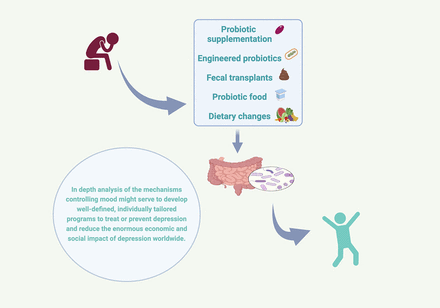
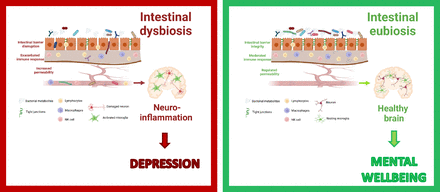
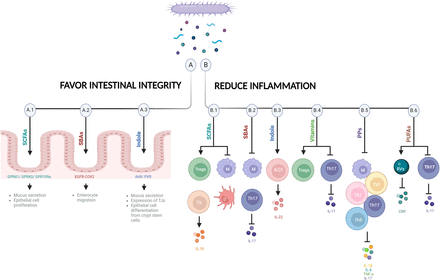
Visual Overview
Abstract
Depression is a highly prevalent disorder and a leading cause of disability worldwide. It has a major impact on the affected individual and on society as a whole. Regrettably, current available treatments for this condition are insufficient in many patients. In recent years, the gut microbiome has emerged as a promising alternative target for treating and preventing depressive disorders. However, the microbes that form this ecosystem do not act alone but are part of a complicated network connecting the gut and the brain that influences our mood. Host cells that are in intimate contact with gut microbes, such as the epithelial cells forming the gut barrier and the immune cells in their vicinity, play a key role in the process. These cells continuously shape immune responses to maintain healthy communication between gut microbes and the host. In this article, we review how the interplay among epithelial cells, the immune system, and gut microbes mediates gut-brain communication to influence mood. We also discuss how advances in our knowledge of the mechanisms underlying the gut-brain axis could contribute to addressing depression.
Significance Statement This review does not aim to systematically describe intestinal microbes that might be beneficial or detrimental for depression. We have adopted a novel point of view by focusing on potential mechanisms underlying the crosstalk between gut microbes and their intestinal environment to control mood. These pathways could be targeted by well defined and individually tailored dietary interventions, microbes, or microbial metabolites to ameliorate depression and decrease its important social and economic impact.
I. Introduction
Depression is a highly prevalent and heterogeneous disorder characterized by low mood and loss of interest or pleasure in all or nearly all activities in addition to symptoms such as changes in appetite or weight, sleep, and psychomotor activity; decreased energy; feelings of worthlessness or guilt; difficulty concentrating or making decisions; and suicidal thoughts or behaviors. More than 280 million people worldwide suffer from depressive disorders. Furthermore, depression is a leading cause of disability and premature death, making it a significant contributor to the global burden of disease with an elevated economic impact (GBD 2017 Disease and Injury Prevalence Collaborators, 2018). Despite the ongoing development of new pharmacological and psychological therapies, there are many unmet needs in the treatment of depressive disorders. More than 50% of people treated with existing therapies show an inadequate response or fail to achieve remission (Ormel et al., 2019). Consequently, new therapeutic options are needed to reduce the economic burden and alleviate the suffering of patients, as depression is a debilitating and often fatal disease.
Microbes that populate our gut, also known as the gut microbiome, communicate with the central nervous system in a bidirectional manner and influence our mood (Margolis et al., 2021). For this reason, researchers are beginning to consider targeting or using these microbes as alternative therapeutic agents for mood disorders such as depression. Bacteria that exert a health benefit on the host are called probiotics. They can be obtained through supplements, naturally fermented food, or artificially enriched food. The specific name for probiotics that benefit mental health is “psychobiotics” (Dinan et al., 2013; Sarkar et al., 2016).
In recent years, an increasing number of studies have focused on the gut microbiome in depressed people to identify potential beneficial microbes. Interest in the microbiome’s influence on mental health has transcended the realm of the scientific community to the wider public, becoming the subject of news stories, wellness forums, social networks, and advertising campaigns. However, most published studies on the gut microbiome in the context of depression are merely descriptive, reporting differences in depressed people compared with healthy controls (Alli et al., 2022). In addition, the great variability of the individual microbiome resulting from the many factors that influence its composition and function (e.g., microbes inherited at birth, diet, use of antibiotics or other medications) makes it very difficult to reach a consensus on a microbial signature associated with depression. Nonetheless, preclinical studies that have replicated depression-like behaviors in animals after the transfer of fecal microbiota from depressed people point to a causal role of the gut microbiota (Kelly et al., 2016; Knudsen et al., 2021). Researchers have confirmed this hypothesis by comparing the behavior of germ-free or microbiota-depleted rodents to that of rodents with normal gut colonization (Kunugi, 2021).
The effects of intestinal microbes depend, first, on communication with the host’s intestinal epithelial cells and the surrounding environment, which can initiate the cascade of signals that eventually reach the central nervous system and influence mental health. Thus, the epithelial cell barrier, which is in close communication with the immune, endocrine, and nervous systems, serves as a first meeting point between the microbes and the host. Later, signals can be transmitted to the central nervous system by: 1) the physical gut-brain connection through the autonomic nervous system, the vagus nerve, and the enteric nervous system; 2) neuroendocrine mediators from the hypothalamic-pituitary-adrenal (HPA) axis; and 3) the immune system, whose immune cells or their immune mediators such as cytokines travel to the brain where they influence mood.
The third mechanism is one focus of this review. Researchers have used various approaches to investigate how gut microbes interact with the host epithelial and immune cells in the intestine to mediate depression, but our understanding of these mechanisms is far from complete.
In the present review, we summarize the current evidence on the interactions among intestinal microbes, epithelial cells, and the intestinal immune system in the context of well-being and depression. We also discuss the gaps and future perspectives of microbiome-based approaches in the field of psychiatry. This review does not provide a systematic description of intestinal microbes that might be beneficial for treating depression; rather, we have adopted a novel perspective by highlighting potential mechanisms of the crosstalk between gut microbes and their intestinal environment that have significant implications for brain function and behavior. These pathways could be targeted by dietary interventions, microbes, or microbial metabolites to ameliorate depression. Therefore, despite our incomplete understanding of the gut-immune-brain axis, this review provides insights into the potential translation of research from bench to bedside.
II. Structure and Function of the Gut Epithelial Barrier
Trillions of microorganisms, including bacteria, archaea, fungi, and viruses, can live in the digestive tracts of animals without upsetting their health. This implies a very well organized physical, molecular, and immune defense that confines the microorganisms to the gut lumen and protects the host.
The first line of defense in the gut is a mucus layer composed of mucins (highly glycosylated proteins); it provides a crucial barrier between the microbiome and the host cells (Johansson et al., 2013). Lying beneath this mucus layer is the intestinal epithelium: a monolayer of specialized cells such as enterocytes or intraepithelial cells, Paneth cells, goblet cells, and microfold cells. Each cell type has a different function. The most abundant cells are intraepithelial cells or enterocytes, whose main role is to maintain epithelial barrier integrity while also producing cytokines, chemokines, and immunological factors against luminal bacteria (Goto, 2019). Paneth cells are secretory cells residing in small clusters at the base of crypts of Lieberkühn. They have large secretory granules containing antimicrobials, which are discharged into the crypt lumen and diffuse into the mucous layer overlying the mucosal epithelium, thus preventing microbial invasion (Bevins and Salzman, 2011). Goblet cells secrete the mucus that forms the mucus layer while also secreting antimicrobial proteins, chemokines, and cytokines (Knoop and Newberry, 2018). Microfold cells transfer microbial antigens from the lumen that are essential for the induction of efficient immune responses (Gutzeit et al., 2014). Therefore, the epithelial cells have immune functions beyond physical barrier maintenance.
The epithelial cells are linked to each other by junctional complexes consisting of tight junctions, adherens junctions, desmosomes, and gap junctions (Schoultz and Keita, 2020). Tight junctions, also called zonula occludens, are multiprotein complexes embedded in the plasma membrane that interact with the adjacent cells. They include the tight junction–associated MARVEL proteins (TAMPs), claudins, and members of the junctional adhesion molecule protein family. Adherens junctions are molecules pertaining to the cadherin family. Adherence junctions and tight junctions form the apical junctional complex. The desmosomes comprise the desmoglein and desmocollin families of desmosomal cadherins as well as connecting proteins such as desmoplakin and keratin. The gap junctions link the interior of adjacent cells (Schoultz and Keita, 2020). All of these junctions maintain the barrier composed by the epithelial cells and prevent microbes leaking from the gut. Gut epithelial barrier integrity is crucial for maintaining a balanced bacteria-host interaction.
Underneath the epithelial monolayer is a thin layer of connective tissue called the lamina propria. Epithelial cells communicate with the microbiota and with immune cells of the lamina propria. This communication among the microbes, the epithelium, and the immune system of the host plays a crucial role in health and disease, including mental health and mental disorders such as depression.
III. Intestinal Immune System
As commented above, the epithelial cells help to control the microbiome by secreting antimicrobial molecules. In addition, the intestinal mucosa is in close contact with cells of the innate and adaptative immune systems to control the microbes and trigger a suitable immune response when homeostasis is disrupted (Zheng et al., 2020). The interplay between the intestinal epithelium and microbiota maintains a basal state of inflammation, which prevents excess inflammatory responses under physiological conditions.
The immune cells in the gut are located in the intestinal lamina propria as well as interleaved in the epithelial layer. The gut contains cells of the innate immune system such as macrophages, dendritic cells, and innate lymphoid cells (ILCs) and of the adaptative immune system such as T and B cells (Yuan and Walker, 2004). Intestinal macrophages are highly heterogeneous in terms of their function and localization within the gut. There are three main types of macrophages: monocyte-derived mature macrophages, monocyte-derived inflammatory macrophages, and self-maintaining macrophages. Monocyte-derived mature macrophages (Ly6C+, CD64+, MHCII+, CX3CR1hi) are located mainly in the lamina propria and secrete anti-inflammatory cytokines. Monocyte-derived inflammatory macrophages (CD64+, MHCII+, CX3CR1int) also reside in the lamina propria, but they secrete proinflammatory cytokines. Self-maintaining macrophages (Tim-4+, CD4+) arise from embryonic precursors and adult bone marrow–derived monocytes; they have a relatively low replenishment rate from blood monocytes compared with the other two types of macrophages, which suggests they are maintained locally. They are located predominantly within the muscularis externa layer, although they are also present in the lamina propria (Yip et al., 2021). Macrophages play a key role in the gut immune system and the regulation of gastrointestinal physiology, including gut motility and secretion. Although the gut is in constant contact with foreign antigens, macrophages protect it from chronic inflammation and thus contribute to intestinal homeostasis. They also contribute to neuroimmune interactions.
Dendritic cells are bone marrow–derived antigen-presenting cells that can be divided into two major subsets: conventional (or classical) and plasmacytoid. Although they are developmentally distinct from macrophages, both cell types share many phenotypic markers (CD11c, MHCII, and CX3CR1). However, research suggests that CD64 expression can differentiate intestinal macrophages from dendritic cells (Tamoutounour et al., 2012). Dendritic cells constantly sample luminal content to monitor for pathogens while also mediating tolerance to food antigens and controlling reactivity to the gut microbiota. Collectively, they generate both regulatory and effector T cell responses (Stagg, 2018).
Innate lymphoid cells are an important family of immune cells that share characteristics with T cells but do not express antigen receptors or undergo clonal selection and expansion when stimulated. Instead, ILCs react promptly to damage signals and produce cytokines. Thus, natural killer cells can be considered the innate counterparts of cytotoxic CD8+ T cells, whereas ILC1s, ILC2s, and ILC3s may represent the innate counterparts of CD4+ T helper 1, T helper 2, and T helper 17 cells (Eberl et al., 2015; Vivier et al., 2018). In addition to all of these innate immune cells, T lymphocytes, as cells of the adaptative immune system, have adapted to intestinal environments, constantly discriminating between natural stimuli (from commensal microbiota and food) and pathogens that need to be eliminated and helping to maintain the integrity of the intestinal barrier and immune homeostasis. T lymphocytes are interleaved in the epithelial layer between the enterocytes (intraepithelial lymphocytes). Due to this specific location, intraepithelial lymphocytes are in direct contact with enterocytes and are in the immediate proximity of antigens in the gut lumen, representing an important part of the gut lymphoid tissue (Vitale et al., 2016). They have regulatory and effector capabilities, so they can prevent pathogenic invasion while maintaining tolerance to the commensal microbes.
Intraepithelial lymphocytes can be divided into “type a” intestinal T cells, which express TCRαβ along with CD4 or CD8αβ as coreceptors, and “type b,” which express either TCRαβ or TCRγδ and, typically, CD8αα homodimers. Intestinal T cells such as CD4+ T helper cells 1, 2, and 17, CD8+ cytotoxic T cells, or FoxP3+ regulatory T cells can also be found in the lamina propria. Together with intraepithelial lymphocytes, these lamina propria lymphocytes fight harmful microbes and contribute to mucosal tolerance. Lamina propria lymphocytes are derived from conventional T cells that are developed in the thymus, primed in secondary lymphoid organs, and reach the lamina propria with an effector memory phenotype (Ma et al., 2019). Finally, B cells are also key players in the symbiotic maintenance of the gut system because activated and differentiated B cells (plasma cells) produce secretory immunoglobulin A, which binds commensals, preventing their adhesion to the enterocytes and limiting microbial motility without activating the complement cascade. Thus, B cells prevent inflammatory damage to the epithelial barrier and preserve a healthy microbial ecosystem.
The immune function carried out in the intestine involves a group of organs and structures called the gut-associated lymphoid tissue, which coordinates B cell function and drains mesenteric and caudal lymph nodes. Gut-associated lymphoid tissue includes Peyer’s patches, isolated lymphoid follicles, and the appendix. Peyer’s patches are located primarily along the small intestine and constitute important sites for the initiation of the immune response in the gut since they are in close contact with the microbes in the lumen. These structures contain a large number of follicles with small T cell areas and germinal centers in which B cells differentiate and proliferate. They are a major immunoglobulin A induction site and play a key role in the maintenance of commensal bacteria and host protection (Botía-Sánchez et al., 2021). Therefore, similarly to an intact intestinal barrier, the efficient crosstalk among microbes, epithelial cells, and immune system cells guarantees a safe relationship between the microbes and their host. Host health can be compromised when the intestinal barrier is breached, as may occur with pathogen invasion or other factors causing bacterial dysbiosis. Increased permeability of the intestinal barrier, known as “leaky gut,” allows the uncontrolled extravasation of microbes and their structural components out of the gut, where they can reach other tissues and cause an exacerbated immune response and numerous diseases, including mental health disorders (Fig. 1).
Intestinal bacteria interplay with the host intestinal epithelium and the immune system to maintain mental well-being. Disruption of this well orchestrated microbe-host ecosystem leads to depression (figure created with biorender.com).
Bacterial components, such as the lipopolysaccharide from the membrane of gram-negative bacteria, have been found at increased levels in the serum of depressed people (Stevens et al., 2018; Caso et al., 2021). These bacterial components may leak from the intestinal lumen and activate an innate immune response in the intestine. Thus, on penetrating the lamina propria, antigens are recognized and trigger the production of proinflammatory cytokines, including interleukin-1β, interleukin-6, tumor necrosis factor alpha (TNFα), interferon-γ, and cytokines involved in the interleukin-23/T helper 17 pathway, in local tissues (Sano et al., 2015; Guan, 2019). Subsequently, the intestinal barrier dysfunction facilitates the passage of local inflammation to the peripheral circulation and other organs, thereby contributing to depression. Research suggests that the T helper 17 pathway plays a key role in depression (Beurel et al., 2013, 2022; Medina-Rodriguez et al., 2020), as does the abundance of circulating cytokines. Indeed, elevated levels of TNFα, interleukin-6, and interleukin-1β have been found in blood and cerebrospinal fluid of individuals with depression compared with healthy controls (Medina-Rodriguez et al., 2018).
In summary, maintaining a robust gut barrier and a balanced immune response is important not only for the physical well-being of the host but also for their mental health.
IV. The Gut Microbiome and Manipulation of the Gut Microbes and Their Metabolites As a Strategy to Treat Depression
The gut microbiome is the ecosystem of microorganisms that populate the gastrointestinal tract. The most common bacterial phyla among the microbiome are Bacteroidetes and Firmicutes, although more than 50 phyla have been described in the human gut. Ample research has shown that the effects of the gut microbes go beyond the gastrointestinal system. Apart from participating in human metabolism and nutrition and being linked to gastrointestinal conditions such as inflammatory bowel disease, irritable bowel syndrome, and obesity, the gut microbiome appears to participate in immune function and cardiovascular, respiratory, liver, kidney, and mental diseases (Hou et al., 2022). Regarding depression, observational studies have shown that the gut microbiome and its metabolites differ between depressed people and healthy controls (Yang et al., 2020). Further, different teams of researchers found that transferring the intestinal microbial community from depressed people to rodents was sufficient to induce depressive-like behaviors in the recipient animals (Kelly et al., 2016; Medina-Rodriguez et al., 2023). In addition, accumulating data indicate a negative impact of antibiotics on mental health. Overall, antibiotics increase depression prevalence, which is likely linked to a decrease of microbial diversity and intestinal barrier disruption (Dinan and Dinan, 2022). Thus, manipulating or reestablishing the microbiome composition might help to ameliorate depression.
A. Probiotic Supplementation
Although still controversial, the use of some probiotics has demonstrated a beneficial effect on depression. For instance, one randomized controlled trial (RCT) reported that add-on probiotic treatment [Vivomixx, containing eight different strains: Streptococcus thermophilus NCIMB 30438, Bifidobacterium breve NCIMB 30441, Bifidobacterium longum NCIMB 30435 (reclassified as B. lactis), Bifidobacterium infantis NCIMB 30436 (reclassified as B. lactis), Lactobacillus acidophilus NCIMB 30442, Lactobacillus plantarum NCIMB 30437, Lactobacillus paracasei NCIMB 30439, Lactobacillus delbrueckii subsp. Bulgaricus NCIMB 30440 (reclassified as L. helveticus)] led to changes in the gut microbiota and brain, as assessed by functional magnetic resonance imaging (fMRI), and improved depressive symptoms (Schaub et al., 2022). In another clinical trial, NVP-1704 (a mixture of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98) alleviated subclinical depression and exerted anti-inflammatory effects, with a decrease in serum interleukin-6 concentrations after 8 weeks of intervention (Lee et al., 2021). Another study found that Bifidobacterium breve CCFM1025 attenuated major depressive disorder (MDD) by regulating the gut microbiome and tryptophan metabolites, measured in feces by ultrahigh-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS); however, participants’ diet and medication (other than antibiotics) were not strictly controlled, which could have influenced the results (Tian et al., 2022). Another clinical trial with Lactobacillus plantarum 299v showed a decrease in kynurenine concentration determined by high-performance liquid chromatography (HPLC) in blood, together with ameliorated cognitive functions in people with major depression. However, there were no differences in depression score (HAMD-17 scale) observed between probiotic and placebo groups over an 8-week trial (Rudzki et al., 2019), nor did other probiotics such as Lactobacillus casei Shirota decrease depression scores over a 9-week period (Zhang et al., 2021).
Owing to these inconsistencies across different studies, together with the growth of big-data analysis, researchers have begun to synthesize data from different clinical trials. One meta-analysis of five clinical trials (183 cases and 182 controls) showed that probiotics significantly lowered depression scores in people aged under 60 years with MDD (Huang et al., 2016). Another meta-analysis, which included the same five clinical trials plus five more (1349 participants in total), showed no difference in mood between the probiotic-treated group and the placebo group. However, a separate subgroup analysis of studies conducted in healthy individuals versus those conducted in individuals with MDD or mild to moderate anxiety and/or depression revealed that probiotic supplementation significantly improved the moods of individuals with mild-moderate depressive symptoms (Ng et al., 2018). Interestingly, one recent systematic review of five studies in people with MDD found adjuvant probiotic or synbiotic treatment to be more efficacious for improving symptoms than antidepressant medication alone (Forth et al., 2023).
Thus, probiotic treatment may be effective as monotherapy for depression or as an adjunct to standard antidepressant medication.
In general, most studies agree that common probiotic strains for depression belong to the genus Lactobacillus, Bifidobacterium, Akkermansia, Clostridium, Streptococcus, and Enterococcus (Gao et al., 2023). However, because these genera include a huge number of strains and because of interpersonal variability, determining a microbial signature and a universal set of probiotics for depression is likely unfeasible. Nevertheless, techniques for individual microbiome screening are advancing fast, and further studies of the gut microbiome composition and potential probiotics might open new avenues for more precise, effective, and personalized ways to improve depression.
B. Engineered Probiotics
Based on genetic engineering, advances in psychiatric therapies might include the use of genetically modified bacteria. Bacteria can be engineered with immune regulatory proteins, chemotactic response systems, and protein delivery systems (Hou et al., 2022).
Engineered probiotics have been tested in preclinical models of colitis, diabetes, cancer, obesity, and pathogenic infections. Lactococcus lactis genetically modified to deliver interleukin-10 has been proven safe and effective in people with Crohn’s disease (Braat et al., 2006). This may constitute a new approach to continuous expression of beneficial metabolites after intestinal colonization to achieve therapeutic effects, although it has yet to be tested in psychiatric diseases.
C. Fecal Transplants
Another way of manipulating the gut microbiome that has garnered considerable scientific interest is fecal transplantation. The process consists of collecting feces from a healthy donor and introducing them into patient’s intestine. Fecal transplantation is usually performed by colonoscopy, but the medically processed feces can also be introduced though a nasoduodenal tube, an oral capsule, or an enema. Fecal transplants are safe and well tolerated. They are showing good results in people with a Clostridium difficile infection, but their effects in other conditions such as inflammatory bowel disease, obesity, or mental diseases remain unclear. Although evidence from preclinical studies in animal models suggests that this approach may improve depression (Knudsen et al., 2021), there are few published studies in humans. One study in people with gastrointestinal disorders found that fecal transplant may improve depressive symptoms (Kurokawa et al., 2018). Furthermore, a case report of a 79-year-old woman with depression who underwent antidepressant and probiotic treatments for 6 months with no beneficial outcome showed an improvement in her symptoms after a transplant via gastroscope with a bacterial solution from her 6-year-old great-grandson (Cai et al., 2019). Another recent publication reported improved depressive symptoms in two people with MDD after they received fecal microbiota transplantation (oral frozen capsules) as add-on therapy; however, because these people received additional pharmacological and psychotherapy treatments, the authors could not attribute the positive outcomes to the fecal transplant alone (Doll et al., 2022). Further studies are needed to corroborate the efficiency of fecal transplantation in depressed individuals.
D. Probiotic Food
Diet-mediated approaches to shaping the gut microbiome, such as the ingestion of food containing beneficial bacteria (probiotic foods), might constitute another therapeutic approach to improving mental health. Fermented foods represent a source of live microbes that may be beneficial for depression. These include fermented vegetables (i.e., kimchi) or fermented milk products (i.e., yogurt or kefir), among others (Rao and Samak, 2013). For instance, studies have shown that kefir improves depression in menopausal women (Özcan et al., 2019; Peluzio et al., 2021). Similarly, one cross-sectional study found that consumption of kimchi and fermented milk products reduced the severity and prevalence of depression, although only in men (Kim and Shin, 2019). One recent meta-analysis of eight studies found a significant association between yogurt consumption and decreased depression risk (Luo et al., 2023).
These types of studies are not exempt from limitations, and readers must interpret their results with special caution. Many other dietary components can influence mental health and might be confounding factors in studies of probiotic dietary interventions. Distinguishing the health benefits of fermented food versus other components of the diet is often difficult, and researchers must control for many other confounding factors (e.g., physical activity, socioeconomic status, education, and quality of life) to avoid biased results.
However, observational studies of this nature can still give insights into potential solutions for improving mental health.
E. Dietary Changes
As well as probiotic food, some other dietary patterns that directly impact gut microbiome composition may be beneficial for the treatment of depression (Dinan et al., 2019). One cross-sectional study of 400 women attending healthcare centers found that diets rich in fruits and vegetables reduced the risk of depression (Baharzadeh et al., 2018). It is known that sulforaphane, present in cruciferous vegetables such as broccoli or sprouts, may prevent the onset of chronic inflammation-related depression (Zhang et al., 2017). In addition, one meta-analysis of 18 studies concluded that total dietary fiber intake is associated with a 10% lower odds of depression in adults and a 57% lower odds in adolescents (Saghafian et al., 2023). Conversely, high-salt diets may lead to greater depressive symptom severity in adolescents (Mrug et al., 2019).
The effects of specific diets on our mental health are mediated by different dietary components, which are related to the gut microbiome:
1. Short-Chain Fatty Acids and Lactate
Short-chain fatty acids (SCFAs) are the main metabolic products of anaerobic bacterial fermentation in the intestine. Dietary fibers are converted to SCFAs after being broken down by bacteria. SCFAs and their metabolites have antidepressant properties (Hamad et al., 2022). Accordingly, compared with healthy controls, people with depression have reduced levels of SCFAs such as acetic acid, propionic acid, and pentanoic acid (Skonieczna-Żydecka et al., 2018) and elevated levels of the saturated fatty acids stearic acid and myristic acid (Frost et al., 2014). Similarly, several studies support an association between depression and other bacterial metabolites abnormalities such as lactate. For example, one study found increased concentrations of urinary lactate in people with severe MDD compared with controls (Chen et al., 2017).
2. Secondary Bile Acids
The primary bile acids are synthetized from cholesterol in the liver and released into the intestine in response to dietary fat. The action of intestinal bacteria on primary bile acids results in the formation of secondary bile acid species. The secondary bile acid tauroursodeoxycholic acid has been found at increased levels in the blood of people with depression (Pu et al., 2021). One study found that people with MDD had significantly higher levels of 23-nordeoxycholic acid and significantly lower levels of taurolithocholic acid, glycolithocholic acid, and lithocholic acid 3-sulfate compared with healthy controls (Sun et al., 2022).
3. Tryptophan Metabolites
Tryptophan is an essential amino acid necessary to produce proteins. It is found naturally in red meat, poultry, eggs, and dairy products. Thus, diet can also influence the production of several tryptophan metabolites that have been linked to depression. The intestinal microbiome plays a key role in regulating tryptophan metabolism toward the synthesis of molecules that influence immunity, metabolism, and neurotransmission (Hou et al., 2023). Kynurenine is one of the main products from tryptophan metabolism. Elevated kynurenine levels are found in social anxiety disorder patients with a history of suicide attempts (Marin et al., 2017; Giménez-Gómez et al., 2021; Butler et al., 2022). However, several meta-analyses have shown decreased kynurenine levels across MDD patients (Ogyu et al., 2018; Marx et al., 2021). Kynurenine can be metabolized into either kynurenic acid, an N-methyl-D-aspartate (NMDA) receptor antagonist involved in neuroprotection, or quinolinic acid, a potentially neurotoxic NMDA agonist. One meta-analysis showed a reduction in serum kynurenic acid/quinolinic acid in individuals with MDD (Marx et al., 2021), and the decrease in this ratio has been correlated with lower hippocampal and amygdalar volumes (Paul et al., 2022). Levels of quinolinic acid are also elevated in the cerebrospinal fluid of people with a history of suicide attempts (Erhardt et al., 2013). Another recent meta-analysis including 51 studies (7056 participants) found that people with current episodes of MDD had significantly reduced levels of tryptophan, kynurenic acid, kynurenic acid/quinolinic acid, KYNA/3-hydroxykynurenine, and kynurenic acid/kynurenine and significantly increased levels of kynurenine/tryptophan (Ou et al., 2023). Another intestinal bacteria-derived tryptophan metabolite, known as indole acetic acid, may also be reduced in the cerebrospinal fluid of depressed people (Coppen, 1972; Chen et al., 2022).
Clinical trials on tryptophan supplementation have shown that high tryptophan diets result in fewer depressive symptoms and decreased anxiety (Lindseth et al., 2015).
4. Vitamins
Vitamins from diet can modify the composition and metabolic activity of intestinal bacteria (Pham et al., 2021). Vitamins also appear to affect stress and depression (Bell et al., 1991; Young et al., 2019; Mahdavifar et al., 2021). Studies have linked moderate intake of niacin (vitamin B3) to lower odds of anxiety (Mahdavifar et al., 2021) and moderate intake of folic acid (vitamin B9) to a reduced likelihood of depression (Mahdavifar et al., 2021). High dietary intake of vitamins B5 and B8 appears to be inversely associated with the prevalence of depression but only in women (Mahdavifar et al., 2021). Lastly, vitamin A intake has led to significant improvements in depression in people with multiple sclerosis (Bitarafan et al., 2016).
5. Polyphenols
Dietary polyphenols change the gut microbiome composition; at the same time, they are metabolized by the intestinal microbes to produce small active and bioavailable products, which further regulate host functions (Wang et al., 2022). Polyphenols are the most abundant natural compounds in plants, occurring naturally in fruits, vegetables, tea, coffee, cocoa, and wine. In one meta-analysis, 12 of 18 included RCTs showed significant improvements in depression with polyphenol use (Lin et al., 2021). However, many of the data were for postmenopausal women after short-term interventions. In addition, there was a high level of heterogeneity among the trials, possibly because of differences in dosage, forms of supplementation, and duration of the intervention.
6. Polyunsaturated Fatty Acids
Modulation of dietary polyunsaturated fatty acids (PUFAs) by gut microbiome enzymes results in bioactive compounds that could influence human health (Brown et al., 2023). It is known that PUFAs such as omega-3 and omega-9, derived from plant-based products and fish, have beneficial effects on depression (Rao et al., 2008). In this respect, some omega-3 fatty acids, such as eicosapentaenoic acid (EPA), have been found to elicit antidepressant effects in humans, whereas docosahexaenoic acid does not exhibit such benefits (Liao et al., 2019). EPA also alleviated MDD in overweight individuals with elevated inflammatory markers in a randomized dose-finding clinical trial (Mischoulon et al., 2022; Lamon-Fava et al., 2023). Another RCT showed that increases in 18-hydroxyeicosapentaenoic acid (18-HEPE), a metabolite of omega-3 fatty acid, correlates with a reduction in depressive symptoms (Lamon-Fava et al., 2023). One cross-sectional study found a positive association between serum omega-9 fatty acid (oleic acid) levels and depression (Yin et al., 2023).
Therefore, the above dietary strategies might represent an alternative approach to alleviating or preventing depression by acting on the gut to reach the brain. However, the results of these studies must be interpreted with caution. Observational studies of the effects of one specific compound are usually biased due to the difficulty of eliminating the rest of compounds from the regular diet of participants, which leads to strong confounding effects derived from individual dietary habits. However, diets enriched in several or most of the above components have demonstrated beneficial effects on mental health. For instance, cross-sectional studies and RCTs have found lower risk of depression in people who follow a Mediterranean diet (Oddo et al., 2022; Seethaler et al., 2022).
Consequently, experts in the field recommend that people with depression or susceptibility to depression should supplement a plant-based diet with a high intake of grains/fibers and fish (Sanz et al., 2018; Dinan et al., 2019).
V. Diet-Mediated Pathways Involving the Microbiome, the Host Intestinal Barrier, and the Immune System as Potential Targets To Treat Depression
This review has presented evidence on the contribution of intestinal microbes and their metabolites, epithelial barrier integrity, and the immune system on depression. However, we have yet to discover how the gut microbiome, the epithelium, and the immune cells interact to control mood. The following section of the review will present potential pathways of microbial-epithelium-immune system communication that might form the basis of future depression therapies (Fig. 2). Intestinal epithelial cells collect signals from intestinal microbes to modulate the mucosal barrier and transfer signals to immune cells in the intestine. Indeed, the immune system is intimately linked to the intestinal microbes: early-life colonization of the gut by microbes plays a pivotal role in maturation of the host’s immune system (Gensollen et al., 2016), and changes in the microbiota throughout life affect the intestinal immune function. Likewise, diet has an important direct or indirect effect on intestinal immunity (through the metabolism of the microbiota) and might represent a therapeutic aid to strengthen the intestinal barrier and control inflammation. For instance, some studies have shown that fermented food such as kefir controls inflammation in humans (Bellikci-Koyu et al., 2019). Conversely, high-salt diet induces interleukin-17–dependent gut inflammation (Aguiar et al., 2018).
Potential mechanisms controlling mood. Some bacterial metabolites favor intestinal integrity (A) and/or reduce inflammation (B), potentially alleviating depression and promoting mental well-being. (A.1) SCFAs bind to receptors in epithelial cells such as GPR41, GPR43, and GPR109a to favor intestinal integrity (e.g., enhance mucus release by goblet cells and facilitate the proliferation of epithelial cells). (B.1) SCFAs reduce inflammation by increasing Tregs and IL-10–producing Th cells, repressing macrophages and suppressing dendritic cell maturation. (A.2) SBAs protect against intestinal barrier breakdown by promoting enterocyte migration via EGFR- and COX-2–dependent mechanisms. (B.2) SBAs downregulate proinflammatory cytokines in macrophages and decrease IL-17 and Th17, which improves inflammation. (A.3) Indole is recognized by AhR or PXR expressed by epithelial cells to maintain epithelial barrier and participates in mucus secretion, in the expression of tight junction molecules, and in the appropriate epithelial cell differentiation from crypt stem cells. (B.3) Indole stimulates ILC3s increasing IL-22. (B.4) Vitamins are recognized by many receptors expressed by immune cells such as Tregs and Th17. Vitamins induce differentiation and survival of Tregs and modulate Th17 cells. (B.5) PPs have immunomodulatory effects on macrophages and decrease Th1, Th2, Th17, and Th9 cells and inflammatory cytokines (IL-17, IL-1β, IL-6, and TNF-α). (B.6) PUFAs decrease immune response by changing plasma CRP levels and Rv concentration and reducing Th17 signaling. COX, cyclooxygenase; CRP, C-reactive protein; D, dendritic cell; EGFR, epidermal growth factor receptor; IL, interleukin; M, macrophage; PP, polyphenol; PXR, pregnane X receptor; Rv, resolvin; SBA, secondary bile acid; Th, T helper cell; TJ, tight junction; Treg, regulatory T cell (figure created with biorender.com).
Diet-linked signals can be triggered by microbial metabolites through different pathways, but the microbes themselves can also control the intestinal barrier and inflammation in different ways.
A. SCFAs and Lactate-Linked Pathways
Bacterial metabolites such as SCFAs appear to play important roles in the maintenance of gut epithelial integrity and intestinal immunity (Kayama et al., 2020) (Fig. 2, A.1 and B.1). They can be regulated by dietary habits, as mentioned before. SCFAs such as butyrate facilitate the proliferation of epithelial cells as an energy source (Salvi and Cowles, 2021) and modulate their immunological function. Epithelial cells express receptors for SCFAs such as G-protein–coupled receptor 41 (GPR41), GPR43, and GPR109a, and their activation controls the production of cytokines and chemokines by the intestinal epithelium (Kim et al., 2013; Singh et al., 2014). SCFAs also enhance mucus production and release by goblet cells (Finnie et al., 1995; Burger-van Paassen et al., 2009). In the innate immune system, butyrate maintains hyporesponsiveness to the commensal bacteria by repressing the expression of proinflammatory mediators in macrophages and suppressing dendritic cell maturation, inducing therein anti-inflammatory properties to accumulate Foxp3+ regulatory T cells and interleukin-10–producing CD4+ T cells (Singh et al., 2010, 2014; Chang et al., 2014). Acetate induces both effector T cells and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway (Park et al., 2015) (Fig. 2). Lactate, also generated by microbial fermentation, accelerates intestinal stem cell–mediated epithelial development (Lee et al., 2018).
B. Secondary Bile Acids Routes
Bile acids such as deoxycholic acid and ursodeoxycholic acid also regulate epithelial cell activity and protect against intestinal barrier breakdown by promoting enterocyte migration via epidermal growth factor receptor (EGFR)- and cyclooxygenase (COX)-2-dependent mechanisms (Maran et al., 2009; Golden et al., 2018; Mroz et al., 2018) (Fig. 2A.2). Bile acids downregulate the production of proinflammatory cytokines by macrophages through recognition by several receptors, such as the farnesoid X receptor (FXR), the sphingosine 1 phosphate receptor 2 (S1PR2), the vitamin D receptor, or the G-protein–coupled receptor 5 (TGR5), expressed in macrophages (Vavassori et al., 2009; Guo et al., 2016; Biagioli et al., 2017; Calzadilla et al., 2022). In addition, bile acids bind retinoic acid–related orphan receptor (ROR)γt as inverse agonists and decrease interleukin-17 and T helper 17 cells with a resulting improvement in inflammation (Calzadilla et al., 2022) (Fig. 2B.2).
C. Tryptophan-Linked Pathways
Indole generated by bacteria from dietary tryptophan is recognized by the aryl hydrocarbon receptor (AhR) expressed by epithelial cells and plays an important role in the maintenance of epithelial barrier integrity by participating in mucus secretion, in the expression of cell-junction-associated molecules, and in the appropriate epithelial cell differentiation from crypt stem cells (Shimada et al., 2013; Metidji et al., 2018). Tryptophan-derived phytochemical indole-3-carbinol, found in cruciferous vegetables, acts on the AhR, and the absence of AhR ligands affects intraepithelial lymphocytes and results in heightened immune activation (Li et al., 2011). In addition, indole stimulation of ILC3s, which also express the AhR, leads to interleukin-22 increase, which has numerous beneficial effects on epithelial cells and the intestinal barrier (Miljković et al., 2021). Microbial-specific indoles can also regulate intestinal barrier function through the xenobiotic sensor, pregnane X receptor. For instance, indole 3-propionic acid as a ligand for pregnane X receptor upregulates tight junction protein expression (Venkatesh et al., 2014). (Fig. 2, A.3 and B.3).
D. Vitamin-Mediated Pathways
Vitamins have known effects on the immune system since many receptors for vitamins [such as GPR109a, RORs, retinoic acid receptors, retinoid X receptors, liver X receptors, and peroxisome proliferator-activated receptors (PPARs)] are expressed by immune cells such as regulatory T cells and T helper 17 (Veldhoen and Brucklacher-Waldert, 2012). Vitamin B3 (niacin), as a ligand of GPR109a, induces differentiation of regulatory T cells (Singh et al., 2014); vitamin B9 (folic acid) is a survival factor for regulatory T cells (Yoshii et al., 2019); and vitamin A modulates the imbalance of T helper 17 and regulatory T cells (Abdolahi et al., 2015) (Fig. 2B.4).
E. Polyphenol-Induced Routes
Polyphenols have immunomodulatory effects on macrophages; induce B cell proliferation; and decrease T helper 1, T helper 2, T helper 17, and T helper 9 cells and the inflammatory cytokines interleukin-1β, interleukin-6, and TNF-α (Shakoor et al., 2021; Tayab et al., 2022) (Fig. 2B.5).
F. PUFA-Related Pathways
PUFAs are potent regulators of inflammation (Veldhoen and Brucklacher-Waldert, 2012) (Fig. 2B.6). EPA reduces plasma levels of the inflammatory marker C-reactive protein in MDD patients, which is correlated with depression symptom reduction (Mischoulon et al., 2022; Lamon-Fava et al., 2023). EPA is the precursor of specialized proresolving lipid mediators (SPMs) known as resolvins, which are involved in the reduction of inflammation (Lamon-Fava et al., 2021). One very recent RCT of EPA-supplemented MDD patients reported a strong association between changes in 18-HEPE and 15-HEPE, the precursors of resolvins E1–4, with changes in depression symptoms and plasma C-reactive protein concentrations (Lamon-Fava et al., 2023). The inverse association of 18-HEPE with both systemic inflammation and symptoms of depression highlights the activation of inflammation resolution as a likely mechanism in the treatment of MDD with omega-3 fatty acid supplementation. These findings suggest a mechanistic involvement of EPA-derived SPMs on inflammation and depression improvement. In addition, omega-9 (oleic acid) has demonstrated antidepressant effects through the reduction of stress-induced T helper 17 signaling (Medina-Rodriguez et al., 2020).
G. Pathways Induced by Microbial Components
Regarding bacterial components, epithelial cells express pattern recognition receptors such as toll-like receptors and NOD-like receptors, which sense bacterial components such as lipopolysaccharide, flagellin, or muramyl dipeptides and contribute to epithelial cell proliferation and the production of cytokines, antimicrobial molecules, and mucus (Kayama et al., 2020). Toll-like receptor–mediated microbial recognition is required for constitutive anti-inflammatory interleukin-10 production by macrophages in the intestine (Ueda et al., 2010). Toll-like receptor 4 or toll-like receptor 2 activation stimulates regulatory T cells and enhances their immunosuppressive activity (Caramalho et al., 2003; Liu et al., 2006).
Furthermore, some intestinal bacteria, such as Citrobacter rodentium or segmented filamentous bacteria (SFB), attach to the mucosal surface, inducing T helper 17 cell differentiation in the lamina propria through the induction of reactive oxygen species (ROS) or inflammatory molecules in the epithelial cells (Atarashi et al., 2015). Interestingly, SFB can directly induce changes in the host cells by transferring microbial content into epithelial cells through the generation of endocytic vesicles (Ladinsky et al., 2019).
Thus, the aforementioned bacterial signals induce rapid responses, which can later be converted to specific responses by the adaptative immune system. Crosstalk between host cells in direct contact with microbes, their antigens, or their metabolites and the adaptative immune system is performed by inflammatory mediators such as cytokines and by cells with antigen-presenting properties such as some epithelial cells, dendritic cells, macrophages, or ILCs (Flannigan et al., 2015; Wosen et al., 2018; Heuberger et al., 2021).
Therefore, although the gut immune system has evolved to live and cooperate with high microbial loads by maintaining a basal level of inflammation and the intestinal barrier function (which together contribute to homeostasis), a failure in the regulatory mechanisms of the intestinal milieu can lead to psychiatric disorders.
VI. Conclusion
Our understanding of the complex crosstalk among the brain, the gut microbiome, and the immune system in depression is still very limited despite the large amount of scientific data generated in recent years. To date, most studies on the subject have been observational, meaning that they could infer associations but not establish true causal links. In addition, clinical trials have included insufficient numbers of participants and failed to control for many influencing sociodemographic, medical, dietary, and lifestyle variables. As a result, we are unable to draw robust conclusions from the available evidence.
There is a need for large-scale longitudinal and clinical cohort studies including multiomics data sets and parallel translational studies in experimental models to progress from associations to causality, decode the mechanisms underlying microbiota-immune interactions, and enable a more precise identification of biomarkers and therapeutic targets of depression. Microbiome-based biomarkers could be useful for predicting, diagnosing, and establishing the prognosis of depression, as current clinical practice is still based on questionnaires and rating scales. New information on the mechanisms underlying the gut microbiota–mediated effects on the host’s immune system could also serve as a basis for designing adequate drug- and lifestyle-based therapies and identifying new therapeutic targets to improve current management strategies. Furthermore, given the wide variety of causes and phenotypes of the condition and the considerable individual variability of the gut microbiota, it is reasonable to envisage future progress toward personalized, microbiome-informed management programs to treat or prevent depression.
Data Availability
This article contains no datasets generated or analyzed during the present study.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Medina-Rodríguez, Martínez-Raga, Sanz.
Footnotes
- Received January 30, 2024.
- Revision received May 28, 2024.
- Accepted May 29, 2024.
This work was funded by the Spanish State Research Agency (AEI, Spain) [Grant PID2022-142106OA-I00] and the Miguel Servet program from Carlos III Health Institute (ISCIII, Spain) [Grant CP22/00030], cofunded by the European Union, and supported by the EarlyCause Project of the European Union’s Horizon 2020 Research and Innovation Program [Grant 848158], the European Commission’s NextGenerationEU through the CSIC Interdisciplinary Thematic Platform (PTI+) NEURO-AGING+ (PTI-NEURO-AGING+) and Severo Ochoa grant of the National Agency for Research (AEI)- Spanish Ministry of Science and Innovation (MCIU) (Ref. CEX2021-001189-S).
The authors declare that they have no conflicts of interest with the contents of this article.
ABBREVIATIONS
- AhR
- aryl hydrocarbon receptor
- EPA
- eicosapentaenoic acid
- GPR
- G-protein–coupled receptor
- HEPE
- hydroxyeicosapentaenoic acid
- ILC
- innate lymphoid cell
- MDD
- major depressive disorder
- PUFA
- polyunsaturated fatty acid
- RCT
- randomized controlled trial
- SCFA
- short-chain fatty acid
- TNF
- tumor necrosis factor
- Copyright © 2024 by The Author(s)
This is an open access article distributed under the CC BY-NC Attribution 4.0 International license.