Abstract
Cathepsin B (CTSB) is a powerful lysosomal protease. This review evaluated CTSB gene knockout (KO) outcomes for amelioration of brain dysfunctions in neurologic diseases and aging animal models. Deletion of the CTSB gene resulted in significant improvements in behavioral deficits, neuropathology, and/or biomarkers in traumatic brain injury, ischemia, inflammatory pain, opiate tolerance, epilepsy, aging, transgenic Alzheimer’s disease (AD), and periodontitis AD models as shown in 12 studies. One study found beneficial effects for double CTSB and cathepsin S KO mice in a multiple sclerosis model. Transgenic AD models using amyloid precursor protein (APP) mimicking common sporadic AD in three studies showed that CTSB KO improved memory, neuropathology, and biomarkers; two studies used APP representing rare familial AD and found no CTSB KO effect, and two studies used highly engineered APP constructs and reported slight increases in a biomarker. In clinical studies, all reports found that CTSB enzyme was upregulated in diverse neurologic disorders, including AD in which elevated CTSB was positively correlated with cognitive dysfunction. In a wide range of neurologic animal models, CTSB was also upregulated and not downregulated. Further, human genetic mutation data provided precedence for CTSB upregulation causing disease. Thus, the consilience of data is that CTSB gene KO results in improved brain dysfunction and reduced pathology through blockade of CTSB enzyme upregulation that causes human neurologic disease phenotypes. The overall findings provide strong support for CTSB as a rational drug target and for CTSB inhibitors as therapeutic candidates for a wide range of neurologic disorders.
Significance Statement This review provides a comprehensive compilation of the extensive data on the effects of deleting the cathepsin B (CTSB) gene in neurological and aging mouse models of brain disorders. Mice lacking the CTSB gene display improved neurobehavioral deficits, reduced neuropathology, and amelioration of neuronal cell death and inflammatory biomarkers. The significance of the compelling CTSB evidence is that the data consilience validates CTSB as a drug target for discovery of CTSB inhibitors as potential therapeutics for treating numerous neurological diseases.
I. Introduction
Evidence for the role of the cysteine protease cathepsin B (CTSB) in neurologic diseases has been extensively investigated and supported by CTSB gene knockout (KO) studies in mouse models of brain disorders. These animal model studies address the role of CTSB upregulation, observed in human patients with neurologic diseases, by evaluating the behavioral, pathologic, and biomarker outcomes of CTSB gene KO in disease models. This review compiles and analyzes these CTSB gene KO data, showing that the absence of the CTSB protease results in improved behavioral deficits, amelioration of neuropathology, and regulation of biomarkers in mouse models of epilepsy, multiple sclerosis, traumatic brain injury, hypoxia/ischemia, tolerance to chronic opioid use, inflammatory pain, aging, Alzheimer’s disease (AD), and chronic periodontitis-associated AD.
This review includes information on CTSB neurobiology, describes gene KO strategies for drug target validation and drug discovery, and discusses advantages and limitations of gene KO studies. This CTSB knowledge is used for elucidating the mechanistic roles of lysosomal CTSB in cell death and inflammation in such neurologic disease mouse models.
The consilience of the CTSB KO data in mouse models of neurologic disorders is that CTSB KO results in improvements in behavioral deficits, pathology, and dysregulated biomarkers compared with sufficient wild-type (WT) mice. Notably, CTSB KO in normal mice are generally healthy, fertile, and indistinguishable from WT mice. These significant results demonstrating CTSB-dependent mechanisms in models of brain disorders provide support for the proposition that inhibiting CTSB proteolytic activity is a logical therapeutic approach for such neurologic diseases.
II. Cathepsin B is Elevated in Human Neurologic Disorders
Clinical findings show that CTSB levels are increased in many neurologic conditions, including several neurodegenerative diseases (Table 1). In AD, CTSB protein levels in the brain temporal cortex from patients with AD were 80% higher than that of age-matched controls (Batkulwar et al., 2018). AD brain autopsy samples showed that high CTSB protein concentration and enzymatic activity are abnormally localized at amyloid brain plaques (Cataldo and Nixon, 1990), although no difference in CTSB activity was reported for homogenized AD frontal cortex tissue (Mantle et al., 1995). Serum and plasma CTSB protein levels were 50% higher in AD patients relative to age-matched controls (Sundelöf et al., 2010; Sun et al., 2015). Importantly, in patients with AD, high serum CTSB levels were strongly correlated with cognitive dysfunction (Sun et al., 2015). Interestingly, blood monocytes and lymphocytes from patients with AD had about 50% less CTSB protein than those from controls (Tiribuzi et al., 2014), suggesting that a redistribution of CTSB from such cellular to extracellular compartments may occur. Cerebrospinal fluid (CSF) CTSB protein levels from patients with AD were significantly higher when analyzed by proteomic methods (Zhang et al., 2005) and appeared higher when assayed by Western immunoassays (Sundelöf et al., 2010; Armstrong et al., 2014), but CSF CTSB activity was not different from controls (Nagai et al., 2000). However, evaluation of the stability of CTSB activity in postmortem samples is necessary since it is known that CTSB has a finite period of stability at extracellular neutral pH (Yoon et al., 2021).
Elevation of cathepsin B in patients with neurologic disorders
In chronic periodontitis linked to AD, serum CTSB levels were 43% higher relative to controls (Rong et al., 2020). In addition, the higher levels of serum CTSB in these patients correlated with reduced minimental state exam scores that measure cognitive function. Thus, patients with chronic periodontitis and AD display elevated serum CTSB that correlates with cognitive dysfunction.
In human immunodeficient virus- (HIV) associated neurocognitive disorders, elevated CTSB was observed in hippocampus and basal ganglia brain regions (Rodriguez-Franco et al., 2012). Furthermore, monocytes from plasma of patients with HIV-associated dementia showed higher CTSB levels compared with controls (Rodriguez-Franco et al., 2012; Cantres-Rosario et al., 2013).
Patients with amyotrophic lateral sclerosis (ALS) displayed a threefold elevation of CTSB expression (Saris et al., 2013) in spinal tissue compared with controls (Dangond et al., 2004; Offen et al., 2009). Moreover, CTSB protein levels were higher and abnormally distributed in dying neurons from the anterior horn of patients with ALS relative to controls, suggesting that CTSB may be associated with neuronal cell death (Kikuchi et al., 2003).
CTSB activity or protein levels were significantly elevated in CSF from patients with Guillain-Barre syndrome, chronic demyelinating polyneuropathy, and multiple sclerosis (MS) neuroinflammatory disorders relative to controls (Nagai et al., 2000). Also, levels of cystatin C, an endogenous inhibitor of CTSB, were reduced in these inflammatory neurologic diseases, consistent with elevated CTSB activity. These findings suggest that CTSB activity may be closely involved in the pathophysiology of these inflammatory neurologic diseases.
Patients with polytrauma, which can include patients with traumatic brain injury (TBI), exhibit neurologic complications and have elevated CTSB (Assfalg-Machleidt et al., 1990; Jochum et al., 1993). Plasma CTSB activity was significantly increased at one day after trauma injury and remained at higher levels at the third day to two weeks post-trauma. The sixfold elevation in plasma CTSB activity correlated with the severity of injury and clinical outcomes of fatal organ failures. Related to polytrauma, in patients with TBI, CTSB protein levels in the CSF were increased twofold compared with controls (Boutte et al., 2020). In multiple trauma with sepsis, plasma CTSB was increased (Jochum et al., 1993). Brain aneurysm trauma resulted in elevated CTSB protein levels within the brain vascular endothelial layer at the aneurysm site (Aoki et al., 2008).
These human clinical data show that numerous neurologic disorders are associated with increased CTSB. Notably, several studies showed a correlation between elevated CTSB and clinical outcomes including cognitive dysfunction of AD and organ failures in trauma. These data provide support for the hypothesis that inhibition of CTSB may be a logical approach for treating such brain disorders.
III. Cathepsin B Genetic Mutations Increase Cathepsin B and Cause Human Diseases
Keratolytc winter erythema (KWE) is an orphan skin disease (Ramsay et al., 2019) caused by a mutation upstream of the CTSB gene located at an intergenic region containing an enhancer element known to be active in skin development (Oti et al., 2016; Ngcungcu et al., 2017). Duplication of the enhancer results in the mutation causing overexpression of CTSB in the skin stratum granulosum of affected individuals. South African and Norwegian KWE families each carry different duplications that overlap in the enhancer region. Environmental conditions, such as cold winter weather and certain physiologic conditions, trigger the enhancer element to upregulate CTSB expression resulting in erythema and hyperkeratosis.
Palmoplantar keratoderma (PPK) is a heterogeneous group of keratinization skin disorders classified as sporadic or genetically inherited forms, with the latter caused by a plethora of mutations in many genes (reviewed in Schiller et al., 2014). A genetic analysis of the CTSB gene was conducted in a patient with PPK who presented with a KWE-like phenotype, finding a mutation in the CTSB gene that caused the disease (Mohamad et al., 2021). A family pedigree analysis suggested an autosomal dominant mode of gene inheritance in the disease, and gene sequencing found a single nucleotide mutation in the CTSB gene that resulted in substitution of a methionine residue for valine at position 255 at the active site. The mutant CTSB was found to have 10% higher in vitro activity relative to normal CTSB. The authors concluded that the gain in CTSB activity resulted from the CTSB gene mutation of the patient with PPK.
Mutation of CTSB may be associated with pancreatitis. CTSB activity is involved in the initiation of acute pancreatitis in an animal model (Halangk et al., 2000). A significantly higher SNP (single nucleotide polymorphism) allele frequency was found in Southern Indian patients with tropical calcific pancreatitis relative to controls (Mahurkar et al., 2006). That mutation caused a leucine to valine substitution at position 26 in the propeptide region of CTSB, which may alter CTSB maturation. However, a subsequent study evaluated the position 26 mutation in European patients with idiopathic chronic pancreatitis and found no difference in the allele frequency compared with controls and other ethnic groups, indicating that the mutation was not a susceptibility factor for pancreatitis in the population of patients studied (Weiss et al., 2007). The findings suggest that CTSB gene mutations of pancreatitis may vary in patient groups of different ethnic backgrounds.
The CTSB gene mutations identified thus far have resulted in increased CTSB. On the other hand, we are not aware of a human disease in which a CTSB loss of function mutation causes a disease. Genetic mutations also occur in other cathepsin proteases, including cathepsin A, cathepsin D, cathepsin F, cathepsin K, cathepsin C, and cathepsin H, and cause rare diseases (Ketterer et al., 2017).
IV. Cathepsin B Is Elevated in Animal Models of Neurologic Diseases
Brain CTSB is elevated in a wide variety of neurologic animal models, including AD, TBI, ischemia, inflammatory injury, ALS, and epilepsy (Table 2). This suggests that elevated CTSB in the brain commonly occurs in neurologic pathologies.
Elevation of Cathepsin B in Animal Models of Neurologic Disorders
Transgenic AD mouse models have been developed that overexpress human amyloid precursor protein (APP) and presenilin 1 (PS1) containing familial mutations found in people genetically predisposed to develop some of the pathology and behavioral deficits observed in patients with AD (Parent and Thinakaran, 2010; Sasaguri et al., 2017). CTSB expression is elevated by 32% in the 5X familial Alzheimer’s disease (FAD) mouse model, which expresses three APP mutations consisting of APP KM670/671NL (Swedish mutation), APP 716V (Florida mutation), and APP V171I (London mutation) combined with two human PS1 mutations consisting of PSEN1 M146L and PSEN1 L286V (Bouter et al., 2014). In the APPSwe/PS1 AD mouse model, expressing human APP with the Swedish FAD mutation and PS1 with FAD mutations, CTSB enzyme levels were increased by 50% in the cortex and hippocampus brain regions (Sun et al., 2015).
In TBI models, such as the severe open skull controlled cortical impact (CCI) mouse, penetrating ballistic-like brain injury (PBBI) rat model, and closed head weight drop mouse and rat models, CTSB in the brain was elevated in its RNA (Natale et al., 2003) and protein levels (Zhang et al., 2006; Luo et al., 2010; Boutte et al., 2020), as well as its proteolytic activity (Hook et al., 2014a; Boutte et al., 2020). These TBI-induced elevations in CTSB occurred in various brain regions, including the hippocampus, for various times postinjury from two days to one month and was accompanied by cognitive deficits or neuromuscular dysfunction. In a moderate closed head weight drop TBI rat model, increased CTSB was observed by histologic expression that occurred in the hippocampus and numerous other brain regions (Martinez-Vargas et al., 2014). In a CCI TBI mouse model, brain CTSB activity was significantly elevated by 64% at two hours after injury relative to controls (Hook et al., 2014a). In a severe PBBI TBI rat model, CTSB levels and activity were significantly increased in the cortex and hippocampus at 3–7 days after PBBI (Boutte et al., 2020). In a weight drop TBI mouse model, brain CTSB was elevated at 6 hours after injury and peaked at 2 days post-trauma, with continued elevation for up to one week (Luo et al., 2010). In a rat TBI model, brain CTSB was elevated within one hour after injury, which maximally increased at 8 days postinjury and remained elevated for more than one month (Zhang et al., 2006). These models of moderate to severe TBI together demonstrate that a rapid and sustained CTSB increase can occur after trauma.
Ischemia and inflammatory brain injuries in animal models also resulted in elevated CTSB. For example, increased CTSB of 2- to 3-fold above controls occurred in brains of acute and chronic rat and nonhuman primate ischemic models (Seyfried et al., 1997; Yamashima et al., 1998; Tsuchiya et al., 1999; Tsubokawa et al., 2006). Inflammation due to bacterial meningitis (Hoegen et al., 2011), sepsis (Ruff and Secrist, 1984; Hummel et al., 1988), neuroinflammation (Terada et al., 2010; Wu et al., 2017; Ni et al., 2019), and inflammatory pain (Sun et al., 2012) resulted in elevation of brain CTSB that was 50% to sixfold above controls.
In ALS models, CTSB expression was elevated by threefold in the spinal cord (Offen et al., 2009). CTSB was also upregulated in patients with ALS (Saris et al., 2013). In animal models of epilepsy, CTSB protein levels are elevated (Ni et al., 2013). Furthermore, in excitotoxicity related to Huntington’s disease, cathepsin B is elevated (Wang et al., 2006). These findings indicate association of elevated brain CTSB in multiple animal models of neurologic diseases.
V. Cathepsin B Neurobiology
A. Cathepsin B Is a Cysteine Protease
1. Cathepsin B Protease Activity
CTSB is a lysosomal protease that cleaves peptide bonds of proteins and peptides to generate smaller fragments that often have distinct biologic functions. CTSB is a cysteine protease based on its reactive cysteine residue within its active site. CTSB belongs to the CA clan of cysteine proteases (Rawlings et al., 2014) and is a member of the C1A family whose members are closely related to papain (cysteine protease from papaya) and includes the cysteine cathepsins B, C, F, H, K, L, O, S, V, W, and Z (also called cathepsin X). In addition to the cysteine cathepsins, cathepsins include aspartyl proteases cathepsin D and cathepsin E and the serine proteases cathepsin A and cathepsin G (Mort, 2004; Turk et al., 2012; Hsu et al., 2018).
CTSB has been extensively studied since its discovery in 1939 (Fruton and Bergmann, 1939). Forty-four years later, the first amino acid sequences of CTSB were determined (Takio et al., 1983), followed shortly thereafter by the first gene sequences (Chan et al., 1986) and then the first X-ray crystal protein structure (Musil et al., 1991). Most proteases have either endopeptidase activity, which cleaves peptide bonds within the polypeptide substrate, or exopeptidase activity, which removes amino-terminal or carboxy-terminal residues. CTSB is unusual in having both endopeptidase (Mort, 2004) and exopeptidase activities (Aronson and Barrett, 1978; Takahashi et al., 1986) regulated by its occluding loop structure at the active site (Illy et al., 1997).
Endopeptidases and exopeptidases recognize specific amino acids flanking the cleaved peptide bond (Schechter and Berger, 1967). When acting as an endopeptidase, CTSB prefers to cleave peptide bonds with an adjacent positively charged arginine or lysine amino acid (P1 position) and a bulky hydrophobic or arginine amino acid at the next distal position (P2 position) (Mort, 2004; Gosalia et al., 2005; Choe et al., 2006; Yoon et al., 2021). CTSB and other proteases possess selectivity for cleavage preferences that may be unique or overlapping.
CTSB protease activity in biologic samples is typically assayed with fluorogenic peptide-AMC substrates that mimic peptide bond cleavages to generate free, fluorescent AMC (7-amino-4-methylcoumarin) that is quantitatively measured. A typical CTSB fluorogenic substrate used in the field is Z-Phe-Arg-AMC, where Z is an N-terminal carboxybenzyl blocking group (Murata et al., 1991; Ruzza et al., 2006). But such a substrate can be cleaved by multiple proteases in addition to CTSB (Hwang et al., 2005). Therefore, a cautionary note regarding such fluorogenic activity data is that such results can indicate multiple protease activities in addition to CTSB. Thus, in heterogeneous tissue samples containing many proteases, CTSB protease activity is best identified with use of a specific inhibitor of CTSB, CA-074 (L-3-trans-(Propylcarbamyl)oxirane-2-carbonyl)-L-proline) (Towatari et al., 1991). Activity that is inhibited by CA-074 is designated CTSB activity (Boutté et al., 2020). It is also noteworthy that CTSB requires a reduced state of the active site cysteine for its proteolytic activity.
2. Cathepsin B Maturation
Active CTSB is generated through a maturation process involving gene transcription, translation, and conversion of the inactive pro-CTSB to mature active CTSB (Fig. 1). Human CTSB is encoded as a single gene on chromosome 8 at position p22-23.1 (Fong et al., 1992), consisting of >21 kilobases and contains 12 exons (Gong et al., 1993). Mouse CTSB is also a single gene containing about 20 kilobases, 10 exons, and nine introns on chromosome 14 at position 33.24 (Qian et al., 1991; Deussing et al., 1997). Human and mouse CTSB nucleic acid sequences encoding the active form of the protease have 82% homology (Chan et al., 1986). In normal human tissues, CTSB mRNAs consist of 2.3 and 4.0 kilobase transcripts, which differ in the untranslated regions (Gong et al., 1993), with the normal brain containing primarily the 2.3 kilobase mRNA form (Sivaparvathi et al., 1995).
Maturation of CTSB: zymogen conversion to active CTSB. Mature, active CTSB is generated from its inactive zymogen that is converted to the active CTSB enzyme. Preprocathepsin B is generated from its mRNA and its N-terminal signal sequence (SP) is removed by signal peptidase to result in procathepsin B. Procathepsin B undergoes autoproteolysis to remove the propeptide (Pro) to generate the mature CTSB. CTSB may also undergo additional processing into light and heavy chains linked by disulfide bonds. Cys108 (*) represents the active cysteine residue. These sequences of human CTSB were obtained from National Center for Biotechnology Information (NCBI) and UniProt databases.
CTSB mRNA is translated into its preproenzyme of 339 amino acids, which is cotranslationally processed to remove the N-terminal signal peptide to result in proCTSB. The proenzyme is then glycosylated in the rough endoplasmic reticulum, translocated to the Golgi apparatus, and undergoes trafficking to the lysosome (reviewed in Mort 2004; Katunuma, 2010; Turk et al., 2012). The proenzyme undergoes autohydrolysis to form the shorter mature CTSB (∼27 kDa), which can be further processed into a double chain form consisting of heavy (∼22 kDa) and light (∼5 kDa) chains covalently linked by disulfide bonds (Fig. 1) (Hook et al., 2020). Both the mature and double chain forms are enzymatically active.
B. Cathepsin B in Brain and Subcellular Organelles
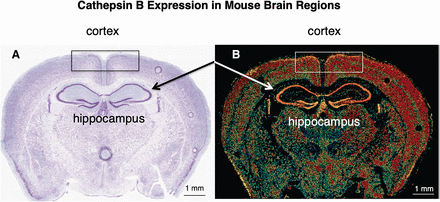
In normal human and rodent brains, CTSB protein is concentrated in neuronal cells of the cortex, hippocampus, neostriatium, and cerebellum (Howie et al., 1985; Bernstein et al., 1990; Nakanishi et al., 1994). In the human brain, CTSB gene expression is consistently and highly expressed in 16 brain regions from early prenatal to young adult and is one of the four most abundantly transcribed brain cathepsins (Hsu et al., 2018). A view of CTSB gene expression in the mouse brain indicates its high abundance in hippocampus and cortex, regions involved in memory and cognitive functions (Fig. 2) (Hook et al., 2015). Brain tissues release CTSB to the cerebrospinal fluid (CSF), and elevated CTSB in CSF occurs with increasing age (Nilsson et al., 2013).
CTSB expression in mouse brain regions. (A) Nissl stain of mouse brain. A coronal section of adult mouse brain was subject to Nissl staining (from the Allen Brain Institute (http://www.brain-map.org/). (B) CTSB mRNA expression in mouse brain. In situ hybridization of mouse brain sections was conducted with antisense mRNA to cathepsin B. The relative levels of CTSB mRNA expression are shown at high levels by shades of yellow to red (yellow, highest express); lower relative expression levels are shown in green to blue (blue, lowest level of expression). CTSB displays high expression of the hippocampus and cortex regions (adapted from Hook et al., 2015, DOI: 10.3389/fneur.2015.00178 indicating Frontiers as the original publisher).
Within cells, CTSB is normally sequestered primarily in lysosomes but is also found in vesicles of the regulated secretory pathway (Tu et al., 2008). CTSB in lysosomes participates in protein degradation to maintain cellular homeostasis, functioning at the acidic pH within lysosomes (Hanewinkel et al., 1987; Mort and Buttle, 1997; Jung et al., 1999). The CTSB concentration within lysosomes is among the highest compared with other cathepsin enzymes of cathepsins L and H (Katunuma, 2010). CTSB resides in lysosomes together with other cysteine cathepsins (cathepsins C, F, H, K, L, O, S, V, W, and Z), aspartyl cathepsins D and E, serine cathepsins A and G, and other proteases, such as legumain and tripeptidyl-peptidase, which together function in lysosomal protein degradation (Hsu et al., 2018).
Autophagosomes are present in cells for degradation of accumulated proteins, damaged organelles, and microorganisms via a process called macroautophagy (Xie and Klionsky, 2007; Uchiyama et al., 2008). Lysosomes fuse with autophagosomes to provide the proteases needed for protein degradation, including CTSB as a key protease in autophagy catabolism.
Secretory lysosomes are another subcellular organelle where CTSB resides (Tu et al., 2008). Secretory lysosomes function in protein degradation and as secretory vesicles for release of its contents, including CTSB to the extracellular environment (Blott and Griffiths, 2002). In the mammalian central nervous system, astrocytes contain secretory lysosomes (Verkhratsky et al., 2016), which can secrete active CTSB in response to a neurotoxin (Verderio et al., 2012). Hippocampal pyramidal neurons also secrete CTSB via lysosomal exocytosis in response to back-propagating action potentials (Padamsey et al., 2017).
CSTB functions in nuclei have been shown by several studies. Such reports have shown that nuclear CTSB functions in, for example, cell viability (Bestvater et al., 2005), thyroid carcinoma (Tedelind et al., 2010), sirtuin degradation in microglia nuclei (Meng et al., 2020, and mitotic chromosome segregation (Hämälistö et al., 2020).
Neurons and endocrine cells contain regulated secretory vesicles that contain neurotransmitters and hormones that are released in response to stimulation by electrical action potentials and receptors (Lin and Salton, 2013). In the central nervous system (CNS), electrical signals activate neurons to secrete chemical neurotransmitters from secretory vesicles at synapses for neurotransmission. CTSB is present in regulated secretory vesicles of pancreatic acinar (Kukor et al., 2002), pancreatic beta (Kuliawat et al., 1997), kidney juxtaglomerular (Wang et al., 1991), neuroendocrine adrenal cells (Hook and Reisine, 2003; Jiang et al., 2021), and neuronal cells (Klein et al., 2009).
In the extracellular environment, CTSB is able to degrade protein scaffolds, such as fibrinogen (Gabrijelcic et al., 1988), collagen (Maciewicz et al., 1990; Li et al., 2016), and the basement membrane components of laminin, fibronectin, and collagen type IV (Buck et al., 1992). Furthermore, extracellular CTSB damages tight junctions of endothelial cells, resulting in permeability of the vessel wall (Wang, et al., 2016). Thus, extracellular CTSB is particularly dangerous in causing biologic damage.
C. Cathepsin B Lysosomal Leakage Causes Inflammation and Cell Death in Neurologic Disorders
CTSB is virtually absent in cytosol of normal healthy cells that sequester CTSB in lysosomes, which protects cytosolic proteins from CTSB degradation (Chiappini et al., 2015). However, in numerous neurologic disease conditions, lysosomal leakage allows CTSB to move into the cytosol where it triggers apoptosis and activates production of proinflammatory cytokines that participate in neurodegeneration and behavioral deficits (Fig. 3). Cytosolic CTSB may also induce necrotic cell death, causing inflammatory reactions at the necrotic site. CTSB in the cytosol induces assembly of the NACHT, LRR, and PYD domains containing protein 3 (NLRP3) inflammasome whose cell death-inducing interleukin-1 converting enzyme (caspase-1) matures the potent proinflammatory interleukin (IL)-1β, (Campden and Zhang, 2019). Further detrimental effects of cytosolic CTSB are the induction of tumor necrosis factor alpha (TNFα) and oxidative cell stress (reviewed in Hook et al., 2020). Based on those mechanisms, cytosolic CTSB participates in the pathogenesis of many neurologic conditions (Hook et al., 2020).
Cathepsin B lysosomal leakage leads to cell death and neuroinflammation in behavioral deficits and neurodegeneration of neurologic disorders. CTSB is normally located within lysosomes. In numerous brain trauma and neurodegenerative disease conditions, lysosomal membrane permeabilization (LMP) results in translocation of CTSB to the cytosol. It is hypothesized that pathogenic cytosolic CTSB activates pathways for cell death and inflammation that result in behavioral deficits and neurodegeneration pathology. CTSB in the cytosol is involved in proteolysis to generate proapoptotic tBid and degrades antiapoptotic Bcl-xL to mediate cell death (Repnik and Turk, 2010; de Castro et al., 2016). Cytosolic CTSB activates production of IL-1β and IL-18 pro-inflammatory factors (Hentze et al., 2003; Bai et al., 2018; Campden and Zhang, 2019) that are released through the gasdermin D pore (GSDMD) (Tsuchiya et al., 2021). Cell death and inflammation result in neurodegeneration and behavioral deficits of numerous neurologic disease conditions.
Importantly, at the neutral pH condition of the cytosol, CTSB possesses proteolytic activity (Mort et al., 1984; Linebaugh et al., 1999; Yoon et al., 2021). This contrasts with many other lysosomal proteases that are only active at the acidic pH within lysosomes and are not active at the neutral pH of the cytosol (Turk et al., 2012; Yoon et al., 2021). CTSB activity in the cytosol cleaves substrates that lead to activation of cell death and inflammation (Fig. 3). In addition, CTSB also translocates to the nucleus where it degrades sirtuins and promotes aging (Meng et al., 2020). The precise cytosolic substrates degraded by CTSB are not yet well-characterized. It will be important in future studies to define the cytosolic CTSB-mediated pathways of cell death and inflammation in neurodegeneration.
D. Endogenous Inhibitors of Cathepsin B
Cysteine cathepsin protease activity is controlled in vivo by potent endogenous protease inhibitors consisting of cystatin type 1 (the stefins), cystatin type 2, and kininogens (reviewed in Turk and Bode, 1991). The cystatin type 1 family is composed of cystatin A and cystatin B, which reside primarily in the cytosol and nucleus of cells where they are the primary means for inhibiting unwanted lysosomal cysteine protease activity (Turk et al., 2002). In extracellular fluids, CTSB activity is controlled by cystatin C, which is a member of the cystatin 2 family. Human CSF and plasma cystatin C concentrations are 16,000- and 170-fold greater than that of their CTSB concentrations, respectively (Hook et al., 2015). CSF and plasma cystatin C concentrations reduce CTSB activity by half in 0.69 and 3.5 seconds, respectively (Abrahamson et al., 1986), showing that cystatin C is an effective CTSB inhibitor. As an example of the importance of endogenous inhibitors, loss of cystatin B inhibition causes a rare childhood form of epilepsy called Unverricht-Lundborg type (discussed in section VIII.C of this review) (Houseweart et al., 2003). Clearly, there is a considerable biologic investment made in endogenous inhibitors to control cytosolic and extracellular cysteine protease activity, including CTSB.
VI. Gene Knockout Mice for Target Validation and Drug Discovery
A. Mouse Gene Knockout Studies to Understand Mechanisms of Human Diseases
1. Gene Knockout Approach
The mouse (mus musculus) has been extensively used as a model for studying human diseases for over a century. Mouse models of neurologic diseases provide a bridge for understanding the roles of genes that are dysregulated in human brain disorders with respect to behavioral dysfunctions and cellular mechanisms. Understanding of mechanisms participating in human disease pathogenesis has been advanced through investigation of transgenic mice with knockout of specific genes. The KO technology allows individual genes to be silenced and the biologic effects of the gene’s absence can be evaluated in the living animal. Mario R. Capecchi, Martin J. Evans, and Oliver Smithies developed this technology for which they were awarded the Nobel Prize in 2007 (Hansen, 2007) with the first gene knockout studies published in 1990 (Koller et al., 1990; Zijlstra et al., 1990).
Mice are advantageous as models for human diseases since 99% of mouse genes have human homologs with a mean amino acid identity of 78.5% (Waterston et al., 2002). Also, mice share common mammalian processes for development, body formation, physiology, behavior, and diseases with humans. Mouse gene KO studies have advanced understanding of the biologic functions of numerous gene products (Palmiter and Brinster, 1985; Paigen, 2003).
Gene KO mice are generated from in vitro mouse embryonic stem cells (ESC). Briefly, homologous recombinant genes (which cannot be transcribed) are inserted into ESC at a predetermined location in the genome to thereby replace the gene at that position and prevent transcription of the targeted gene (reviewed in Mak, 2007). Hence, that gene is knocked out. The ESC with the modified, nonfunctional gene are selected and transferred into nonmodified mouse blastocysts, which are implanted into pseudopregnant mice. The resulting progeny contain the target gene in some cells but not others (chimeras); the progeny that carry the target gene in germ cells are selected for and bred to produce offspring in which the gene is inactivated in all tissues. A variant of this method, called gene trapping, was subsequently developed that allows larger numbers of genes to be replaced more rapidly by randomly inactivating genes (reviewed in Stanford et al., 2001). Both methods are used, and each has its advantages.
2. Limitations and Controls for Gene Knockout Studies
Use of gene knockout mice should consider limitations of the approach, which include developmental lethality, tissue specificity, genetic background effects, and passenger mutations. Such limitations can be minimized with appropriate controls that are important for interpretation of gene KO results. It is also important to assess gene knockout effects in both male and female genders of animals.
A limitation of gene knockout studies is that about 15% of the constitutive gene KO are developmentally lethal, such that genetically altered homozygous embryos cannot develop into adult mice. In some cases, offspring having only one copy of the wild-type gene may be viable and can be studied. With respect to KO of mutant genes that are lethal, heterozygous offspring having one copy of the mutant gene may be viable for experimental studies.
Also, constitutive KO mice cannot be used to evaluate effects of deleting the protein in one particular tissue because the germline transmitted KO removes the protein from all tissues. Therefore, conditional KO approaches for deletion of the gene at a defined age or in a defined tissue are increasingly used in the field. Nonetheless, all CTSB KO studies described in this review have been made by constitutive KO models, and useful information on CTSB functions have resulted from those KO studies.
The animal’s genetic background can affect outcomes of gene KO conditions. For example, commonly used inbred strains of mice exhibit differences in morphine and pain sensitivity (Mogil and Wilson, 1997; reviewed in Lariviere et al., 2001) as well as cognitive ability (Brooks et al., 2005). Moreover, substrains of the most commonly used strain C57BL/6 may exhibit behavioral differences (Bryant et al., 2008). It is, therefore, important to define the genetic background.
Another potential problem of gene KO studies is the issue of “passenger” mutations, consisting of unwanted genetic alterations that accompany the knockout modification that can confound outcome results (reviewed in Gerlai, 1996). Knockout mice are often congenic, which is to say they contain genetic material from another mouse strain. This occurs because the ESC and the blastocyst/mouse are often from different mouse strains. In the first decade of gene targeting in mice, 129 mouse strain ESC cells were implanted into the C57BL/6 blastocyst and mouse because C57BL/6 mice are easy to breed and well-characterized. The C57BL/6 mice with the gene deletion are repeatedly backcrossed to reduce the 129 gene pool by recombination, but segments of the 129 gene pool around the targeted gene locus usually remain because during meiosis the homologous recombination frequency for allelic exchange near the transgene is low. The passenger genes from the ESC cells can produce different proteins from that of the wild-type surrogate strain. An example of a passenger confound effect occurred in caspase-1 KO C57BL/6 mice that were generated with 129 ESC that resulted in no mIL-1β as expected but surprisingly reduced septic shock mortality in response to lipopolysaccharide treatment (Li et al., 1995). The genome of those animals was subsequently shown to contain a mutant strain 129 caspase-11 gene, which provided resistance to septic shock (Kayagaki et al., 2011). In another example, caspase-3 KO C57BL/6 mice produced using 129 ESC were also resistant to septic shock mortality due to the strain 129 caspase-11 passenger gene (Vanden Berghe et al., 2013). There are over a thousand 129 strain genes, which diverged from the C57BL/6 strain sequence (Vanden Berghe et al., 2015) and 12 backcrosses over 2 years of breeding have been estimated to result in C57BL/6 mice with 1% 129 genes or about 300 129 genes (Festing, 1992). However, by now most newly generated KO strains are based on targeting ES cells derived from C57BL/6 mice, allowing for defined background genetics in the animal studies (Pettitt et al., 2009). Further, control studies can resolve confounding passenger effects, which include generating ESC in cells of the surrogate strain (e.g., Uccellini et al., 2020), conducting rescue experiments in which the functional gene is reinserted into the knockout, or generating “knock-in” mice in addition to the knockout mice (reviewed in Gerlai, 1996).
Gene knockout studies should assess whether similarities or differences occur for male and female genders. Neuropathological CTSB KO studies to date have not compared male and female genders (Table 3). It will be important to assess if gender effects may occur in CTSB KO mice since such effects have been shown to occur in models of anxiety (Czibere et al., 2011) and cholesterol (Wong et al., 2013).
Cathepsin B Knockout Improves Behavioral Deficits in Animal Models of Neurologic Disorders
B. Gene Knockout Mice in Drug Discovery
1. Drug Targets Assessed by Gene Knockout
Gene knockout animals are a powerful tool for identifying potential drug targets. Knockouts of the druggable genome are particularly important for target validation. Genes of the druggable genome encode proteins having a function that can be pharmacologically affected and include genes encoding enzymes, receptors, transporters, channels, and secreted proteins. A review of KO mice for the top drug targets showed there is a strong correlation between phenotypes, mechanism of action, and utility of the associated therapeutics that have led to the conclusion that KO animals provide a path forward for the biopharmaceutical industry to discover next generation targeted therapeutics (Zambrowicz and Sands, 2003).
A large public effort by a pharmaceutical company to use gene knockout animals to identify drug targets has been achieved by Lexicon Pharmaceuticals in its Genome5000 campaign in partnered alliances (Tang et al., 2010; reviewed in Brommage et al., 2019). From 2001 to 2008, 4654 knockout animals of the druggable genome were created and data were published on 100 gene knockouts focusing on obesity and bone phenotypes. An example of a drug that resulted from this effort is XERMELO (telostritat), a treatment of carcinoid syndrome diarrhea caused by intestinal serotonin (reviewed in Rendell, 2019). Deletion of the serotonin-synthesizing gene tryptophan hydroxylase resulted in improved physiology of the carcinoid disease condition; as a result, small molecule inhibitors of tryptophan hydroxylase were developed for therapeutics. To our knowledge, the Genome5000 campaign has not published on CTSB KO animals.
Gene KO mouse approaches and resources are benefiting investigation of the mechanistic roles of specific genes, including CTSB, as candidate drug targets in human brain disorders modeled in transgenic models of neurologic diseases.
2. On- and Off-Target Drug Effects Assessed in Gene Knockout Mice
Gene KO animals can be advantageously used to evaluate the potential of candidate drugs for on- versus off-target effects. For example, if the drug targets inhibition of a particular gene product, then the candidate drug will result in no changes when administered to animals with KO of that target gene. Alternatively, effects of the drug in animals with KO of the target gene can indicate off-target effects.
Such inhibitor effects in target gene KO mice are useful for protease inhibitor drug candidates.
Analyses of drug effects in mice with KO of the target gene may reveal specific actions of the drug at the target, off-target effects, or a combination of on- and off-target effects. This approach has been used in CTSB and other cysteine cathepsin KO mice to evaluate an inhibitor’s efficacy by on- and off-target effects (Hook et al., 2014b).
VII. Cathepsin B Knockout Mice Are Generally Indistinguishable from Wild-Type Healthy Animals
The first CTSB KO mouse article reported that the CTSB KO mice (generated in the 129 strain and later backcrossed for more than 10 generations to C57BL/6) appeared normal and had the same major histocompatibility complex class II-mediated antigen presentation as wild-type mice (Deussing et al., 1997). CTSB KO mice were reported to be healthy and indistinguishable from heterozygous and wild-type littermates, based on histologic inspection of external and internal organs, which showed normal tissues in the brain, heart, lung, liver, spleen, thyroid, pancreas, stomach, intestine, ovary, kidney, skeletal muscle, and lymph nodes in the CTSB KO animals (Halangk et al., 2000; Reinheckel et al., 2001). Moreover, those studies reported that CTSB KO mice were fertile, reproduce normally, and had no abnormalities in T or B lymphocytes. Thus, deletion of the CTSB gene results in animals that are healthy and similar to wild-type mice.
While CTSB KO mice are generally phenotypically indistinguishable from wild-type mice, several biochemical differences have been considered. For example, CTSB KO animals display higher levels of thyroid prohormone thyroglobulin relative to wild-type animals. Nonetheless, CTSB KO mice maintain normal blood thyroid hormone thyroxine levels and thus do not display hyperthyroid pathology (Friedrichs et al., 2003). Analysis of the CTSB KO mouse skin proteome determined that nine proteins in the ubiquitin proteasome and vascular endothelial growth factor signaling pathways are more abundantly expressed compared with wild-type mice, and six proteins in the serine protease inhibitor class are less abundantly expressed relative to wild-type mice (Tholen et al., 2013). But such differences do not phenotypically affect CTSB KO animals since they have normal skin and fur.
CTSB KO mice exhibit several subtle behavioral differences compared with wild-type mice. For example, female CTSB KO mice display slightly higher anxiety than female wild-type mice as measured in a swim test (Czibere et al., 2011). In another study, running improves memory and increases hippocampal neuronal cell density in wild-type mice compared with sedentary animals, but in CTSB KO mice these effects of running were not observed (Moon et al., 2016). But, as described in this review article (next section VIII), eliminating CTSB gene expression generally improves memory and pathology in animal models of brain disorders. Thus, eliminating CTSB appears to cause different effects in normal versus neurologic disease models.
CTSB functions in concert with other lysosomal proteases, most importantly with the other cysteine type cathepsins of which a total of 11 proteases that share considerable homology are encoded in the human genome (Ketterer et al., 2017). In mice, the CTSB gene has been simultaneously deleted with the cathepsin S or cathepsin Z gene and even a triple KO of the three proteases has been generated. Interestingly, mice with those gene KO combinations, like single CTSB KO mice, are viable, healthy, and reproduce normally. (Sevenich et al., 2010; Allan and Yates 2015; Akkari et al., 2016).
However, double deficiency of CTSB and cathepsin L genes is lethal a few weeks after birth due to neurodegeneration resembling human ceroid-lipofuscinoses (Felbor et al., 2002; Koike et al., 2005). This lethal phenotype can be rescued by expression of human cathepsin L in CTSB/cathepsin L double deficient mice, indicating reciprocal functional compensation between the two enzymes (Sevenich et al., 2006).
Generally, the CTSB KO mice are healthy, indicating physiologic functions that are operational. These findings suggest that inhibition of CTSB by chemical molecules as candidate drug agents will generally display a healthy phenotype.
VIII. Cathepsin B Knockout Improves Behavioral Deficits in Neurologic Disease Animal Models
Numerous neurologic animal model conditions are improved by knockout of the CTSB gene. CTSB deletion ameliorates behavioral deficits, neuropathology, and biomarkers in numerous brain disorders including traumatic brain injury, ischemia, epilepsy, multiple sclerosis, opioid tolerance, and inflammatory pain (Table 3), assessed in this section.
A. Cathepsin B in Neuromotor Dysfunction, Brain Tissue Loss, and Hippocampal Cell Death in Traumatic Brain Injury
TBI occurs when external forces to the brain result in a range of injuries of mild to severe injuries, closed and open head injuries, and nonpenetrating or penetrating injuries (reviewed in Saatman et al., 2008; Dixon, 2017). These injuries lead to severe brain dysfunction, tissue damage, and neurodegeneration, resulting in death and severe disability among all ages (Faul et al., 2010). TBI is suffered by over 10 million people per year worldwide (reviewed in Hyder et al., 2007).
In the CCI model of TBI, CTSB gene expression in the brain is increased (Natale et al., 2003), and CTSB activity and enzyme protein levels are increased in the brain (Luo et al., 2010; Hook et al., 2014a). In the penetrating ballistic-like brain injury animal model of TBI, substantial increases in CTSB activity and enzyme levels occur at 1, 3, and 7 days after the injury (Boutte et al., 2020).
The role of CTSB in mediating the behavioral deficits and neuropathology of CCI-TBI has been demonstrated by CTSB KO studies (Hook et al., 2014a). CTSB KO eliminated the CCI-induced increases in CTSB and resulted in improved neuromotor dysfunction at 1 day and 7 days postinjury, amelioration of brain tissue loss at 7 days post-CCI, and reduced hippocampal cell death in CCI-TBI at 7 days post-CCI (Hook et al., 2014a). Furthermore, CTSB KO blocked the CCI-induced rise in Bax, a proapoptotic cell death protein. The paper concluded that “These results validate cathepsin B as a new TBI therapeutic target” (Hook et al., 2014a, page 515,” Abstract,” last sentence).
In TBI, CTSB has been found to undergo lysosomal leakage translocation to the cytosol in brain neurons during increased intracranial pressure (ICP) in a moderate TBI rat model (Lafrenaye et al., 2012). Brain neurons in these TBI animals displayed chronic membrane poration in the elevated ICP condition compared with animals with only transient ICP. In fact, neuronal membrane disruption occurs acutely after injury and continues with a biphasic time-dependent elevation during 6 hours to 3 days post-TBI and 2 to 4 weeks after TBI in subsets of neurons in the central fluid percussion injury TBI model in rats (Hernandez et al., 2019).
The CTSB KO results suggest that chemical inhibition of CTSB will have beneficial effects for TBI. Indeed, chemical inhibition of CTSB and cathepsins by E64c improved motor dysfunction and ameliorated brain tissue and neuronal loss in CCI-TBI mice (Hook et al., 2014a). The chemical molecule (+)-(2S,3S)-3-(1-[N-(3-methylbutyl)amino]-leucylcarbonyl) oxirane-2-carboxylic acid, known as E64c, is a potent pan cysteine cathepsin inhibitor (Tamai et al., 1986), which is administrated (oral) as a prodrug form, E64d (EST, Aloxistatin, L-trans-epoxysuccinly(OEt)-Leu3methybutamide). E64d treatment reduced neuromotor disability, reduced brain tissue loss, and decreased neuronal cell loss in the CCI mice.
Furthermore, reduction of CTSB activity with the cathepsin B inhibitor, CA-074, also improved CCI-induced motor and cognitive deficits with reduced neurodegeneration in this TBI model (Luo et al., 2010). The inhibitor attenuated TBI-induced cell death, lesion volume, and motor and cognitive dysfunction.
CTSB studies have demonstrated the prominent role of CTSB in mediating TBI-induced brain deficits (Luo et al., 2010; Hook et al., 2014a; Boutte et al., 2020). It is realized that TBI injuries are diverse, and it will, therefore, be advantageous to investigate the role of CTSB in multiple TBI models.
B. Cathepsin B in Hippocampal Cell Death and Neuroinflammation in Hypoxia/Ischemia
Hypoxic-ischemic (HI) brain injury, caused by deficient oxygen supply, is a leading cause of death and severe disability. The high metabolic rate of the brain is compromised in hypoxic-ischemia such that ATP energy demands cannot be met, which results in dysfunctional neurons, synaptic dysfunction, and cell death. Notably, in human neonates, HI is the most common cause of death and disability, and occurs as a result of asphyxia of the umbilical blood supply to the fetus (reviewed in du Plessis and Volpe, 2002). Among those who survive, high rates of disability occur, including cognitive impairment, neuromotor dysfunction, and seizures.
In a neonatal HI model, CTSB gene KO resulted in substantial improvements in outcomes shown by reductions in neuronal cell death and neuroinflammation involving neurotoxic M1 microglia (Ni et al., 2015). HI increased CTSB and activated the neurotoxic M1 microglia type (Hu et al., 2012; Orihuela et al., 2016). which displayed elevated inducible nitric oxide synthetase, TNF-α, and IL-1β mRNA levels by 200-, 8-, and 50-fold, respectively, above noninjured controls. These data show that CTSB KO blocked the HI-induced neurotoxic M1 phenotype in HI mice. CTSB KO also resulted in enhancement of the neuroprotective M2 phenotype of microglia with anti-inflammatory IL-4 and IL-10. Furthermore, the aspartic protease cathepsin E was found as an upstream regulator of increased CTSB, which activates nuclear factor κB (NF-κB) to polarize microglia into a neurotoxic phenotype and increase CTSB (Ni et al., 2015).
In vitro cell studies then assessed mechanisms of CTSB activation of microglial in HI, by subjecting cells to oxygen-glucose deprivation followed by reoxygenation (Ni et al., 2015). Oxygen-glucose deprivation of microglial cells initiated autophagy, increased CTSB levels, and increased NF-κB. The CTSB inhibitor CA-074Me prevented the activation of NF-κB by inhibiting degradation of its inhibitor of NF-κB during oxygen-glucose deprivation of microglia cells. These findings suggest that CTSB activated NF-κβ to promote the neurotoxic M1 phenotype. NF-κB is a master gene regulator that is activated by diverse stimuli, including oxidative stress, and is a three-component system consisting of NF-κB, inhibitor of NF-κB, and Iκβ kinase complex (Ghosh et al., 2012). When activated, NF-κB translocates from the cytoplasm to the nucleus where it activates genes that control inflammation, cell death, and other functions controlled by phosphorylation of NF-κB subunits (Christian et al., 2016).
Overall, these studies of CTSB KO in the neonatal HI mouse model provide strong evidence for involvement of CTSB, regulated by upstream cathepsin E, in HI-induced neuronal death (Ni et al., 2015). The paper concluded that “inhibitors of cathepsin B or E as promising pharmacological agents for the treatment of ischemic brain injury” (Ni et al., 2015, page 12488, “Significance Statement,” last line).
C. Cathepsin B in Brain Neuronal Cell Death in Epilepsy
Progressive myoclonic epilepsy of the Unverricht-Lundborg (EPM1) is a rare genetically inherited autosomal recessive form of epilepsy that develops in childhood, resulting in lighting-like myoclonic jerks and tonic-clonic seizure attacks, and causes mental retardation and severe ataxia (reviewed in Kälviäinen et al., 2008). Notably, knockout of the CTSB gene in a EPM1 neuropathological model resulted in decreased neuronal apoptotic cell death in the brain, indicating that CTSB contributes to cell apoptosis in EPM1 (Pennacchio et al., 1996; Houseweart et al., 2003). This was one of the first studies to evaluate CTSB KO in a neurologic disease model.
EPM1 is a genetic disease caused by loss of function mutations in the cystatin B gene, an endogenous inhibitor of CTSB and cysteine cathepsins (Pennacchio et al., 1996). The cystatin B KO mice were used as an EPM1 model having seizures and loss of cerebellar granule neurons (Pennacchio et al., 1998; Houseweart et al., 2003). The absence of cystatin B in EPM1 leads to elevated cysteine cathepsin activity, which induces apoptotic cell death. To assess the roles of the cysteine cathepsins CTSB, cathepsin L, or cathepsin S in the EPM1 phenotypes, knockout of each of these cathepsin genes was conducted (Houseweart et al., 2003). Knockout of CTSB, but not of cathepsin L or cathepsin S, reduced apoptosis in the EPM1 mice, but had no effect on seizures. These findings suggest a role for CTSB in cell death of EPM1, and other mechanisms participating in EPM1 seizures.
Further evidence for a role of CTSB in cell death was provided by studies of overexpression of the CTSB gene in the cystatin B KO EPM1 model (Houseweart et al., 2003). In heterozygote CTSB and cystatin B KO EPM1 mice, increased apoptosis was observed, but the low number of heterozygote mice precluded statistical analyses. Further, the low birth number of transgenic CTSB and cystatin B KO mice showed that CTSB expression and eliminating its endogenous inhibitor, cystatin B, was a lethal combination. The authors concluded that “these findings establish cathepsin B as a contributor to apoptotic phenotype of cystatin B-deficient mice and humans with EPM1” (Houseweart et al., 2003, page 315, “Abstract,” second to last sentence).
D. Cathepsin B and Cathepsin S in Multiple Sclerosis
MS is a complex inflammatory CNS disease resulting in the destruction of the insulating myelin surrounding neuronal axons, which results in neurologic impairments, inflammation, and lesions (reviewed in Correale et al., 2017). The disease is driven by an autoimmune reaction of CNS-infiltrating myelin-specific autoreactive lymphocytes. Widespread activation of macrophages and microglia in the CNS occurs along with extensive immune infiltration of reactive lymphocytes and macrophages. A commonly used model of MS is the experimental autoimmune encephalomyelitis (EAE) model in which myelin oligodendrocyte glycoprotein antigen in Freund’s adjuvant and pertussis toxin are administered (subcutaneous and intraperitoneal routes) to animals to drive activation of CD4+ T lymphocytes in an autoimmune response mimicking MS (Nakahara et al., 2010).
Lysosomal cysteine cathepsins, including CTSB, are highly expressed in antigen presenting cells and are critical for processing antigens and the major histocompatibility-class II invariant chain in autoimmune conditions. Cathepsin S has also been thought to play an important role in MS because a cathepsin S inhibitor, a peptide containing the amino acids leucine-histidine-valine-serine (LHVS), affected antigen presentation (Riese et al., 1998) and attenuated EAE in wild-type mice (Fissolo et al., 2008). But since LHVS is a broad spectrum cysteine protease inhibitor (Wilson et al., 2009), only gene KO studies can assess the specific role of cathepsin S.
Evaluation of CTSB KO and cathepsin S KO mice in the EAE model (Allan and Yates, 2015) showed that CTSB and cathepsin S represent dual targets for a candidate MS therapeutic approach. Double cathepsin S and CTSB KO in EAE mice resulted in improvements consisting of 40% reduction in major histocompatibility-class II invariant chain expression, 82% reduction in CD4+ cells presenting CD69 antigen challenged with small myelin oligodendrocyte glycoprotein antigen peptides, 82% improvement in clinical score, 21% increase in the time of onset, and approximately 100% fewer infiltrating leukocytes macrophages, CD4+ T cells, and CD8+ T cells in spinal cord and about 75% lower for microglia relative to wild-type EAE animals. However, EAE animals with CTSB KO or cathepsin S KO each displayed similar EAE phenotypes as wild-type EAE mice. Thus, inhibition of both CTSB and cathepsin S represents a new poly target therapeutic approach for MS that is yet to be exploited. The authors concluded that cathepsin B and S are redundant in the EAE model and that “inhibition of multiple cysteine cathepsins may be needed to modulate autoimmune disorders, such as multiple sclerosis” (Allan and Yates, 2015, page 1, “Abstract,” last sentence).
E. Cathepsin B in Inflammatory Pain
Chronic pain is a detrimental condition that causes widespread disability in the world. Chronic pain is not merely a symptom of a disease or condition but rather a distinct condition that can result from inflammatory diseases (inflammatory pain) or nerve injury (neuropathic pain). Chronic pain contrasts with normal pain sensitivity that is essential for avoiding dangerous conditions.
CTSB participates in inflammatory pain as demonstrated in studies of CTSB KO mice (Sun et al., 2012). CTSB deficiency inhibited the induction of tactile allodynia induced by complete Freund’s adjuvant (CFA) in mice, without affecting peripheral inflammation. But CTSB KO did not affect nerve injury-induced allodynia for neuropathic pain, indicating the selective role of CTSB in inflammatory pain. In inflammatory pain, CTSB KO inhibited CFA induction of spinal IL-1β, IL-18, and cyclooxygenase-2. CTSB KO also reduced CFA-induced increases in spinal microglia cell size. These data indicate that CTSB participates in the development of chronic inflammatory pain through maturation of IL-1β and IL-18 by spinal microglia. These findings support the strategy of using inhibitors of CTSB to selectively treat inflammatory pain, but not nerve injury-induced neuropathic pain.
Inflammatory and neuropathic pain cause microglial activation to the neurotoxic M1 phenotype for production of inflammatory cytokines (Carniglia et al., 2017; Zhao et al., 2017). A key difference between the two forms of pain is that different proteases participate in production of inflammatory cytokines. In inflammatory pain, lysosomal CTSB leaks into the cytosol where the enzyme activates the NLRP3 inflammasome and caspase-1 (Halle et al., 2008; reviewed in Tschopp and Schroder, 2010). However, in neuropathic pain, metalloproteases participate in the process to activate caspase-1 (Kawasaki et al., 2008). The caspase-1 enzyme is necessary for cleavage of procytokines to generate the proinflammatory cytokines IL-1β and IL-18 (Martinon et al., 2002). Subsequent to cytokine production, the molecular pathways to pain diverge in these two pain conditions. In inflammatory pain, IL-1β causes an increase in COX-2 and prostaglandin E2 in the spinal cord that contributes to pain hypersensitivity (Samad et al., 2001; Ren and Torres, 2009). In neuropathic pain, IL-1β does not affect spinal COX-2 and activates other molecular pain mechanisms (Sweitzer et al., 2001; Broom et al., 2004).
Based on the evidence for a significant role of CTSB in inflammatory pain, the authors concluded that CTSB is essential for activation of IL-1β in inflammatory, but not neuropathic, pain and that “CTSB-specific inhibitors may represent a useful new strategy for treating inflammation-associated pain” (Sun et al., 2012, page 11341, right column, last sentence).
F. Cathepsin B in Tolerance with Chronic Opioid Use
Opiates are the most effective therapeutic agent for controlling severe pain, but long-term use results in the problem of antinociceptive tolerance in which opioids lose their analgesic efficacy, requiring dose escalation for pain relief (reviewed in Martyn et al., 2019). Importantly, CTSB participates as a mechanism by which chronic morphine causes antinociceptive tolerance through activation of autophagy involving increased neuroexcitatory transmitter release (Zhao et al., 2010). Significantly, deletion of the CTSB gene prevents tolerance to chronic morphine assessed by allodynia of the von Frey test (Hayashi et al., 2014). These CTSB knockout studies show that opiate tolerance is dependent on CTSB.
CTSB KO mice do not develop chronic morphine-induced increases in autophagy, and CTSB KO inhibited glutamate neurotransmitter release during chronic morphine (Hayashi et al., 2014). Whereas wild-type mice exhibit increased glutamate release in lamina I neurons with chronic morphine treatment, CTSB KO mice do not exhibit such changes in glutamate during chronic morphine. These findings indicate that chronic morphine causes excessive CTSB-dependent autophagy in GABAergic neurons, which causes dysregulation and a reduction of GABAergic neuronal inhibition that increases excitatory glutamate release.
The authors of the CTSB KO studies in chronic morphine concluded that “[CTSB] inhibitors can be beneficial in the blockade of opioid antinociceptive tolerance” (Hayashi et al., 2014, page 392, “Conclusion,” last sentence).
G. Cathepsin B in Memory Deficits in Aging
Aging is a main risk factor in neurodegenerative diseases and neurologic conditions that involve CTSB mediation of neuroinflammation and memory impairment (Terada et al., 2010; Stoka et al., 2016; Ni et al., 2019). Significantly, knockout of the CTSB gene reduced inflammation and improved cognitive impairment in aged mice but not in young mice (Ni et al., 2019). These studies showed that aging hippocampal microglia display increases in CTSB combined with lysosomal leakage of CTSB that mediates generation of mitochondria-derived reactive oxygen species (ROS) and activation of proinflammatory IL-1β and TNF-α (Ni et al., 2019). Knockout of CTSB attenuated elevation of ROS and inflammation in aging. Importantly, knockout of CTSB resulted in significant improvement of memory deficits in aged mice. These findings demonstrate that CTSB participates in activation of innate immunity related to cognition during aging.
The relationship of lysosomal leakage of CTSB and ROS production was assessed in microglia cell cultures (Ni et al., 2019). Induction of lysosomal leakage by L-leucyl-L-leucine methyl ester, a lysosome destabilizing agent, resulted in CTSB-dependent increases in cellular ROS generation and inflammatory responses in microglia. Furthermore, induction of oxidative stress and inflammation with rotenone treatment of microglia cells was blocked by an inhibitor of CTSB, CA-074Me. Leaked CTSB participated in degradation of cytosolic pre-TFAM, a potential substrate of CTSB that functions as a mitochondrial transcription factor and stabilizer of mitochondria. The same research group also found increased nuclear location of CTSB in microglia with aging; nuclear CTSB was involved in the degradation in sirtuins and activation of NFκB (Meng et al., 2020). Notably, impairment of learning and memory occurred in aged mice after injection of L-leucyl-L-leucine methyl ester-treated CTSB-overexpressing microglia cells into the lateral ventricle of the brain, but not by untreated CTSB-expressing microglia. These findings indicate that increased CTSB participates in activation of mitochondria-derived ROS and proinflammatory mediators that result in memory impairment in aging (Ni et al., 2019; Meng et al., 2020).
Overall, the CTSB dependence of memory deficits in aged mice, shown by CTSB KO studies, led the authors to conclude that “the increase and leakage [from lysosomes] of CTSB in microglia during aging are responsible for the increased generation of mitochondria-derived ROS and proinflammatory mediators, culminating in memory impairment” (Ni et al., 2019, Abstract, last sentence).
The endogenous chromogranin A (CgA) peptide neurotransmitter (neuropeptide) participates in lysosomal leakage of CTSB, with elevation of CTSB enzyme levels, leading to activation of IL-1β production (Terada et al., 2010). Studies of primary microglia cells from CTSB KO and wild-type mice brain cortex (in culture) showed that CTSB gene deletion prevented CgA induction of IL-1β secretion and also reduced CgA activation of cell death. CTSB KO attenuated CTSB processing of pro-caspase 1 to active caspase 1 that cleaves pro-IL-1β to generate IL-1β. These findings demonstrate participation of lysosomal CTSB in production of IL-1β in CgA-activated microglia cells that accumulate neuroinflammation in Alzheimer’s disease, Parkinson’s disease, and Pick neurodegenerative disease conditions in aging (Weiler et al., 1990; Brion et al., 1991; Yasuhara et al., 1994). Based on these findings, authors concluded that “either pharmacological or genetic inhibition of [CTSB] may provide therapeutic intervention in inflammation-associated neurological diseases” (Terada et al., 2010, page 114, “Abstract,” last line).
H. Cathepsin B in Memory Deficits, Neuroinflammation, and Amyloid-β in Chronic Periodontitis-Associated Alzheimer’s Disease
AD results in severe memory loss (reviewed in Masters et al., 2015; Lane et al., 2018) and neuroinflammation (reviewed in Akiyama et al., 2000; Heneka et al., 2015; Ardura-Fabregat et al., 2017). Infectious AD etiology by Porphyromonas gingivalis, the major periodontal bacteria, and numerous chronic viral, fungal, and bacterial infections have been shown to participate in AD (reviewed in Sochocka et al., 2017). Strong clinical evidence indicates a positive link between periodontitis and AD with respect to cognitive dysfunction and inflammation (reviewed in Singhrao et al., 2015;Te ixeira et al., 2017), supported by the presence of Porphyromonas gingivalis lipopolysaccharide (PgLPS) in the human AD brain (Poole et al., 2013). Significantly, CTSB has been found to participate in PgLPS-induced periodontitis and memory deficits (Wu et al., 2017).
CTSB KO studies in the neuroinflammatory periodontitis model of AD demonstrated that CTSB drives memory deficits through activation of microglia inflammation and neuronal amyloid-beta (Aβ) production (Wu et al., 2017). Knockout of CTSB in the PgLPS mice significantly improved memory deficits in middle-aged mice (12 months old), but not in young mice (2 months), treated with PgLPS for 5 weeks (i.p., 1 mg/kg daily). PgLPS elevated CTSB in hippocampus and this elevated CTSB was absent in CTSB KO mice treated with PgLPS. Further, CTSB KO reduced inflammatory responses, indicated by reductions IL-1β and toll-like receptor 2 in microglia. These effects of CTSB KO for improving memory deficits and reducing inflammation occurred in middle-aged mice, but not in young mice, treated with PgLPS. The amelioration by CTSB KO of PgLPS induced memory loss and neuroinflammation support CTSB as a candidate drug target for discovery of therapeutic agents for cognitive decline in chronic periodontitis-associated AD (Wu et al., 2017).
CTSB KO blocks PgLPS-induced elevation of Aβ(1–42) in the mouse brain (Wu et al., 2017), indicating that Aβ(1–42) production is dependent on CTSB. In cellular studies, PgLPS induces release of IL-1β from microglia cells, and incubation of the resultant microglia IL-1β-containing conditioned medium with neurons resulted in increased CTSB, APP, and Aβ. IL-1β regulation of neurons may involve CTSB activation of NF-κΒ that drives expression of APP and CTSB, shown to function as an alternative β-secretase for Aβ production (Hook et al., 2005, 2008b). These results show that CTSB participates in Aβ production for amyloidosis of periodontitis related to AD.
The role of CTSB in elevation of Aβ in chronic periodontitis (Wu et al., 2017) has been corroborated by several studies (Rong et al., 2020). PgLPS increases CTSB and elevates Aβ(1–40) and Aβ(1–42) in neuroblastoma cells expressing wild-type human APP, but had no effect on APP or beta-site amyloid precursor protein cleaving enzyme (BACE) 1 levels. The CTSB inhibitor CA-074Me blocked PgLPS-induced increases in Aβ(1–40) and Aβ(1–42), indicating involvement of CTSB in Aβ peptide production. Clinical data show that serum CTSB levels are significantly elevated in patients with chronic periodontitis, and higher serum CTSB levels correlate with cognitive deficits in periodontitis patients. These findings support involvement of CTSB in Aβ production and memory deficits in chronic periodontitis related to AD (Rong et al., 2020).
CTSB-dependent increases in Aβ have been reported in advanced glycation end (AGE) products, another model of neurodegeneration (Batkulwar et al., 2018). AGE results from a nonenzymatic reaction of glucose or other glycolytic intermediates with proteins to induce toxic neuronal effects through the AGE receptor involved in neurodegeneration. AGE treatment of mouse neuro2a or cortical neurons increased CTSB and Aβ(1–42), which was reduced by CA-074Me inhibition of CTSB. Also, CTSB is elevated in human brain cortex of patients with AD compared with age-matched controls. These findings suggest that cathepsin B may have a role in AGE-RAGE [receptor for AGE (RAGE)] signaling that exacerbates the onset and development of Aβ(1–42) pathology of AD (Batkulwar et al., 2018).
Additional support for CTSB-dependent processing of wild-type APP has been provided in studies of a mouse model of mucopolysaccharidosis type I (MPS I). MPS I is a rare disease resulting in neurologic deficits, caused by a genetic deficiency of α-L-iduronidase (IDUA) involving an impairment of lysosomal catabolism, which is modeled by knockout of the IDUA gene in mice (Viana et al., 2020). This MPS 1 model displayed elevated CTSB, activation of microglia and astrocytes, and elevated APP β-secretase processing, and no effect on BACE1 levels. CTSB was significantly increased in the cytoplasm of hippocampal pyramidal neurons from IDUA KO mice, indicating lysosomal leakage of CTSB. The study concluded that CTSB represents an alternative amyloidogenic pathway in MPS I brain involving lysosomal leakage of CTSB that may lead to neurodegeneration (Viana et al., 2020).
CTSB regulation of Aβ production from multiple neurodegenerative disease models of periodontitis, AGE, and MPS I that express WT APP are relevant to patients with sporadic AD expressing normal WT APP, representing the majority (>95%) of the AD population. A small percentage of FAD, involving AD genetic mutations, comprise less than 5% of AD cases (Price and Sisodia, 1998; Van Cauwenberghe et al., 2016). Since most patients with AD are sporadic (∼95%), the role of CTSB in Aβ production from WT APP is relevant for the major portion of the population with AD.
Evidence supports CTSB as an alternative β-secretase used for conversion of APP, together with γ-secretion, into Aβ peptides (Hook et al., 2008a, 2008b, 2009, 2014b; Kindy et al., 2012; Wu et al., 2017). Although BACE1 has been viewed as the only β-secretase (Laird et al., 2005; Willem et al., 2009; Hampel et al., 2021), based in part on findings that BACE1 KO mice had no β-secretase activity (Roberds et al., 2001), the data do not rule out CTSB since the assay included an inhibitor of CTSB and lacked reducing conditions necessary for CTSB activity. It is noteworthy that APP β-secretase processing involves several proteases in addition to BACE1 (reviewed by Vassar, 2004; Andrew et al., 2016; Hasanbasic et al., 2016), which include CTSB (Hook et al., 2008b; Terada et al., 2010; Wu et al., 2017; Batkulwar et al., 2018; Ni et al., 2019; Rong et al., 2020; Viana et al., 2020), delta-secretase (Zhang et al., 2015; Wu et al., 2020), meprin (Becker-Pauly and Pietrzik, 2017; Schlenzig et al., 2018), and matrix metalloproteinases (Garcia-Gonzalez et al., 2019).
Overall, evidence for involvement of CTSB in Aβ production and memory deficits in chronic periodontitis related to AD have led authors to conclude that “CTSB might be the link between chronic periodontitis and AD” (Rong et al., 2020, page 9, last paragraph, second sentence).
I. Cathepsin B in Memory Deficits and Amyloid-β in Transgenic Human Amyloid Precursor Alzheimer’s Disease Models
CTSB KO studies have been conducted in transgenic AD mouse models overexpressing human amyloid precursor protein (hAPP) by two groups, Hook (Hook et al., 2009; Kindy et al., 2012; Hook et al., 2014b) and Gan (Mueller-Steiner et al., 2006; Wang et al., 2012). These models produce human Aβ,and some develop brain Aβ plaque and memory impairment. Six different transgenic CTSB KO and hAPP AD mouse models were studied (summarized in Table 4). The two groups obtained confirming and conflicting data, with the latter being likely due to differences in the hAPP transgene used.
Cathepsin B Gene Knockout Improves Memory Deficits in AD and Aging Models
1. Transgenic Human Amyloid Precursor Protein Used in Cathepsin B Knockout Alzheimer’s Disease Models Differ in the β-Secretase Site Sequence, Amyloid Precursor Protein Isoform, and Gene Construct
Transgenic AD models used to evaluate CTSB differed in three significant aspects consisting of the hAPP amino acid sequence at the β-secretase cleavage site, the hAPP isoform expressed, and the genetic engineering construct of the hAPP. The models expressed hAPP with either the WT β-secretase site found in the vast majority of patients with AD or the familial Swedish mutation of amyloid precursor protein (Swe) mutant β-secretase site sequence (K670N/M671L) found in one extended family (Mullan et al., 1992). This difference has a major effect on CTSB ability to cleave that site as it readily cleaves the WT but does not cleave the Swe β-secretase site sequence (Hook et al., 2008a). Both groups used both types of hAPP models, and, as discussed below, the Hook group found that CTSB gene deletion caused a major reduction in β-secretase cleavage products, including Aβ, brain plaque, and memory deficits in models expressing hAPP with WT β-secretase site sequence but had no effect in models expressing the Swe mutant β-secretase site sequence (Table 4). The Gan group found that CTSB KO had no effect on Aβ production and a slight brain Aβ degradation effect in a model expressing hAPP with WT β-secretase site sequence (Table 4). In a model expressing Swe mutant β-secretase site sequence, the Gan group confirmed the Hook group’s finding that CTSB had no effect on Aβ production but had a degradative effect on brain plaque.
The hAPPs used by the two groups differed in isoforms of hAPP that were expressed. The Hook group studied the hAPP 695 isoform expressed in AD mice, whereas the Gan group used the hAPP 751/770 isoform in mouse studies. The hAPP is expressed as isoforms containing either 770, 695, or 751 amino acids. hAPP-695 is the most abundant, is exclusively expressed in neurons, and is processed into Aβ (Fig. 4Ai) (Sandbrink et al., 1993; Rockenstein et al., 1995; Rohan de Silva et al., 1997). APP-751 and APP-770 isoforms are present at much lower levels (Tanaka et al., 1989; Kang and Müller-Hill, 1990; Jacobsen et al., 1991; Rockenstein et al., 1995; Rohan de Silva et al., 1997), which are expressed primarily in glia cells and generate nonamyloidogenic sAPPα (Fig. 4Bi). (Kametani et al., 1993; Nalivaeva and Turner, 2013). With respect to trafficking, APP695 forms cis-dimers within the endoplasmic reticulum of cells, whereas APP-751/770 prevents that from occurring and APP-751/770 is present only as trans-dimers (Isbert et al., 2012). As a result, APP-695 is preferentially trafficked through the amyloidogenic endosomal pathway in lipid rafts (Cordy et al., 2003; Ehehalt et al., 2003). In contrast, APP-751/770 has been found to undergo trafficking and processing by the nonamyloidogenic cell-surface pathway (Cordy et al., 2003; Ehehalt et al., 2003; Ben Khalifa et al., 2012; Nalivaeva and Turner, 2013). The Hook group used models expressing hAPP-695, whereas the Gan group used models expressing hAPP-751/770. As discussed below, the difference in isoform may have caused different CTSB KO outcomes found by the two groups.
APP-695 and APP-751/770 expression and processing in normal and transgenic mouse models of Alzheimer’s disease. (A) Mouse APP-695 (mAPP-695) isoform: (i) normal neuronal expression of mAPP-695 produces mouse Aβ (mAβ) and (ii) transgenic neuronal expression of human APP-695 (hAPP-695), driven by the PDGF promoter, produces human Aβ (hAβ). Panel (i) shows that in normal mouse brain, APP-695 is exclusively expressed in neurons for the production of amyloidogenic Aβ peptides, reported by several studies (Sandbrink et al., 1993; Rohan de Silva et al., 1997). APP-695 is the most abundant APP isoform expressed in the normal brain (Tanaka et al., 1989; Kang and Müller-Hill, 1990; Jacobsen et al., 1991; Rockenstein et al., 1995; Nalivaeva and Turner, 2013). Panel (ii) shows that in transgenic mice expressing hAPP-695 driven by the PDGF promoter, hAPP-695 is present in neurons and produces Aβ (Hook et al., 2009; Kindy et al., 2012; Hook et al., 2014b), which models the normal (nontransgenic) neuronal expression of hAPP-695 and production of Aβ. (B) Mouse APP-751/770 (mAPP-751/770) isoforms: (i) normal glia expression of mAPP-751/770) produces sAPPα and (ii) transgenic neuronal expression of hAPP-751/770, driven by the PDGF promoter, produces hAβ. Panel (i) shows that in normal mouse brain, APP-751/770 is expressed in glia cells (Sandbrink et al., 1993) and produces the nonamyloidogenic sAPPα fragment (Kametani et al., 1993; Nalivaeva and Turner, 2013). APP-751/770 is a minor APP isoform in the brain (Tanaka et al., 1989; Kang and Müller-Hill, 1990; Jacobsen et al., 1991; Rockenstein et al., 1995; Nalivaeva and Turner, 2013). Panel (ii) shows that in transgenic mice expressing hAPP-751/770 driven by the PDGF promoter, with deletions of segments within introns 6 and 7 and an insertion (4 bp) in intron 7 (Games et al., 1995; Rockenstein et al., 1995; Mucke et al., 2000; Mueller-Steiner et al., 2006; Wang et al., 2012), hAPP-751/770 is present in neurons and produces Aβ, which differs from the normal (nontransgenic) glia expression of hAPP-751/770 and production of sAPPα (Kametani et al., 1993; Sandbrink et al., 1993; Nalivaeva and Turner, 2013).
The hAPP genetic engineered construct differed between the two groups. The Hook group used the naturally occurring hAPP-695 gene sequence, whereas the Gan group used a highly modified hAPP-751/770 gene in which introns were deleted and nucleic acid base-pairs added (Rockenstein et al., 1995). Both groups used the platelet derived growth factor (PDGF) promoter in the transgene to induce hAPP expression in neurons and not in other brain cells (Sasahara et al., 1991). This resulted in the hAPP-695 used by the Hook group being expressed in neurons that naturally occur (Fig. 4Aii). However, the PDGF promoter forced the hAPP-751/770 used by the Gan group to be expressed in neurons where those isoforms are not naturally produced (Fig. 4Bii), which contrasts with the normal glia expression of hAPP-751/770 (Fig. 4Bi). Thus, the genetic engineering differences may contribute to the differences in results obtained by the two groups.
2. Major Cathepsin B Dependency of Memory Deficits and Amyloid-β in Human Amyloid Precursor Protein with Wild-Type β-Secretase Site Sequence and Amyloid Precursor Protein Isoform 695 Alzheimer’s Disease Models, Representative of Most Alzheimer’s Disease Patients
The transgenic WT hAPP-695 (no mutations) model most closely mimics the hAPP processing that occurs in the neurons of most patients with AD. Knockout of the CTSB gene in these WT hAPP AD mice reduced human Aβ40 and Aβ42 by 70%, reduced CTFβ by 40%, and increased sAPPα by 60% compared with control hAPP mice (Hook et al., 2009). The reduction in Aβ and CTFβ showed that the CTSB gene deletion reduced β-secretase activity for processing the WT β-secretase cleavage site of APP for Aβ production. The increase in sAPPα in these CTSB KO hAPP AD mice are consistent with increased availability of APP for nonamyloidogenic processing by α-secretase, which cleaves within the Aβ peptide sequence. These data provide support for CTSB-dependent WT β-secretase activity involved in Aβ production in the brain (Hook et al., 2009).
Whereas WT hAPP-695 mice do not develop memory deficits (Mucke et al., 1994), mice expressing hAPP-695 with the WT β-secretase site and London mutation of amyloid precursor protein (Lon) near the γ-secretase site sequence (hAPP-WT-Lon-695) display memory loss (Moechars et al., 1999). Thus, this model allows evaluating CTSB effects on Aβ, brain plaque, and memory deficits in a model having WT β-secretase activity. CTSB KO resulted in substantial improvements in memory deficits and decreased levels of Aβ42 and Aβ40 with reduced amyloid plaque load in the hAPP-WT-Lon-695 model (Kindy et al., 2012; Hook et al., 2014b). CTSB KO ameliorated memory dysfunction of the hAPP-WT-Lon-695 mice, assessed by Morris water maze behavioral assay, to nearly normal levels of the control WT AD mice (Kindy et al., 2012). Improved memory function was demonstrated by the increased latency time for mice to swim to a submerged platform (after training) and by the decreased distance traveled by the mice to reach the submerged platform. These results show that the memory impairment in these AD mice was dependent on CTSB.
With respect to APP-derived biomarkers, CTSB KO in the hAPP-WT-Lon-695 AD mice resulted in decreased brain levels of Aβ40 and Aβ42 by ∼85%, reduced CTFβ by 60%, and increased sAPPα by 60% (Kindy et al., 2012). Furthermore, CTSB knockout reduced amyloid plaque load by ∼85%. The effects of overexpressing the CTSB gene were also evaluated in these AD mice; CTSB expression resulted in increased levels of Aβ40 and Aβ42 that were 50% and 100% above controls (Hook et al., 2014b). These results demonstrate that CTSB participates in regulating WT β-secretase activity for Aβ production.
Importantly, the CTSB KO studies in the transgenic hAPP-WT-Lon-695 AD mice demonstrate that memory deficits and Aβ production were dependent on CTSB. These findings led to the authors’ conclusion that “[CTSB] may be an effective drug target for improving memory deficits in most AD patients” (Kindy et al., 2012, Abstract, last line).
3. Major Cathepsin B Dependency of Pyroglutamate Amyloid-β in Human Amyloid Precursor Protein with Wild-Type β-Secretase Site Sequence and Amyloid Precursor Protein Isoform 695 Alzheimer’s Disease Model
Human AD brains possess elevated levels of truncated pyroglutamate-modified amyloid-beta (pGlu-Aβ)(3–40) and pGlu-Aβ(3–42) peptides that contribute to neurotoxicity (Mori et al., 1992; Saido et al., 1995; Iwatsubo et al., 1996). The pGlu-Aβ(3–42) species are a dominant fraction of Aβ forms in human brains with AD (Hosoda et al., 1998; Harigaya et al., 2000; Piccini et al., 2005; Portelius et al., 2010). The pGlu-Aβ(3–40/42) peptides enhance aggregation of Aβ peptides to result in greater neurotoxicity (Saido et al., 1996; Russo et al., 2002; Schilling et al., 2006; Nussbaum et al., 2012). Therefore, there is keen interest in the field to find approaches to reduce pGlu-Aβ peptides as a therapeutic approach (Jawhar et al., 2011; Cynis et al., 2016; Hettmann et al., 2020).
The hAPP-WT-Lon-695 AD model discussed above produces pGlu-Aβ (Tanghe et al., 2010). Significantly, the Hook group showed that CTSB KO results in substantial decreases in pGlu-Aβ(3–40) and pGlu-Aβ(3–42) by 65% and 90%, respectively, in the hAPP-WT-Lon-695 mouse model of AD (Hook et al., 2014b). Knockout of CTSB also reduced pGlu-Aβ amyloid plaque load by 46% in the brain. These data demonstrate the CTSB dependence of pGluAβ production and pGluAβ amyloid plaque accumulation.
Overexpression of human CTSB increased pGlu-Aβ(3–40) and pGlu-Aβ(3–42) by 50% and 100%, respectively, and increased Aβ(1–40), and Aβ(1–42) by 50% and 100%, respectively, relative to control AD mice (Hook et al., 2014b). Overexpression of CTSB also increased pGlu plaque load by 178% relative to controls. These data show that CTSB drives pGlu-Aβ and Aβ production.
Formation of the pGlu-Aβ peptides utilizes the N-truncated Aβ(3–40) and Aβ(3–42) as substrates for N-terminal cyclization of glutamate by glutaminyl cyclase (QC) to generate pGlu-Aβ(3–40) and pGlu-Aβ(3–42) (Schilling et al., 2004; Cynis et al., 2008). The QC substrates may be generated by β-secretase cleavage of APP to produce Aβ(1–40) and Aβ(1–42) followed by aminopeptidase removal of N-terminal residues to generate Aβ(3–40) and Aβ(3–42). Recently, meprin was found to function as both endopeptidase and N-terminal dipeptidyl peptidase to generate the N-truncated Aβ substrates for QC formation of pGlu-Aβ peptides (Schlenzig et al., 2018). Alternatively, direct cleavage of APP could produce Aβ(3–40) and Aβ(3–42). It will be of interest to define the proteolytic mechanisms for pGlu-Aβ formation to understand how CTSB participates in pGlu-Aβ peptide production.
The significant reduction of neurotoxic pGlu-Aβ peptides by CTSB KO led the authors to conclude that CTSB inhibitors “have potential as new AD therapeutics based on their ability to reduce both pGlu-Aβ and Aβ produced from AβPP containing the [wild-type] β-secretase site expressed in the majority of AD patients” (Hook et al., 2014b, Discussion, last paragraph, last sentence).
4. Minor Cathepsin B Dependency of Amyloid-β Degradation in Human Amyloid Precursor Protein with Wild-Type β-Secretase Site Sequence and Amyloid Precursor Protein Isoforms 751/770 Alzheimer’s Disease Model
Brain Aβ was studied in CTSB KO mice expressing the hAPP-WT-751/770 transgene (known as PDAPP hAPP-WT-751/770 mice) by the Gan group. CTSB knockout in these engineered mice resulted in small increases of Aβ(1–42) in cortex, Aβ(1–x) in cortex, and hippocampal Aβ(1–x) brain regions that were 12%, 24%, and 18%, respectively, above controls (Wang et al., 2012). But CTSB KO had no effects on hippocampal Aβ(1–42). There were no changes in cortical CTFβ or sAPPα resulting from the CTSB KO condition (Wang et al., 2012). These findings lead to the conclusion that CTSB was not involved in WT hAPP-751/550 conversion to Aβ and that CTSB had a minor role in degrading Aβ in the hAPP-WT-751/770 expressing mice.
Overexpressing CTSB in the hAPP-WT-751/770 mice resulted in small decreases in brain Aβ(1–42) in hippocampus and cortex (by 20% and 9%, respectively), combined with no change in Aβ(1–x) in hippocampus and cortex, and no change in β-CTFβ or α-CTF in cortex relative to controls (Wang et al., 2012). In vitro assays showed that CTSB can degrade Aβ. These data implicate a modest role for CTSB degradation of brain Aβ(1–42) in mice expressing the hAPP-751/770 isoforms (Wang et al., 2012).
Elevation of CTSB activity was also achieved by knocking out the cysteine protease inhibitor of CTSB, cystatin C, in the hAPP-WT-751/770 transgenic mice (Wang et al., 2012). The absence of the endogenous CTSB inhibitor resulted in modest reductions of Aβ(1–42) and Aβ(1–x) by 14% and 6%, respectively, in brain cortex. These results also suggested that CTSB has a small role in Aβ degradation. But in addition to CTSB, other proteases for Aβ degradation may participate since cystatin C inhibits several cysteine proteases including cathepsins L, S, and H (Barrett, 1986; Abrahamson, 1994). Results from studies of the hAPP-WT-751/770 mice suggested that CTSB did not affect processing of the hAPP-751/770 isoforms and that CTSB has a modest role in Aβ degradation (Wang et al., 2012). The authors concluded that “enhancing [CTSB] could lower Aβ, especially Aβ42, in AD patients” (Wang et al., 2012, Abstract, last line).
It must be noted that studies of the hAPP-WT-751/770 mice did not assess memory function, (Wang et al., 2012) and, therefore, the role of the hAPP-WT-751/770 isoform and modest effects on Aβ in cognitive deficits is unknown. Evaluation of memory function in the CTSB KO condition of hAPP-WT-751/770 mice will be important in future studies.
5. Differences in Cathepsin B Dependency of Amyloid-β in Human Amyloid Precursor Protein with Wild-Type β-Secretase Site Sequence Alzheimer’s Disease Models Likely Due to Different Isoforms or Constructs
As discussed above, the Hook group found a major CTSB dependence of Aβ production generated from hAPP-695 with the WT β-secretase site sequence (Hook et al., 2009, 2014b; Kindy et al., 2012; Wang et al., 2012), whereas the Gan group found no CTSB dependency for Aβ production in mice expressing the hAPP-751/770 isoforms with the WT β-secretase site (Wang et al., 2012). The differences in Hook and Gan group data are likely due to the different isoforms and constructs used in the models of the two groups.
The PDGF promoter in the hAPP-695 transgene used by the Hook group resulted in neuronal expression of the transgene. That mimics the native expression of hAPP-695, which is the most abundant brain isoform, expressed exclusively in neurons and is processed into Aβ peptides in the brain (Rockenstein et al., 1995; Belyaev et al., 2010; Nalivaeva and Turner, 2013) (Fig. 4A). As such, the hAPP-WT-Lon-695 precursor is subjected to the endogenous neuronal brain pathways for production of Aβ peptides that occur in vivo in AD. Notably, knockout of CTSB KO in the hAPP-WT-Lon-695 AD mice resulted in substantial reductions in Aβ1–40, Aβ1–42, and amyloid plaque load in the brain and significant improvements in memory deficits (Hook et al., 2009, 2014b; Kindy et al., 2012). Therefore, the CTSB dependency for Aβ production and memory deficits in the hAPP-WT-Lon-695 AD mice represents a model representing the main population with AD.
In contrast, the PDGF promoter artificially forced the hAPP-751/770 transgene used in the Gan studies to be expressed at high levels in neurons where these APP isoforms are normally not expressed (Fig. 4B). hAPP-751/770 expression resulted in processing of these APP isoforms to amyloidogenic Aβ (Fig. 4Bii) (Wang et al., 2012), but APP-751/770 isoforms are normally processed to nonamyloidogenic sAPPα (Fig. 4Bi) (Kametani et al., 1993; Nalivaeva and Turner, 2013). APP-751/770 isoforms in normal mouse and human brain are expressed primarily in glia cells (rather than in neurons) (Sandbrink et al., 1993) for production of nonamyloidogenic sAPPα (Fig. 4Bi). Therefore, the transgenic neuronal hAPP-WT-751/770 expression and production of Aβ does not represent the normal glia expression of hAPP-751/770 and production of sAPPα in the brain. Thus, the transgenic hAPP-WT-751/770 expression may reflect an experimental artifact rather than that which naturally occurs.
The hAPP used by the two groups also differed in the engineered APP gene constructs. The hAPP-695 transgene used by the Hook group contained the naturally occurring sequence that produces hAPP-695 (Hook et al., 2009, 2014b; Kindy et al., 2012). On the other hand, the hAPP-751/770 used by the Gan group was not the naturally occurring sequence but rather a highly engineered gene construct with intronic deletions and insertions (Rockenstein et al., 1995; Mucke et al., 2000; Wang et al., 2012). The differences in the APP gene constructs could have resulted in differences in hAPP processing in AD models used by the Gan group compared with the Hook group.
Importantly, mice expressing the PDGF-driven expression of hAPP-695 transgene in neurons, representing the usual AD condition, show that CTSB KO results in reduced levels of neurotoxic Aβ peptide.
6. No Cathepsin B Dependency of Memory Deficits and Amyloid-β in Human Amyloid Precursor Protein with Swedish Mutant β-Secretase Site Sequence Models, Representative of Rare Familial Alzheimer’s Disease Patients
In the hAPP-Swe-Lon-695 AD mice, the Hook group found that CTSB KO resulted in no change in memory deficits, Aβ peptides (Aβ40 and Aβ42), and CTFβ and sAPPα (Kindy et al., 2012). Studies by the Gan group using hAPP-Swe-Ind-751/770 mice also found no effect of CTSB KO on Aβ peptide production; memory deficits were not assessed in these mice (Mueller-Steiner et al., 2006). Data shows that CTSB does not cleave the Swe mutant β-secretase site sequence (Hook et al., 2008a), which provides an explanation for the lack of CTSB KO effect in models expressing hAPP with Sweβ-secretase site. But the important point is that such models mimic the Aβ production that only occurs in a few individuals and is, thus, not relevant to most AD patients.
J. The Consilience of Aging, Chronic Periodontitis-Associated Alzheimer’s Disease, and Transgenic Alzheimer’s Disease Data Are that Memory Deficits Are Cathepsin B Dependent, which Provides Rationale for Cathepsin B Inhibitor Development
The consilience by numerous studies demonstrates that CTSB KO improves memory deficits in cognitive aging, chronic periodontitis-associated AD, and AD of representative animal models (Table 4) (Terada et al., 2010; Kindy et al., 2012; Hook et al., 2014b; Ni et al., 2019). The amelioration of memory dysfunction by CTSB KO occurred in aged mice but not in young mice and was associated with CTSB-dependent reductions in the inflammatory biomarkers of IL-1β and TNF-α (Terada et al., 2010; Ni et al., 2019). CTSB KO attenuated memory loss in the periodontitis model of AD, induced by PgLPS from Porphyromonas gingivalis, in middle-aged mice but not in young mice; CTSB KO reduced inflammatory responses, shown by reduction in microglia IL-1β, toll-like receptor 2, as well as Aβ. Importantly, knockout of CTSB in the hAPP-WT-Lon-695 AD mouse model resulted in substantial improvement in memory deficits to levels similar to that of normal mice (Kindy et al., 2012), accompanied by CTSB-dependent reductions in the neurotoxic Aβ peptides consisting of Aβ42, Aβ40, pGlu-Aβ(3–42) and pGlu-Aβ(3–40) (Kindy et al., 2012; Hook et al., 2014b).
In the AD model studies, improvements in memory deficits by CTSB KO occurred in transgenic mice expressing the WT β-secretase site of the hAPP-WT-Lon-695 AD mice, which represents the majority of patients with AD of the sporadic type (>95%) (Masters et al., 2015) who express hAPP with the WT β-secretase site. Therefore, effective amelioration of memory deficits in the CTSB KO studies in the hAPP-WT-Lon-695 AD mice is relevant to the major population with AD. Importantly, the CTSB KO findings indicate CTSB as an excellent candidate drug target for development of inhibitors as a therapeutic approach for AD.
IX. Cathepsin B Upregulation Is a Common Response in Neurologic Disorders and Causes Cellular Pathology by Multiple Specific Mechanisms
CTSB upregulation is a common response in neurologic disorders as shown by a wide range of neuropathological conditions in such brain diseases (Table 2). Innate and adaptive immune activation occurs in response to a wide range of damage and infectious signals and results in CTSB upregulation (Yan et al., 2020). Activation of the immune system may be a common mechanism by which the diverse neuropathologies cause CTSB activation, but more research is needed to determine if that is the case. Regardless, the upregulated and uncontrolled CTSB activity that accompanies the neuropathology causes cellular and tissue damage, which can continue long after the precipitating cause has subsided. The CTSB KO data show that the CTSB activity damages cells and tissues via multiple mechanisms including cellular necrosis, apoptotic cell death, vasculature permeation, microglial activation, NLRP3 inflammasome activation, caspase-1 activation, NFκB activation, overproduction of ROS and cytokines (IL-1β, IL-18, and TNF-α), selective neuronal autophagy activation, nuclear sirtuins degradation, and Aβ production and degradation. The extent and importance of each specific mechanism by which CTSB damages cells likely varies with the neuropathological condition and model. Figure 3 summarizes many of these mechanisms of action mediated by CTSB, and Table 3 summarizes how elimination of the CTSB gene improves behavioral and pathologic phenotypes in several brain disorder models.
X. Summary and Conclusion: Cathepsin B Knockout Data Validates Cathepsin B as a Drug Target for Development of Cathepsin B Inhibitors as Potentially New Therapeutics for Neurologic Disorders
The aggregate of the CTSB gene knockout studies demonstrate the prominent role of CTSB in mediating the behavioral deficits, inflammation, and cell death in a multitude of neurologic disease animal models of Alzheimer’s disease, periodontitis AD, aging, traumatic brain injury, ischemia, epilepsy, inflammatory pain, and opioid tolerance. The main conclusion is that the data consilience shows that (1) the powerful proteolytic activity of CTSB becomes increased and uncontrolled in numerous neurologic and aging conditions causing behavioral dysfunction and neuropathology and (2) CTSB gene KO in these models results in substantial improvements in behavioral deficits and amelioration of pathology (Fig. 5). These CTSB KO animal studies advance our understanding of the role of CTSB upregulation in patients with these neurologic diseases, supporting the hypothesis that these human brain disorders are dependent on CTSB mechanisms in memory deficits, motor dysfunction, cell loss, neuroinflammation, and neuropathology. Significantly, CTSB inhibition has merit and therapeutic potential for drug treatment of many neurologic disease conditions. Importantly, mice lacking the CTSB gene are healthy and generally indistinguishable from normal wild-type animals, which predicts the safety of CTSB inhibitors. It is noted that alternative suggestions in the field for activation of CTSB as a therapeutic approach in various diseases are not desirable because CTSB promotes the detrimental condition of cancer (Buck et al., 1992; Vasiljeva et al., 2008; Gopinathan et al., 2012).
The consilience of CTSB KO data in neurologic disorders modeled in mice demonstrate CTSB-dependent behavioral deficits and pathology. Evidence for elevation of CTSB in models of brain disorders and amelioration of behavioral deficits and neuropathology by CTSB gene knockout in these models is summarized in this figure. (A) Elevation of CTSB expression results in several behavioral deficits and pathology in several neurologic disorders modeled in mice. Increased levels of CTSB in the brain occurs in numerous neurologic disorders modeled in mice (Table 2). The elevated CTSB in the animal models of brain disorders parallels the increased CTSB found in numerous patients with clinical neurologic disease (Table 1). (B) CTSB gene KO results in substantial improvements in behavioral deficits and pathology of several neurologic disorders modeled in mice. The consilience of results of CTSB gene KO studies in numerous animal models of neurologic disease demonstrate that the absence of CTSB results in substantial improvements in behavioral deficits and pathology (Tables 3 and 4).
Indeed, chemical inhibition of CTSB for improvement of behavioral deficits and neuropathology of brain disorders has been investigated in the field for Alzheimer’s disease (Hook et al., 2005, 2007, 2008b, 2011 2014b), TBI and brain trauma (Knoblach et al., 2004; Sun et al., 2013; Luo et al., 2010; Hook et al., 2014a; Ni et al., 2012), ischemia (Inuzuka et al., 1990; Yamashima et al., 1998; Tsuchiya et al., 1999; Seyfried et al., 2001; Yoshida et al., 2002; Tsubokawa et al., 2006), pain (Sun et al., 2012; Nakanishi, 2020), meningitis (Ruff and Secrist, 1984), and other neurologic and neurodegenerative disease animal models (reviewed in Hook et al., 2020; Sharma et al., 2022). These studies have used (a) the selective CTSB inhibitor, CA-074 (Murata et al., 1991; Towatari et al., 1991), administered in vivo to animal models as the prodrug form of CA-074Me (Buttle et al., 1992), (b) the pan-cysteine protease inhibitor E64c (Hashida et al., 1980; Tamai et al., 1986), administered as its prodrug form of E64d, and (c) other inhibitors of CTSB, such as K11777 (Turk et al., 2012), Z-Phe-Arg-FMK (Wang et al., 2006), and other related inhibitors. These CTSB inhibitor studies are summarized in a recent review (Hook et al., 2020).
In summary, the strong evidence for CTSB participation in numerous neurologic disease conditions and efforts in the field for development of chemical inhibitors of CTSB for therapeutics support the hypothesis that CTSB inhibitors can be developed as therapeutic agents for treatment of CTSB-dependent neurologic disease deficits.
XI. Significance
The significance of this review is in its comprehensive compilation of the extensive data that together point to inhibition of the CTSB target as the logical approach for therapeutics development for the plethora of CTSB-dependent neurologic disease conditions. The extensive experimental evidence in support of inhibition of CTSB as the best therapeutic approach for these diseases addresses an ongoing debate in the CTSB research community as to whether CTSB inhibition or activation is the appropriate therapeutic approach. The consilience of the majority of data demonstrates the significant conclusion that inhibition of the CTSB target provides a new opportunity to address the lack of drug treatments available that are needed to improve patient health in neurologic disease conditions.
Acknowledgments
Figure 2 is adapted from Hook et al., 2015, DOI: 10.3389/fneur.2015.00178, originally published by Frontiers. The Frontiers policy states that “The CC-BY Creative Commons attribution license enables anyone to sue the publication freely, given appropriate attribution to the authors and citing Frontiers as the original publisher.”
Authorship Contributions
Wrote or contributed to the writing of the manuscript: G. Hook, Reinheckel, Ni, Wu, Peters, V. Hook.
Footnotes
This work was supported by National Institutes of Health National Institute of Neurological Disease and Stroke [Grant R01-NS109075] (to V.H.) and [Grant R41-NS110147] (to G.H.); the Deutsche Forschungsgemeinschaft, under Germany’s Excellence Strategy [BIOSS-EXC-294] (to T.R.); the Collaborative Research Centre 850 [project B7] (to T.R.); GRK 2606 [Project ID 423813989)] (to T.R.); the German Cancer Consortium DKTK [projects L627 and FR01-371] (to T.R.); and the Grant-in-Aid for Scientific Research [16K11478] research grant for OBT research center from Kyushu University (to Z.W.).
V.H. and G.H. have equity positions at American Life Science Pharmaceuticals (ALSP) and are founders of ALSP. V.H. is an advisor to ALSP. G.H. is vice president of research, corporate counsel, and member of the board of directors at ALSP. V.H.’s conflict has been disclosed and is managed by her employer, the University of California, San Diego. No other author has an actual or perceived conflict of interest with the contents of this article.
Abbreviations
- Aβ
- amyloid-beta
- AD
- Alzheimer’s disease
- AGE
- advanced glycation end
- ALS
- amyotrophic lateral sclerosis
- APP
- amyloid precursor protein
- CA-074
- L-3-trans-(Propylcarbamyl)oxirane-2-carbonyl)-L-proline
- CCI
- controlled cortical impact
- CFA
- complete Freund’s adjuvant
- CgA
- chromogranin A
- CNS
- central nervous system
- CSF
- cerebrospinal fluid
- CTSB
- cathepsin B
- EAE
- experimental autoimmune encephalomyelitis
- EPM1
- progressive myocolonus epilepsy of the Unverricht-Lundborg type
- ESC
- embryonic stem cells
- FAD
- familial Alzheimer’s disease
- hAPP
- human amyloid precursor protein
- HI
- hypoxia/ischemia
- HIV
- human immunodeficient virus
- ICP
- intracranial pressure
- IDUA
- α-L-iduronidase
- IL
- interleukin
- KO
- knockout
- KWE
- keratolytic winter erythema
- Lon
- London mutation of amyloid precursor protein
- MPS 1
- mucopolysaccharidosis type I
- MS
- multiple sclerosis
- NF-κB
- nuclear factor κB
- NLRP3,
- domains containing protein 3
- PBBI
- penetrating ballistic-like brain injury
- PDGF
- platelet derived growth factor
- PgLPS
- Porphyromonas gingivalis lipopolysaccharide
- pGlu-Aβ
- pyroglutamate amyloid-beta
- PPK
- palmoplantar keratoderma
- PS1
- presenilin 1
- QC
- glutaminyl cyclase
- Swe
- Swedish mutation of amyloid precursor protein
- TBI
- traumatic brain injury
- TNFα
- tumor necrosis factor alpha
- WT
- wild-type.
- U.S. Government work not protected by U.S. copyright