Introduction
In less than a decade since their discovery, the study of K2P channels has revealed that background leak of potassium ions via dedicated pathways is a highly regulated mechanism to control cellular excitability. Potassium leak pathways, active at rest, stabilize membrane potential below firing threshold and expedite repolarization. Although the existence of leak currents was proposed in 1952 by Hodgkin and Huxley, they remained a biophysical curiosity for more than 4 decades. Identification of the first molecular correlate of a potassium leak current was preceded by cloning of potassium channels in Saccharomyces cerevisiae and Caenorhabditis elegans with two pore-forming P loops in each subunit and four or eight transmembrane (TM1) domains (Ketchum et al., 1995). Thereafter, K2PØ was isolated by functional expression cloning from the neuromuscular tissue of Drosophilia melanogaster (Goldstein et al., 1996). Biophysical characterization revealed K2PØ to be a potassium-selective channel with the predicted attributes of a background conductance, that is, a voltage-independent portal showing Goldman-Hodgkin-Katz (open) rectification. When the concentration of potassium is symmetrical across the membrane, K2PØ currents change in a linear manner with voltage; under physiological conditions (high internal and low external potassium), K2PØ passes greater outward than inward currents (Goldstein et al., 2001).
A striking feature of K2P channels is their subunit body plan: each has two P loops and four TM domains. This distinct 2P/4TM topology can be found in more than 70 predicted homologs in genome databases. Fifteen mammalian genes in the family are designated as KCNK genes encoding the K2P channels (Fig. 1); most readily reveal ion channel function upon expression. As expected for regulators of excitability, K2P channels are under tight control by a plethora of chemical and physical stimuli, including oxygen tension, pH, lipids, mechanical stretch, neurotransmitters, and G protein-coupled receptors; the channels are also the molecular targets for certain volatile and local anesthetics (Lesage and Lazdunski, 2000). Regulation of K2P channels alters the attributes subject to change in any ion channel: number of pores at the site of operation, open probability, and unitary current (Plant et al., 2005). Nonetheless, some regulatory changes are striking; for example, phosphorylation of K2P2 endows the open rectifier with sensitivity to voltage (Bockenhauer et al., 2001), and desumoylation of K2P1 (removal of covalently-bound small ubiquitin-modifier protein) relieves chronic silencing of complexes that reside in the plasma membrane, thereby revealing that the protein can function as an ion channel and operates like K2PØ as an open rectifier (Plant et al., 2005; Rajan et al., 2005). Tables 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 present the properties of K2P1.1 through K2P18.1 channels.
K2P1.1 channels
K2P2.1 channels
K2P3.1 channels
K2P4.1 channels
K2P5.1 channels
K2P6.1 channels
K2P7.1 channels
K2P9.1 channels
K2P10.1 channels
K2P12.1 channels
K2P13.1 channels
K2P15.1 channels
K2P16.1 channels
K2P17.1 channels
K2P18.1 channels
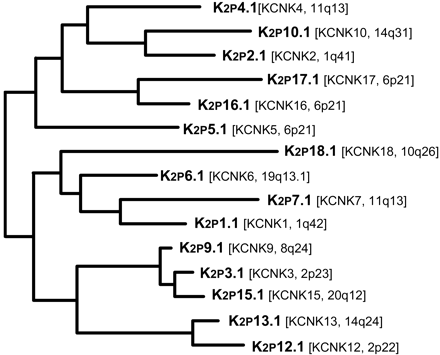
Phylogenetic tree for K2P channels. Amino acid sequence alignments and phylogenetic analysis for the 15 known members of the human K2P family were generated as described in the legend for Fig. 1 of “LIII. Nomenclature and Molecular Relationships of Voltage-Gated Potassium Channels.” K2P18.1 was added to the topology shown in the previous edition of this compendium by use of maximum parsimony and neighbor-joining algorithms. International Union of Pharmacology and HUGO Gene Nomenclature Committee names of the genes are shown together with their chromosomal localization.
Footnotes
-
↵1 Abbreviations: TM, transmembrane.
-
Article, publication date, and citation information can be found at http://pharmrev.aspetjournals.org.
-
doi:10.1124/pr.57.4.12.
- The American Society for Pharmacology and Experimental Therapeutics