with oral cyclophosphamide is twice that of published values after a similar dose of single-agent sorafenib. Metronomic cyclophosphamide does not alter the pharmacokinetics of imatinib. The plasma concentration time curves of ifosfamide, 2-DCEI, 3-DCEI, and 4-OH-IF are not influenced by sunitinib, but ifosfamide significantly reduces the exposure to sunitinib. Similarly, pazopanib does not influence the pharmacokinetics of ifosfamide and these metabolites, but during a continuous 72-hour infusion of ifosfamide, the concentration of pazopanib decreases by approximately 35% and the mean AUC(0–24h) of pazopanib is reduced by about 27%. Ifosfamide does not affect sorafenib pharmacokinetics and vice versa. More work is required to evaluate pharmacokinetic interactions following the use of combinations of nitrogen mustards and small-molecule targeted agents.
XI. Cyclophosphamide in Combination with Vaccine Therapy and Virotherapy
The immunomodulatory effects of cyclophosphamide have led to its use with vaccines and virotherapy. Vaccines induce the immune recognition of tumor cells, which are destroyed by the subsequent antitumor immune response. Low-dose cyclophosphamide improves the activity of tumor vaccines by decreasing the number of Tregs. Cyclophosphamide has been combined with vaccines in the context of clinical trials, but this approach has not entered routine clinical practice. Work involving patients with melanoma and renal cell cancer has shown clinical activity and variable effects on Treg numbers.
Camisaschi and colleagues (2013) found that cyclophosphamide slightly and transiently reduced the number of circulating Tregs in vaccinated patients. Intravenous cyclophosphamide (300 mg/m2) was administered 1 week before vaccination with HLA-A*0201-modified tumor peptides and 7 and 11 weeks after the initiation of vaccination. Their study included patients with early-stage melanoma with baseline circulating Treg levels only marginally above those seen in healthy donors and considerably less than in those with stage IV melanoma. The number of activated Tregs was lower in the lymph nodes of patients receiving cyclophosphamide, which may be more effective at restricting tumor-induced Tregs at the tumor site as opposed to the circulating Treg pool. Immunization did not increase DFS or OS compared with an observation group. In another study of 11 patients with melanoma receiving 300 mg/m2 cyclophosphamide intravenously 1 week before and 7 and 11 weeks after the initiation of vaccination with four melanoma peptides, along with a second cyclophosphamide dose administered on day 56, a more than 2-fold decrease in Tregs was observed in only one patient (de Vries et al., 2011). The combination of cyclophosphamide (50 mg twice daily for 1 week every second week) with an mRNA-transfected dendritic cell vaccine given intradermally in the week without cyclophosphamide also failed to change the number of circulating Tregs in patients with melanoma (Borch et al., 2016).
In a study of patients with metastatic renal cell cancer, patients were randomized to vaccination with IMA901, a multipeptide vaccine, and granulocyte-macrophage colony-stimulating factor (GM-CSF) or the vaccination plus a single intravenous dose of 300 mg/m2 cyclophosphamide (Walter et al., 2012). Cyclophosphamide was given 3 days prior to the first vaccination, resulting in a 20% decrease in Treg numbers. The addition of cyclophosphamide was associated with a prolonged median OS (23.5 vs. 14.8 months). The benefit was only seen in patients with specific immune responses to IMA901, suggesting that cyclophosphamide functioned as an immunomodulator of the vaccine rather than a nonspecific immunomodulatory agent or cytotoxic.
More recently, 50 mg cyclophosphamide given twice daily to patients with metastatic colorectal cancer on weeks 1 and 3 of a 3-week cycle, in combination with modified vaccinia ankara-5T4, depleted Treg numbers and increased PFS (Scurr et al., 2017). Low-dose cyclophosphamide (100 mg daily for 7 days) prior to vaccination with a personalized peptide vaccine increased PFS in patients with biliary tract cancer (Shirahama et al., 2017). Cyclophosphamide is currently being used in combination with vaccines in early clinical trials to treat ovarian, pancreatic, hepatocellular, breast, and prostate cancers as well as non–small-cell lung cancer and neuroblastoma (Vermaelen, 2019).
Oncolytic viruses (OVs) are selective antitumor agents with multiple mechanisms of action (Bartlett et al., 2013). They destroy infected cancer cells by direct oncolysis and uninfected cells via bystander effects. Cyclophosphamide enhances the activity of OVs in preclinical models of reovirus, vaccinia virus, measles, and adenovirus (Meyers et al., 2017). Low-dose cyclophosphamide has immunologic properties that are supportive for oncolytic virotherapy, increasing the delivery and efficacy of OVs. It improves viral replication by diminishing antiviral innate immunity and also enhances adaptive antitumor immunity induced by OVs, probably by the selective depletion and inhibition of Tregs.
Clinical trials have been performed using cyclophosphamide with virotherapy, but the combination is not yet used routinely. An oncolytic adenovirus has been given with low-dose temozolomide and cyclophosphamide (50 mg daily), starting 1 week before virus treatment and continuing until progression, to selectively reduce Tregs. A total of 41 combination treatments were administered to 17 patients who were refractory to chemotherapy. Antitumor activity was observed after 67% of treatments (Liikanen et al., 2013). In another study, patients with refractory solid tumors were treated with three different regimens of low-dose cyclophosphamide combined with an oncolytic adenovirus; 21 patients received metronomic oral cyclophosphamide at 50 mg daily, 7 patients were given a single intravenous dose of 1,000 mg, and 7 patients were treated with both the oral and intravenous cyclophosphamide schedules. Virus was injected into the tumor. Metronomic cyclophosphamide (oral and oral plus intravenous schedules) diminished Tregs but did not compromise the induction of antitumor or antiviral T cell responses. Oncolytic adenovirus administered with metronomic cyclophosphamide increased cytotoxic T cells and induced Th1-type immunity in the majority of patients. All of the cyclophosphamide regimens produced significantly higher rates of disease control than virus alone. The longest PFS and OS were seen in the oral plus intravenous group, with a 1-year PFS and OS of 53% and 42% respectively, figures that are exceptionally high in patients refractory to chemotherapy (Cerullo et al., 2011).
XII. Conclusions
The nitrogen mustards have been established for more than 60 years and have stood the test of time. They are powerful cytotoxic and lymphoablative agents and remain important today. Cyclophosphamide, ifosfamide, melphalan, and chlorambucil all have their specific places in the clinical arena. Cyclophosphamide is one of the most successful chemotherapeutics; it has proved to be safe and has revealed its many capabilities and versatility of clinical uses. Knowledge of the properties of the nitrogen mustards continues to expand. More sophisticated analytical methods have allowed detailed interrogation of the metabolic pathways of the oxazaphosphorines, which are a major influence on the balance between efficacy and toxicity. As a result, new oxazaphosphorine derivatives have been developed in attempts to decrease toxicity while maintaining efficacy, but these have not always been successful. Nevertheless, evofosfamide remains under clinical investigation. Melflufen, a promising melphalan derivative, is also being assessed in clinical trials. As well as damaging DNA, it is now recognized that the nitrogen mustards can act through other mechanisms, such as antiangiogenesis and immunomodulation. This is particularly the case for low-dose continuous cyclophosphamide, leading to the effective adoption of metronomic dosing in clinical practice. The influence of cyclophosphamide on the intestinal microbiome and the subsequent immunomodulatory effects are also of interest. The first decade of the 21st century witnessed the introduction of targeted small molecules and monoclonal antibodies into the routine treatment of malignant and autoimmune diseases, a trend that is becoming more popular, and combination trials with nitrogen mustards have been performed in the phase 1 and 2 settings.
XIII. Perspectives
Cyclophosphamide and melphalan are the most widely used nitrogen mustards. In the future, cyclophosphamide will remain an important agent in the treatment of solid and hematologic malignancies and autoimmune disorders and as a conditioning regimen for SCT. Melphalan will continue to be employed in multiple myeloma, especially as HDM. With the introduction of immunotherapy for melanoma, the subset of patients most likely to benefit from melphalan ILP needs to be identified. Ifosfamide retains a place in the treatment of sarcoma, and perhaps trofosfamide has a role in selected patients with STS and diffuse large B-cell lymphoma as maintenance treatment, but more work is needed. The use of chlorambucil is diminishing, especially now that monoclonal antibodies have become established, but it is still used in selected patients with CLL. Long-term pharmacokinetic data are required to support the use of oral metronomic scheduling. Additional studies are also necessary to clarify the transport mechanisms of the oxazaphosphorines, particularly their transport forms and transporters; such investigations may lead to interventions that decrease tumor resistance and reduce toxicity. Furthermore, it is essential to develop other new derivatives of the nitrogen mustards to improve their therapeutic indices. This can be a fertile area, as seen with the recent success of melflufen. In preclinical work, the identification of signal transduction pathways affected by cyclophosphamide may also result in novel strategies to reduce toxicity.
Pharmacogenetic studies of the nitrogen mustards have not resulted in a personalized approach to therapy. Prospective well powered analyses of larger patient cohorts in clinical trials, including patients of differing ethnicities, will be required to confirm the effect of single nucleotide polymorphisms on the interpatient heterogeneity of response and toxicity, as well as to identify new genetic markers of these two outcomes. More meta-analyses are also desirable. Pharmacogenomic assessments have concentrated on candidate genes. However, a whole genome methodology may be more comprehensive, leading to the detection of other significant factors in the clinical activity of the nitrogen mustards. Proteomic studies could illuminate crucial processes following gene expression. The latest analytical techniques for the nitrogen mustards may aid TDM, the phenotypic aspect of personalized medicine.
Advances in the utilization of cyclophosphamide as an immunomodulator bode well for the future. The advent of PTCy has permitted nearly universal access to allo-SCT. PTCy may have differential effects on T cell subsets influencing antitumor activity, immunity against infections, and GVHD. Additional work is needed to further develop the models used to study the mode of action of PTCy. Identifying specific roles of T cells could lead to innovative methods of regulating post-transplant alloreactivity, thereby improving PTCy GVHD prophylaxis. More studies are also required to elucidate the mechanisms of tolerance induced by PTCy. It is envisaged that the PTCy platform will be increasingly adapted and used in other settings such as combined solid organ and stem cell transplantation. The effects of cyclophosphamide on Tregs merit further study. Low-dose cyclophosphamide may only influence Treg subpopulations that are actively dividing, as the impact of cyclophosphamide on Tregs is transient. Tregs are often analyzed with diverse methods at varying time points, and a more thorough analysis of Tregs is required in future trials. Oncomicrobiotics are substances or microbes that alter the gut microbiome to a desired composition to enable a maximum response to immunomodulatory agents with fewer toxic effects. Combinations of cyclophosphamide with novel oncomicrobiotics need to be explored.
Immunotherapies are a pioneering development in the treatment of malignant disease. Potentially, their effectiveness can be improved by pairing with chemotherapeutic agents, but the initial clinical results of cyclophosphamide and immunotherapy combinations have been disappointing. However, this approach is in its infancy, and the therapeutic value of these combinations remains uncertain. The ideal doses, sequence, timing, and schedule of administration of chemoimmunotherapy for specific tumors are not yet known. To be successful, an increased understanding of the interaction between chemotherapy and immunotherapy is required, with rational and effective synergistic methodologies to improve activity. Investigations of the influence of immunotherapy on the cellular and molecular mechanisms of the chemosensitivity and chemoresistance of tumors are necessary to develop logical chemoimmunotherapy regimens. Furthermore, the promise of therapeutic vaccines is most likely to come to fruition through combination with immunomodulatory agents such as cyclophosphamide. A meticulous reevaluation of the mechanisms of action of cyclophosphamide may indicate a key role for this agent in future cancer immunotherapy treatment.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Highley, Landuyt, Prenen, Harper, De Bruijn.
Footnotes
This paper received no external funding.
No author has an actual or perceived conflict of interest with the contents of this article.
Abbreviations
- ABVD
- doxorubicin, bleomycin, vinblastine, and dacarbazine
- AC
- doxorubicin and cyclophosphamide
- ADH
- alcohol dehydrogenase
- AlcP
- alcophosphamide
- ALDH
- aldehyde dehydrogenase
- allo-SCT
- allogeneic stem cell transplantation
- AML
- acute myelogenous leukemia
- ASCT
- autologous stem cell transplantation
- ATG
- antithymocyte globulin
- AUC
- area under the plasma concentration-time curve
- bcl-2
- B-cell lymphoma 2
- BEACOPP
- bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone
- BEAM
- carmustine, etoposide, arabinoside, and melphalan
- BCRP
- breast cancer resistance protein
- BR
- bendamustine plus rituximab
- CAA
- chloroacetaldehyde
- CBR
- clinical benefit rate
- CD
- cluster of differentiation
- CEA
- chloroethylamine
- CEP
- circulating endothelial progenitor
- CEPM
- carboxyethylphosphoramide mustard
- CHOP
- cyclophosphamide, vincristine, doxorubicin, and prednisone
- CLL
- chronic lymphocytic leukemia
- CMF
- cyclophosphamide, methotrexate, and 5-fluorouracil
- CNM
- N-chloroethyl-1,3-oxazolidine-2-one
- CNS
- central nervous system
- COX-2
- cyclo-oxygenase 2
- CR
- complete response
- CSF
- cerebrospinal fluid
- CTD
- cyclophosphamide, thalidomide, and dexamethasone
- CTLA-4
- cytotoxic T lymphocyte antigen 4
- CVP
- cyclophosphamide, vincristine, and prednisone
- CyBorD
- cyclophosphamide, bortezomib, and dexamethasone
- DBS
- dried blood spot
- DCEC
- dechloroethylcyclophosphamide
- 2-DCEI
- 2-dechloroethylifosfamide
- 3-DCEI
- 3-dechloroethylifosfamide
- DFS
- disease-free survival
- DLBCL
- diffuse large B-cell lymphoma
- DLT
- dose-limiting toxicity
- EGPA
- eosinophilic granulomatosis with polyangiitis
- FAC
- 5-fluorouracil, doxorubicin, and cyclophosphamide
- FC
- fludarabine and cyclophosphamide
- FCR
- fludarabine, cyclophosphamide, and rituximab
- FEC
- 5-fluorouracil, epirubicin, and cyclophosphamide
- 5-FU
- 5-fluorouracil
- GC
- gas chromatography
- GC-MS
- gas chromatography-mass spectrometry
- GnRH
- gonadotropin-releasing hormone
- GPA
- granulomatosis with polyangiitis
- GST
- glutathione S-transferase
- GVHD
- graft-versus-host disease
- HC
- hemorrhagic cystitis
- HDM
- high-dose melphalan
- HiCy
- high-dose cyclophosphamide
- HL
- Hodgkin lymphoma
- HLA
- human leukocyte antigen
- HPLC
- high-performance liquid chromatography
- ICE
- ifosfamide, carboplatin, and etoposide
- IFN
- interferon
- IL
- interleukin
- ILD
- interstitial lung disease
- ILI
- isolated limb infusion
- ILP
- isolated limb perfusion
- iMN
- idiopathic membranous nephropathy
- IPM
- isophosphoramide mustard
- 4-keto CY
- 4-ketocyclophosphamide
- LAT
- L-type amino acid transporter
- LC-MS
- liquid chromatography-mass spectrometry
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- LLOQ
- lower limit of quantification
- MAP
- methotrexate, doxorubicin, and cisplatin
- MAPK
- mitogen-activated protein kinase
- MDR1
- multidrug resistance protein 1
- MGMT
- O6-methyl-guanine-DNA methyl transferase
- MOPP
- mechlorethamine, vincristine, procarbazine, and prednisone
- MP
- melphalan and prednisone
- MPA
- microscopic polyangiitis
- MRP
- multidrug resistance-associated protein
- MS
- mass spectrometry
- MTD
- maximum tolerated dose
- NAC
- N-acetylcysteine
- NF-κB
- nuclear factor κB
- NHL
- non-Hodgkin lymphoma
- NK
- natural killer
- NNM
- nor-nitrogen mustard
- NO
- nitric oxide
- OCTN2
- novel organic cation/carnitine transporter 2
- 4-OH-CY
- 4-hydroxy cyclophosphamide
- 4-OH-IF
- 4-hydroxy ifosfamide
- OR
- overall response
- OS
- overall survival
- P450
- cytochrome P450
- PFS
- progression-free survival
- PHE
- p-N, N-bis (2-chloroethyl) amino phenylacetic acid
- PM
- phosphoramide mustard
- POR
- P450 oxidoreductase
- PR
- partial response
- PSA
- prostate-specific antigen
- PTCy
- post-transplantation cyclophosphamide
- R- (prefix)
- rituximab
- RMS
- rhabdomyosarcoma
- ROS
- reactive oxygen species
- SCMC
- S-carboxymethylcysteine
- SLE
- systemic lupus erythematosus
- SSc
- systemic sclerosis
- STS
- soft tissue sarcoma
- TBI
- total body irradiation
- TC
- topotecan and cyclophosphamide
- TDGA
- thiodiglycolic acid
- TDM
- therapeutic drug monitoring
- TFLC
- turbulent-flow liquid chromatography
- TH
- helper T
- TIP
- paclitaxel, ifosfamide, and cisplatin
- TNF
- tumor necrosis factor
- Treg
- regulatory T cell
- TSP-1
- thrombospondin-1
- UPLC
- ultra-performance liquid chromatography
- VAC
- vincristine, actinomycin D, and cyclophosphamide
- Vd
- volume of distribution
- VEGF
- vascular endothelial growth factor.
- Copyright © 2022 by The Author(s)
This is an open access article distributed under the CC BY Attribution 4.0 International license.