Abstract
Both preclinical and clinical studies implicate functional impairments of several neuroactive metabolites of the kynurenine pathway (KP), the major degradative cascade of the essential amino acid tryptophan in mammals, in the pathophysiology of neurologic and psychiatric diseases. A number of KP enzymes, such as tryptophan 2,3-dioxygenase (TDO2), indoleamine 2,3-dioxygenases (IDO1 and IDO2), kynurenine aminotransferases (KATs), kynurenine 3-monooxygenase (KMO), 3-hydroxyanthranilic acid oxygenase (3-HAO), and quinolinic acid phosphoribosyltransferase (QPRT), control brain KP metabolism in health and disease and are therefore increasingly considered to be promising targets for the treatment of disorders of the nervous system. Understanding the distribution, cellular expression, and regulation of KP enzymes and KP metabolites in the brain is therefore critical for the conceptualization and implementation of successful therapeutic strategies.
Significance Statement Studies have implicated the kynurenine pathway of tryptophan in the pathophysiology of neurologic and psychiatric diseases. Key enzymes of the kynurenine pathway regulate brain metabolism in both health and disease, making them promising targets for treating these disorders. Therefore, understanding the distribution, cellular expression, and regulation of these enzymes and metabolites in the brain is critical for developing effective therapeutic strategies. This review endeavors to describe these processes in detail.:
I. Introduction
For innumerable reasons, scientists with a wide range of expertise and interests have been fascinated with the amino acid tryptophan for more than a century. Tryptophan was first identified and described by Frederick Hopkins, who isolated the compound from casein, a major constituent of dairy products, using the enzyme trypsin (hence the name) (Hopkins and Cole, 1901). During the following decades, analysis of biological fluids and tissues of a wide variety of organisms led to the realization that tryptophan itself, as well as many of its biologically active precursors and degradation products, plays unique roles in the fate and function of all living beings.
As other amino acids, tryptophan is a building block of proteins. In contrast to most other amino acids, however, it has a relatively complex chemical structure and, of special importance in the biological realm, is mainly synthesized by plants, fungi, and micro-organisms (Crawford, 1989; Radwanski and Last, 1995). The dependence on nutritional supply therefore makes tryptophan an “essential” amino acid in mammals and most other eukaryotes.
The biochemical and functional features of the large number and complex bioactive properties of chemicals in the “tryptophan network” have been the subject of ever-increasing scrutiny and analysis, revealing novel insights into the ubiquitous roles of these compounds in physiological and pathological phenomena in every cell and multicellular organism studied (Palego et al., 2016; Comai et al., 2020). Besides being used in protein synthesis, tryptophan is metabolized into serotonin (5-hydroxytryptamine), melatonin, and neuroactive compounds in the kynurenine pathway (KP) (Fig. 1). However, until the late 1960s there was very little evidence indicating that tryptophan or chemically and metabolically related compounds have biologically significant effects in the nervous system. This changed dramatically with the recognition that the tryptophan metabolite serotonin is a neurotransmitter and is causally linked to the pathophysiology of depression and other major brain diseases in humans (Carlsson et al., 1969; Lapin et al., 1972) and that pharmacological modulation of serotonin function in the brain provides remarkable therapeutic benefits (Oxenkrug, 2013). This led to a surge of interest in brain tryptophan metabolism in both experimental animals and humans and, in particular, to an increased awareness that this aspect of tryptophan research has been ignored for too long, holds promise for fundamentally new neurobiological concepts, and may have novel therapeutic implications. In this context, the KP, the major route of tryptophan metabolism, has garnered attention for its potential role in neuroinflammation and cognitive impairment. Metabolites produced along this pathway can exert neurotoxic or neuroprotective effects, influencing brain function and disease progression. Dysregulation of the KP has been implicated in various neurologic and psychiatric disorders, highlighting the need for further research to explore its therapeutic potential and address gaps in our understanding of brain inflammation and cognitive health. Although relevant extracerebral phenomena, including the role of peripheral tryptophan metabolism, will be briefly described and discussed, the present review will highlight these translationally exciting discoveries from a neuroscience perspective, with special emphasis on the latest pharmacological approaches for possible clinical applications in humans.
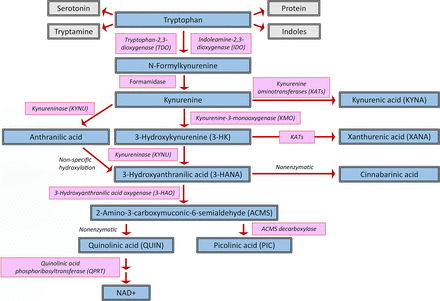
Tryptophan degradation via the kynurenine pathway in mammalian cells.
II. Kynurenine Pathway Enzymes and Their Neuroactive Products
In mammals, only a small proportion of tryptophan is converted to serotonin and other biologically active downstream molecules, such as melatonin (which regulates sleep and the circadian rhythm; Pereira et al., 2020) and indoles, which affect a large number of physiological processes (Roager and Licht, 2018). The large majority (∼95%) of the amino acid is degraded via the KP, named after the pivotal metabolite kynurenine, resulting in a considerable number of neuroactive products that play distinct roles in brain function and dysfunction (Fig. 1). The mechanisms of KP regulation outside and within the brain are highly complex and still require substantive elaboration. However, understanding the basic principles that control this metabolic cascade is critical for the conceptualization of pharmacological interventions to prevent or normalize abnormal KP functions. In the following, we will therefore review the current status of knowledge regarding the neurobiologically and clinically relevant features of the KP. In light of the rapidly expanding information regarding KP biology and its intricacies, and to maintain focus, several related but less directly germane concepts will be referenced appropriately but will not be covered in depth.
A. The Formation of Kynurenine from Tryptophan
In mammals as in other biological systems, tryptophan degradation through the KP is initiated by three heme-containing enzymes, i.e., tryptophan 2,3-dioxygenase (TDO2; originally called “tryptophan pyrrolase”) and indoleamine 2,3-dioxygenases (IDO1 and IDO2), all of which convert tryptophan irreversibly to N-formyl-L-kynurenine (Fig. 1). As briefly summarized below, the three dioxygenases differ substantively in tissue distribution and expression and are differentially involved in physiological and pathological phenomena. Notably, however, the three enzymes show only subtle differences in the affinity of the substrate tryptophan (Rafice et al., 2009; Dolšak et al., 2021).
1. Tryptophan 2,3-Dioxygenase
TDO2 was long considered to be the sole enzyme that catalyzes the first step of the KP (see Raven, 2017 for a brief history). Its activity is by far highest in the liver, but both the protein and its mRNA are also present in extrahepatic tissues. Although information is quite sparse, this includes the brain, where TDO2 mRNA has been demonstrated (Kindler et al., 2020) and where both mature and immature TDO2-positive neurons have been described (Ohira et al., 2010; Wu et al., 2013). Of note, the enzyme recognizes both L- and D-tryptophan as a substrate (Yamamoto and Hayaishi, 1967), and its activity, unlike that of IDO1 (see below), is induced by tryptophan (Knox, 1966) and by glucocorticoids (Knox, 1966). Although TDO2 activity is generally not stimulated by cytokines and other immune regulatory factors (Yoshida and Hayaishi, 1978; Yoshida et al., 1979), evidence has emerged showing that certain cytokines can induce both IDO and, in some cases, TDO activity (Walker et al., 2013; Urata et al., 2014; Sellgren et al., 2016).
Taken together, these properties indicate that TDO2 plays an important and singular role in the control of tryptophan homeostasis both under physiological conditions and in situations that are associated with increased glucocorticoid formation (such as stress), i.e., TDO2 is normally the key regulator of tryptophan levels in the blood and in peripheral organs. Importantly, as tryptophan easily crosses the blood-brain barrier, changes in TDO2 activity and in circulating tryptophan levels readily affect KP metabolism in the brain (Gál et al., 1978; Larkin et al., 2016).
2. Indoleamine 2,3-Dioxygenase 1
Unlike TDO2, IDO—first identified in the 1960s and termed IDO1 after the discovery of a second IDO enzyme (i.e., IDO2; see below)—is ubiquitously distributed in extrahepatic organs of mammals (Yamamoto and Hayaishi, 1967; Yoshida et al., 1980). The enzyme is especially highly expressed in the respiratory system, as well as in the placenta, bone marrow, and lymphoid tissues (Théate et al., 2015). Of significant functional relevance, pioneering studies, also conducted by Yoshida and Hayaishi, (1978), demonstrated that viral and bacterial infections stimulate IDO1 activity in vivo (Yoshida and Hayaishi, 1978; Yoshida et al., 1979) and that the underlying enzyme induction is the result of inflammatory cytokines, such as interferon (IFN)-γ (Yoshida et al., 1981). Of note in this context, tumor necrosis factor (TNF)-α can synergistically increase the transcriptional activation of the IDO1 gene in the presence of IFN-γ (Babcock and Carlin, 2000; Currier et al., 2000). Together with a large number of supportive and clinically relevant findings (Prendergast et al., 2011; Passarelli et al., 2022), as well as the discovery that IDO1 plays a vital role in preserving maternal T-cell tolerance (Munn et al., 1998), it soon became widely accepted that the induction of IDO1 is closely—and probably causally—related to pathological conditions and host defense mechanisms in a large number of neuroinflammatory conditions. Thus, IDO1 provides protection against pathogens by inhibiting potentially harmful inflammatory processes in the body and, more specifically, is critically involved in immune tolerance (Romani et al., 2008). Moreover, like IDO2 (see below), IDO1 plays an important role as a modulator of B-cell function, though, in contrast to IDO2, it inhibits—whereas IDO2 stimulates—inflammatory B-cell responses (Merlo et al., 2022).
In biochemical terms, IDO1 differs substantively from TDO2. Thus, though IDO1 also recognizes D-tryptophan as a substrate (Capece et al., 2010), the enzyme has lower substrate specificity than TDO2 and is able to process, for example, various indoles (Yeung et al., 2015). Notably, and of likely functional significance, its activity in the periphery is not influenced by (changes in) circulating tryptophan levels.
IDO1 expression and enzyme activity in the brain have been repeatedly documented using histological, biochemical, and genetic methods (Browne et al., 2012; Larkin et al., 2016). IDO1 is primarily expressed in vascular endothelial cells in vivo (Hansen et al., 2000) but is also found in cultured neurons, astrocytes, and microglial cells (Guillemin et al., 2005b). All of these studies indicate low functional efficacy of brain IDO1 under regular physiological conditions. In line with the realization that the enzyme may be critically involved in inflammatory processes, however, major enzyme induction/up-regulation is seen in brain tumor cells (Herrera-Rios et al., 2020; Ladomersky et al., 2020; Platten et al., 2021), in IFN-γ–stimulated cultured glioma cells (Takikawa et al., 1991; Adams et al., 2014), and in macrophages and activated microglial cells of mice with experimental autoimmune encephalomyelitis (Kwidzinski et al., 2005). Interestingly, though the biological implications are still unknown, IDO1 in human macrophages and tumor cells often lacks the heme binding domain, which plays a major role in enzyme activity (Thomas et al., 2001; Lewis-Ballester et al., 2017; Nelp et al., 2018; Behmoaras, 2021).
3. Indoleamine 2,3-Dioxygenase 2
More recently, a new IDO isoform—named IDO2—was identified (Metz et al., 2007), and its functional characteristics and biological roles are increasingly understood (Li et al., 2021; Guo et al., 2023). IDO2 likely arose via gene duplication of IDO (Ball et al., 2007) and has distinctive kinetic characteristics and substrate specificity (Austin et al., 2010). Although the enzyme is widely expressed in mammalian organs, including liver and kidney, its low activity seems to argue against a significant role in oxidative tryptophan degradation under normal conditions (Jusof et al., 2017). Distinct cellular localizations of IDO2 have been documented immunohistochemically (Guo et al., 2023). Studied mostly in the cerebral cortex and cerebellum of mice, brain IDO2 has so far been shown to be mainly expressed in neuronal cells (Fukunaga et al., 2012).
Although the physiological relevance of IDO2 is still being elaborated in detail, the enzyme appears to play a distinct role in immunological phenomena. Thus, increased IDO2 expression can lead to the exacerbation of inflammatory responses, and the enzyme is therefore considered to be a proinflammatory mediator of autoimmunity (Merlo and Mandik-Nayak, 2016). Of interest in this context, IFN-γ significantly potentiates IDO2 expression in cultured human glioma cells (Adams et al., 2014). Notably, IDO2 protein is strongly upregulated when IDO1 is eliminated as shown in mice devoid of IDO1 (IDO1−/− mice; Fukunaga et al., 2012).
4. Kynurenine Formamidase
N-formyl-L-kynurenine (formylkynurenine), the product of TDO2, IDO1, and IDO2 under both physiological and pathological conditions, serves as a substrate of kynurenine formamidase, which is singularly responsible for the next step in the KP, i.e., the synthesis of the pivotal pathway metabolite kynurenine. In spite of its proposed key role in tryptophan degradation, the enzyme has received relatively little attention compared with both upstream and downstream KP mechanisms, and the conversion of formylkynurenine to kynurenine may even involve alternative mechanisms (Dobrovolsky et al., 2005). In contrast to other enzymes involved in KP metabolism and function, kynurenine formamidase has been more frequently examined in microbiota than in animals (Shinohara and Ishiguro, 1970; Arndt et al., 1973; Brown et al., 1986; Han et al., 2012), and the cellular localization and functionally relevant regulation of the enzyme in the mammalian brain remains essentially unknown (Cumming et al., 1979). The few studies in this respect have focused mostly on the possible role of the enzyme in cancer biology and immunotherapy (Badawy, 2022).
a. Kynurenine: the pivotal kynurenine pathway metabolite
Kynurenine, whether synthesized endogenously as summarized above, provided from dietary consumption, or originating from the microbiome community in the digestive tract, is present in low micromolar concentrations in the mammalian blood (Mrštná et al., 2023). In addition to being the major determinant of the formation—and therefore function and dysfunction—of all KP metabolites downstream (see below), kynurenine, within the physiological pH range, is readily oxidized to nonenzymatically produce biologically active molecules (Ramírez Ortega et al., 2021). Importantly, kynurenine activates the aryl hydrocarbon receptor (AhR) at physiological concentrations (see Fig. 2) (Mezrich et al., 2010; Opitz et al., 2011) and thus directly affects, and likely controls, a large number of AhR-dependent processes in health and disease (Rothhammer and Quintana, 2019; Opitz et al., 2023). Notably, and in line with the ability of upstream enzymes to recognize D-tryptophan as a substrate (see above), D-kynurenine is present in mammalian cells and shares several biological qualities of its more prominent enantiomer (Kotake and Ito, 1937; Mason and Berg, 1952).
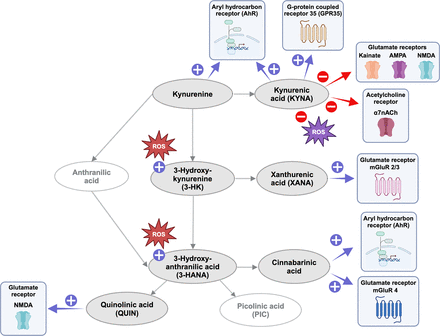
Key biological actions of neuroactive kynurenine pathway metabolites in the mammalian brain. Purple and red signs indicate agonist and antagonist properties, respectively. ROS, reactive oxygen species.
Competing with tryptophan and several other amino acids, circulating kynurenine [both L- and the D-enantiomers (Fukui et al., 1991; Wang et al., 2012)] readily crosses the blood-brain barrier via the large amino acid transporter (Segawa et al., 1999). Because of the comparatively low activity of brain TDO2, IDO1, and IDO2, approximately 60% of brain kynurenine is normally derived from the circulation. However, local neosynthesis of the compound increases dramatically when brain IDO1 is stimulated by an activated immune system (Saito et al., 1992; Thomas et al., 2014), with major impact on brain diseases (Skorobogatov et al., 2021; see below).
Endogenous kynurenine levels in the mammalian brain are in the submicromolar range (see Table 1) (Beal et al., 1992; Heyes et al., 1998; Schwarcz et al., 2001; Linderholm et al., 2012; Notarangelo et al., 2012; Drewes et al., 2015; Clark et al., 2016; Fuertig et al., 2016; Sorgdrager et al., 2019; Rentschler et al., 2024). Although not tested sufficiently in many mammalian species but of interest for both physiological considerations and because of potential clinical implications (see below), the concentration of kynurenine in the cerebrospinal fluid (CSF) is at least 10-fold lower than in the brain, with CSF typically being withdrawn from the lumbar region at the L4 to L5 level in human studies. This suggests that kynurenine is readily taken up by and accumulates in—but may not be released from—brain cells. Kynurenine readily enters neurons and glial cells via Na+-dependent and Na+-independent mechanisms, respectively (Speciale et al., 1989), but uptake into other brain cells has not been studied rigorously so far. Verification will provide significant functional insights since enzymatic or oxidative degradation of kynurenine to biologically active molecules may take place within most, if not all, brain cells.
Central levels of key kynurenine pathway metabolites in adult mammals
Ranges of the concentrations of kynurenine, KYNA, 3-HK and QUIN in postmortem brain tissue, in the cerebrospinal fluid, and in the extracellular milieu (collected by in vivo microdialysis). References are provided in the text.
B. Degradation of Kynurenine: An Array of Enzymes and Neuroactive Metabolites
As the pivotal metabolite of the KP, kynurenine serves as a substrate of several enzymes, which are segregated into three distinct branches and produce neuroactive metabolites either directly or through further degradation downstream. The dynamics and roles of some of these metabolites have attracted significant attention of neuroscientists since the 1980s, whereas others are still just beginning to be understood. Focusing on KP function and dysfunction in the brain, the present status of knowledge regarding the biological activity and role of these catabolic branches (Fig. 2) is summarized in the following sections.
1. Kynurenine Aminotransferases
Kynurenine aminotransferases (KATs) are responsible for the irreversible conversion of kynurenine to kynurenic acid (KYNA) and of 3-hydroxykynurenine (3-HK) to xanthurenic acid (XANA). Four KAT enzymes, termed KAT I–IV, have been identified, and their presence is well documented in several mammalian organs, including liver, muscle, and brain. The Km values of these enzymes for kynurenine are remarkably similar [875 μM for KAT I (= glutamine transaminase K), 660 μM for KAT II (= 2-aminoadipate aminotransferase), 1.5 mM for KAT III (= cysteine conjugate β-lyase 2), and 724 μM for KAT IV (= mitochondrial aspartate aminotransferase)]. The specific contributions of the KAT enzymes to brain-derived KYNA and XANA synthesis have not been fully elucidated; however, it is believed that under normal physiological conditions KAT II accounts for the greatest fraction (60%) of total KAT activity in the rat and human brain (Schmidt et al., 1993; Guidetti et al., 1997; Han et al., 2008; Sathyasaikumar et al., 2017). Although all these enzymes metabolize kynurenine, they differ with regard to their alternate substrates. The classic substrate for KAT II is the lysine metabolite α-aminoadipate, which is found at low micromolar concentrations in the brain of rodents and humans (Guidetti and Schwarcz, 2003). In contrast, the other KATs use amino acids with higher brain concentrations, such as glutamine and aspartate, as substrates. This, as well as the fact that KAT I and KAT III are optimally active at alkaline pH (∼9.0–9.5), argues for a preferential role of KAT II in KYNA and XANA neosynthesis under physiological conditions. Experimental evidence for this supposition is provided by using mice with a genetic elimination of the KAT II gene (Kat II−/− mice) (Yu et al., 2004), which present with a number of biological changes that are expected in animals with reduced brain KYNA levels and function (see below) (Potter et al., 2010). Of interest, and requiring further clarification in this context, the relative contribution of KAT II to KYNA neosynthesis in the mouse brain changes greatly with age and accounts for only 12% of the process in adulthood (Guidetti et al., 2007a).
a. Kynurenic acid
The generation of KYNA has been described in various mammalian cells, including endothelial cells, epithelial cells, fibroblasts, pancreatic islet cells, hepatic cells, skeletal muscle cells, and (mainly) glial cells in the brain. Following its local formation, KYNA is promptly released into the extracellular milieu. Formation of the metabolite in liver and skeletal muscle are major determinants of KYNA found in blood (Cervenka et al., 2017). KYNA is excreted by the kidney (Musajo et al., 1951), effected primarily by the organic anion transporters (OATs) OAT1 and OAT3, which have also been described to be present in the brain (Uwai et al., 2012).
Interest in a role of endogenous KYNA in brain physiology originated with the seminal finding of Perkins and Stone (1982), who described its ability, at mid- to high micromolar concentrations, to inhibit the function of various recently identified ionotropic glutamate receptors in the hippocampus of marmosets. The discovery that KYNA has antiexcitotoxic, i.e., neuroprotective, as well as anticonvulsant, properties (Foster et al., 1984b) then suggested that this compound, which at the time was not known to be present in the brain, deserved further investigation because of its possible link to the pathology of major neurologic disorders. The presence of KYNA in the mammalian brain was indeed soon documented, interestingly revealing substantially higher (low micromolar) levels of the compound in human than in rodent tissue (see Table 1) (Moroni et al., 1988; Turski and Schwarcz, 1988). Additional electrophysiological studies then showed that concentrations of KYNA in the high nanomolar and low micromolar range, i.e., with substantially higher efficacy than reported in the original study of Perkins and Stone (1982), competitively inhibits the strychnine-insensitive glycineB site of the N-methyl-D-aspartate receptor (NMDAR) (Birch et al., 1988; Kessler et al., 1989; Parsons et al., 1997) and noncompetitively interferes with the function of the α7 nicotinic acetylcholine receptor (α7nAChR) (Hilmas et al., 2001; Alkondon et al., 2004; Stone, 2007) (see Fig. 2). Later, KYNA was found to also serve as an agonist of GPR35, a G-protein–coupled receptor (Wang et al., 2006), and of the AhR (DiNatale et al., 2010).
Behavioral studies in experimental animals (so far mainly rodents) have provided convincing evidence that endogenous KYNA, acting through one or more of these receptors, alone or in combination with its antioxidant properties (discussed below), plays a significant role in neurophysiology and that impaired KYNA function is likely to be causally involved in—and in several cases responsible for—pathological events related to psychiatric and neurologic diseases. Thus, in the behavioral realm, increased brain KYNA have been demonstrated, for example, to dampen prepulse inhibition (Erhardt et al., 2004; Linderholm et al., 2010) and impair spatial learning and memory processes (Chess et al., 2007; Pocivavsek et al., 2012, 2014; Buck et al., 2020) as well as attentional-set shifting (Alexander et al., 2012, 2013) and delayed nonmatch to position behavioral performance (Phenis et al., 2014). Moreover, prolonged increases in brain KYNA levels are associated with exaggerated amphetamine-induced locomotor activity (Olsson et al., 2012; Liu et al., 2014; Erhardt et al., 2017a; Tufvesson-Alm et al., 2020; Zheng et al., 2023), and—of special translational significance—elevated KYNA levels disrupt fear memory consolidation and extinction, leading to disrupted fear responses (Chess et al., 2009; Akagbosu et al., 2012; Klausing et al., 2020; 2024). Also with significant ramifications in this context, elevated KYNA levels reduce sleep duration and impact arousal phenotypes (Pocivavsek et al., 2017; Rentschler et al., 2021, 2024).
In light of the increasing appreciation that extracellular KYNA is well dispositioned to impact neurotransmission and that the neurobehavioral effects of KYNA in experimental animals may be of significant relevance for the treatment of a range of major neurologic and psychiatric disorders (see below), the features and dynamics of brain KYNA have received considerable attention; and astrocytes, which contain the vast majority of KAT II in the rat brain (Guidetti et al., 2007b), have received special attention in this context. Since the mammalian brain does not appear to contain processes that degrade KYNA, for example to quinaldic acid, a proposed breakdown product found in rat and human urine (Kaihara et al., 1956; Turski and Schwarcz, 1988), brain KYNA levels are mostly controlled by the bioavailability of kynurenine and fluctuations in energy metabolism within astrocytes (Gramsbergen et al., 1997; Hodgkins and Schwarcz, 1998). Newly produced KYNA is then rapidly, and Ca2+-independently, released into the extracellular milieu (Turski et al., 1989; Kiss et al., 2003). In vivo microdialysis studies in rodents and nonhuman primates indicate that the physiological concentrations in the extracellular compartment are in the low- to midnanomolar range, with little intraspecies variability across brain regions (Swartz et al., 1990; Fukui et al., 1991; Wu et al., 1992, 2010; Beggiato et al., 2013). Since cellular reuptake by OATs or other mechanisms is very modest in the brain (Turski and Schwarcz, 1988; Uwai et al., 2012), extracellular KYNA concentrations in the brain are normally kept in balance by a probenecid-sensitive transport mechanism (Moroni et al., 1988).
Again mainly based on studies in rodents, endogenous, i.e., nanomolar, concentrations of KYNA affect both the α7nAChR and the glycineB site of the NMDAR in the brain—the latter potentially when glycine or D-serine levels are low. These effects, which do not appear to be brain region–specific and include concentration- and time-dependent regulation of several classic neurotransmitters, account for the now widely accepted role of KYNA as an endogenous neuromodulator (see Pocivavsek et al., 2016 for review). Examples include significant, up to 50%, reductions in extracellular glutamate in response to biochemically or pharmacologically induced increases in brain KYNA (Carpenedo et al., 2001; Rassoulpour et al., 2005; Wu et al., 2010; Pocivavsek et al., 2011, 2016). Conversely, extracellular glutamate levels increase quickly when KYNA levels are diminished by pharmacological or genetic interference with, or elimination of, KAT II activity (Konradsson-Geuken et al., 2010; Potter et al., 2010; Wu et al., 2010; Pocivavsek et al., 2011). Similarly, experimentally induced elevations in brain KYNA promptly lower the extracellular levels of GABA and dopamine, whereas KYNA reduction raises extracellular GABA and dopamine, respectively (Rassoulpour et al., 2005; Wu et al., 2007; Beggiato et al., 2013, 2014). Reductions and elevations of KYNA cause the same effects, i.e., up- and downregulation, respectively, of extracellular acetylcholine levels in the rat prefrontal cortex (Zmarowski et al., 2009).
Functional assessments are in line with these biochemical effects. This includes several of the behavioral phenomena associated with KYNA, which are well known to involve glutamatergic (dys)function (see above). Moreover, pharmacologically or genetically elevated levels of brain KYNA increase firing rate and burst activity in the ventral tegmental area and substantia nigra dopamine neurons (Erhardt et al., 2001a; Erhardt and Engberg, 2002; Schwieler and Erhardt, 2003; Nilsson et al., 2006; Olsson et al., 2010; Linderholm et al., 2012; Tufvesson-Alm et al., 2018), and a reduction in endogenous KYNA dampens dopaminergic neuronal activity (Schwieler et al., 2006; Linderholm et al., 2016).
The respective roles of α7nAChRs and NMDARs as functionally significant targets of endogenous KYNA in the brain are still not entirely clear. Thus, KYNA-induced reductions in extracellular glutamate, GABA, and dopamine in vivo can be readily prevented by systemic administration of a low dose of galantamine, which potently and specifically prevents KYNA’s inhibition of the allosteric potentiating site of the α7nAChR (Lopes et al., 2007; Wu et al., 2007; Konradsson-Geuken et al., 2010; Wu et al., 2010; Beggiato et al., 2013, 2014). Choline, another α7nAChR agonist (Hone and McIntosh, 2023), attenuates the actions of KYNA on extracellular dopamine levels (Rassoulpour et al., 2005), and the effects of KYNA on extracellular glutamate and dopamine levels can be duplicated by—but are not additive with—the selective α7nAChR antagonist methyllycaconitine (Carpenedo et al., 2001; Rassoulpour et al., 2005). Other studies, however, suggest that inhibition of NMDARs, rather than blockade of α7nAChRs, plays a central role in the various neurophysiological effects of KYNA, including regulation of dopaminergic activity (Schwieler et al., 2004, 2006; Linderholm et al., 2007, 2010; Stone, 2020).
Compared with the extensive literature discussing the respective roles of α7nAChRs and NMDARs in the neurobiological effects of KYNA, studies of the possible participation of other KYNA targets, specifically GPR35 and the AhR, as well as a role of specific KYNA-related redox phenomena (see below), are still in their infancy. Of note in this context, however, activation at GPR35 impacts cyclic adenosine monophosphate production and influx of Ca2+ into astrocytes and modulates glutamate neurotransmission (Moroni et al., 2012; Berlinguer-Palmini et al., 2013), and KYNA signaling through AhRs, a well as the metabolite’s antioxidant properties, may serve a neuroprotective role (García-Lara et al., 2015; Kubicova et al., 2019).
b. Xanthurenic acid
The transamination of 3-HK, the product of kynurenine 3-monooxygenase (KMO)(see below), to XANA is catalyzed by KAT II, i.e., the same enzyme that is responsible for the conversion of kynurenine to KYNA (Gobaille et al., 2008; Sathyasaikumar et al., 2017; Maitre et al., 2024). XANA is suggested to be functionally significant within the brain as it is stored and transported in neuronal vesicles and subsequently released in an activity-dependent manner (Gobaille et al., 2008). Subsequently, XANA can regulate excitatory neurotransmission by inhibiting the vesicular glutamate transporter and by acting as an agonist at group II mGlu receptors (see Fig. 2) (Copeland et al., 2013; Fazio et al., 2017).
Interestingly, like KYNA, XANA affects dopaminergic neurotransmission (Taleb et al., 2021). Thus, acute local infusion of XANA dose-dependently stimulates dopamine release in the rat prefrontal cortex. Notably, this effect can also be triggered by peripheral XANA administration, which significantly boosts brain XANA levels and results in a dose-dependent increase in dopamine release in the cortex and striatum. Moreover, similar to the effects of increased brain KYNA levels on the activity of dopamine neurons in the ventral tegmental area (see above), daily administration of XANA reduces the number of spontaneously active dopamine neurons (Taleb et al., 2021).
2. Kynurenine 3-Monooxygenase
KMO is an aromatic hydroxylase that catalyzes the hydroxylation of L-kynurenine to 3-HK. A member of the NAD(P)H-dependent flavin monooxygenase family (Okamoto et al., 1967), KMO, which is produced by a single gene and uses flavine adenine dinucleotide as a cofactor, is not only directly responsible for the neosynthesis of 3-HK but also plays a central role in the formation and function of 3-hydroxyanthranilic acid (3-HANA), quinolinic acid (QUIN), and other KP metabolites downstream (see below). Notably, because of its unique position in the KP, illustrated in Fig. 1, KMO is also a key regulator of the formation of XANA in a sidearm of the pathway (see above).
KMO plays a central role in KP metabolism in mammals but is also present in bacteria and fungi. The enzyme, originally named kynurenine hydroxylase, was partially purified for the first time from rat liver mitochondria (Saito et al., 1957), but isolation of the mammalian KMO protein remained challenging, and the crystal structure of human KMO was not described until 2018 (Gao et al., 2018).
In the mammalian brain, KMO is found mainly in microglial cells (Alberati-Giani et al., 1996; Guillemin et al., 2001; Giorgini et al., 2008), though neurons, too, contain a substantive proportion of functional KMO in the adult mouse brain (Sathyasaikumar et al., 2022). The enzyme is essentially absent from astrocytes, however (Heyes et al., 1997; Guillemin et al., 1999; Sathyasaikumar et al., 2022). In light of the lower Km value for kynurenine compared with KATs (see above), KMO is more readily saturated by its substrate, causing KP metabolism to shift toward enhanced KYNA formation with increasing kynurenine levels (Bender and McCreanor, 1982). Moreover, and of substantive significance regarding the role of KP function and dysfunction in the brain, KMO activity is consistently activated under neuroinflammatory conditions (Parrott et al., 2016; Garrison et al., 2018).
Impaired function of KMO is increasingly linked to the pathophysiology of a number of neuropsychiatric disorders (see below). Although Kmo knockout (Kmo−/−) mice, generated by the targeted experimental deletion of the Kmo gene, also show changes in the brain expression of other genes that are critically involved in nervous system development and neurotransmission (Erhardt et al., 2017a), these mutant animals have so far been used only to study the biological role of the KP. Kmo−/− mice show significant changes in KP metabolite levels that affect both brain biochemistry and function. Thus, elimination of KMO results in a significant reduction in cortical and hippocampal 3-HK levels and an increase in cortical KYNA (Giorgini et al., 2013; Tashiro et al., 2017; Erhardt et al., 2017a; Mori et al., 2021; Kubota et al., 2022). This shift in KP metabolism toward elevated KYNA is associated with several cognitive and behavioral impairments, though it also increases neuroprotective properties in the mutant animals (Nahomi et al., 2020; Bondulich et al., 2021). Thus, Kmo−/− mice show significant deficits in hippocampus-dependent contextual memory when tested in a passive avoidance paradigm (Erhardt et al., 2017a). Similarly, Kmo−/− mice perform poorly in a spatial working memory test in a T-maze, indicating dysfunctional long-term potentiation (LTP) in the hippocampus (Imbeault et al., 2021). These mutant mice are also impaired in social interactions and display an array of depressive-like phenotypes, showing anxiety-like behaviors in an open field arena, in the elevated plus maze paradigm, and in a light-dark box (Tashiro et al., 2017; Erhardt et al., 2017a; Mori et al., 2021). On the other hand, no deficits in spontaneous alternation in a Y-maze paradigm (Tashiro et al., 2017) or novel object recognition memory (Mori et al., 2021) were found to be associated with KMO deficiency. These discrepancies suggest that although Kmo−/− mice display certain memory impairments, their cognitive deficits are not seen across all types of memory tasks. Interestingly, behavioral anomalies in Kmo−/− mice are often exacerbated following challenges with psychoactive drugs. For example, the mutant animals exhibit impairments in prepulse inhibition after being treated with phencyclidine (Kubota et al., 2022), and D-amphetamine potentiates their locomotor activity (Erhardt et al., 2017a; Kubota et al., 2022). Together with additional supportive evidence (Erhardt et al., 2004; Stone and Darlington, 2013; Parrott and O’Connor, 2015), these findings indicate that KMO plays a critical role in controlling and modulating a number of translationally relevant behavioral phenomena.
Kmo−/− mice also undergo distinctive changes when exposed to inflammatory challenges, such as lipopolysaccharide (LPS) treatment (Garrison et al., 2018). LPS, a potent inducer of inflammation, has been studied as an activator of the KP (Walker et al., 2013; Larsson et al., 2016; Parrott et al., 2016; Oliveros et al., 2017; Garrison et al., 2018; Clark et al., 2019; Peyton et al., 2019; Imbeault et al., 2020; Tufvesson-Alm et al., 2020; Millischer et al., 2021; Notarangelo and Schwarcz, 2021; Balter et al., 2023; Zheng et al., 2023). Compared with normal mice, LPS administration to KMO-deficient animals causes marked increases in the levels of kynurenine and KYNA and simultaneous reductions in 3-HK and the downstream metabolite QUIN (see below) in the brain (Parrott et al., 2016; Garrison et al., 2018). Interestingly, these effects are associated with an attenuated inflammatory response, evidenced by reduced expression of cytokines such as TNF-α and interleukin (IL)-6 as well as other inflammatory markers (Garrison et al., 2018). This suggests that the changes in KP metabolism in Kmo−/− mice provide resilience to neuroinflammation.
Related, Kmo−/− mice are protected from several LPS-induced depressive-like behaviors. For example, LPS-treated mutant animals do not show increased immobility in the tail suspension test or a reduction in spontaneous alternations in the Y-maze (Parrott et al., 2016). This resistance to inflammation-induced behavioral changes aligns with the biochemical data mentioned above and confirms that KMO plays a functionally significant—and translationally relevant—role in the detrimental behavioral effects of systemic inflammation. Of note, Kmo−/− mice have also been used to evaluate the role of circulating KP metabolites on the nutritional status of niacin after ethanol consumption (Mizutani et al., 2023, 2024).
Given that stress and inflammation heighten KP metabolism both prenatally (Notarangelo and Schwarcz, 2014; Baratta et al., 2020) and in the early postnatal period (Asp et al., 2010; Liu et al., 2014), a dietary strategy was developed to boost kynurenine metabolism during this critical phase (Notarangelo and Pocivavsek, 2017). Kynurenine treatment of dams heterozygous for the Kmo gene potentiates the elevation of KYNA levels, and lowers 3-HK and QUIN levels, in the embryonic brain of offspring heterozygous for the Kmo gene compared with wild-type control offspring (Beggiato et al., 2018). These findings place special attention on the maternal Kmo genotype on modulating fetal brain levels of KP metabolites, which may subsequently have long-lasting impacts on behavioral outcomes and pathophysiological significance toward the study of neurodevelopmental disorders. Interestingly, Kmo−/− mice have also been used to evaluate nutritional status after ethanol exposure, drawing attention to the role of circulating KP metabolites in this regard (Mizutani et al., 2023, 2024).
a. 3-Hydroxykynurenine
3-HK is the first metabolite of the “KMO branch” of the KP. In mammals, plasma and CSF 3-HK concentrations are in the low nanomolar range. Under physiological conditions, 3-HK levels in the mammalian brain are low, with a similar regional distribution as kynurenine (see Table 1) (Notarangelo et al., 2012; Pocivavsek et al., 2012; Giorgini et al., 2013; Clark et al., 2019; Schwieler et al., 2020).
As a toxic metabolite that can induce oxidative damage and cell death, elevated levels of 3-HK are associated with pathological conditions (Guidetti et al., 2006; Sathyasaikumar et al., 2010). Locally, 3-HK levels can be enhanced with the application of kynurenine (Guidetti et al., 1995; Beggiato et al., 2018), and extracellular 3-HK is detectable after kynurenine stimulation (Notarangelo et al., 2012). Immune cells, including KMO-containing microglia in the brain, are likely primarily responsible for these effects.
Notably, 3-HK is readily oxidized in the presence of trace metals, such as Cu2+ and Fe3+, and by iron chelators, like hemoglobin (Lugo-Huitrón et al., 2011). Thiols, including glutathione, cysteine, and ascorbic acid, effectively inhibit the oxidative process. By donating electrons, 3-HK can therefore participate in physiologically relevant redox reactions in the brain, modulating antioxidative processes and inducing oxidative damage at higher concentrations.
3. Kynureninase
Kynureninase catalyzes the conversion of kynurenine to anthranilic acid and, in the branch initiated by KMO (see above), of 3-HK to 3-HANA (Fig. 1). This enzyme therefore plays a critical role in the KP, influencing the production of several key metabolites with significant biological activities. Within the brain, kynureninase is preferentially expressed in microglial cells (Guillemin et al., 2001, 2003a). In cultured human glioma cells, IFN-γ stimulation significantly potentiates the expression of kynureninase (Adams et al., 2014), and a similar increase in kynureninase mRNA expression is observed in IFN-y–stimulated microglial cells (O’Farrell et al., 2017).
a. Anthranilic acid
Although elevated brain levels of anthranilic acid, which are normally in the low- to midmicromolar range (Baran and Schwarcz, 1990), have been linked to inflammatory responses, depression, and oxidative stress, which are contributing factors to various chronic diseases, including neurodegenerative disorders and cancer (Pawlowski et al., 2021; Milusheva et al., 2023), this metabolite has not received sufficient attention so far. However, its role in the brain has recently been proposed to involve the stimulation of a G-protein–coupled receptor (GPR109A) and, as a consequence, may participate in the preservation of myelin integrity (Oxenkrug and Forester, 2024). Notably, ANA—through mechanisms that have not been elucidated so far—can serve as an efficient bioprecursor of 3-HANA in the brain (Baran and Schwarcz, 1990). By modulating the levels or activity of processes that determine the formation and/or the degradation of anthranilic acid in the brain, it may therefore be possible to influence the production of up- and downstream KP metabolites, thereby preventing adverse effects and promoting beneficial outcomes.
b. 3-Hydroxyanthranilic acid
3-HANA easily auto-oxidizes and thereby generates highly reactive products, including hydrogen peroxide and hydroxyl radicals (Liochev and Fridovich, 2001). Thus, 3-HANA is implicated in various physiological and pathological processes. which are exacerbated by superoxide dismutase but, on the other hand, eliminated by catalase (Iwahashi et al., 1988; Goldstein et al., 2000). However, 3-HANA also possesses antioxidant properties and can scavenge free radicals and therefore protect cells from oxidative stress (Iwahashi et al., 1988; Christen et al., 1990). This dual role of 3-HANA, as both a protector against and a contributor to oxidative stress and neurotoxicity, further emphasizes the complex regulatory mechanisms that are associated with KP metabolism.
4. 3-Hydroxyanthranilic Acid Oxygenase
The Fe2+-dependent enzyme 3-hydroxyanthranilic acid oxygenase (3-HAO) catalyzes the oxidative ring cleavage of 3-HANA to produce QUIN. Triggered by the emerging interest in the role of QUIN in brain function and dysfunction (see below), anti–3-HAO antibodies were generated in the 1980s and used for the immunocytochemical localization of the enzyme in the rat brain. Based on double-labeling with glial fibrillary acidic protein, astrocytes were reported as the major source of the enzyme in these early studies, both in the normal brain (Okuno et al., 1987; Köhler et al., 1988) and following nerve cell loss, e.g., in a model of chronic epilepsy (Du et al., 1993). Although still failing to demonstrate the presence of 3-HAO in neurons, subsequent work shifted attention away from astrocytes and toward a substantive role of microglia and macrophages (Heyes et al., 1997). Supported by in vivo experiments in which the neosynthesis of QUIN from 3-HANA was shown microscopically by staining with anti-QUIN antibodies (Lehrmann et al., 2001), microglial cells are now widely believed to be the major source of QUIN formation in the brain under physiological conditions and especially in response to immune stimulation (Sahm et al., 2013).
a. Quinolinic acid
The initial clues pointing toward the involvement of kynurenines in brain function emerged when seizures were observed in mice injected with QUIN directly into the brain (Lapin, 1978). A few years later, Stone and Perkins discovered that QUIN excites neurons in the central nervous system (CNS) by acting as an agonist at NMDARs with approximately 4 times lower potency than the synthetic ligand NMDA (see Fig. 2) (Stone and Perkins, 1981). Together with the subsequent demonstration that intracerebral injections of QUIN cause selective “axon-sparing” (excitotoxic) neurodegeneration in the rat brain, leading to translationally informative neurochemical, physiological, and behavioral changes (Schwarcz et al., 1983), and the identification of QUIN as a normal brain constituent (Wolfensberger et al., 1983), QUIN became the first KP metabolite to attract significant attention from neuroscientists. Endogenous QUIN is found in the brain in nanomolar concentrations (see Table 1) (Schwarcz et al., 1988; Heyes et al., 1992b; Sinz et al., 1998; Medana et al., 2003; Notarangelo et al., 2012; Drewes et al., 2015; Jacobs et al., 2019; Sorgdrager et al., 2019; Orhan et al., 2024).
The neurotoxic effects of QUIN appear to preferentially affect neurons that are richly endowed with NMDA receptors, such as GABAergic cells in the striatum and pyramidal cells in the hippocampus (Nakanishi, 1992). Calcium influx through NR2A and NR2B subunits appears to be particularly critical in this respect (de Carvalho et al., 1996). Notably, QUIN also produces reactive oxygen species, reduces the levels of natural antioxidants (Guillemin, 2012), and induces phosphorylation of structural proteins, leading to cytoskeletal damage (Rahman et al., 2009; Pierozan et al., 2010).
Brain QUIN levels increase significantly following stimulation of the immune system due to inflammatory effects on macrophages, microglia, and dendritic cells (Heyes et al., 1992b; Atlas et al., 2007). Notably, this results both in the harmful buildup of QUIN within cells (Chen and Guillemin, 2009; Chung et al., 2009) and in the extracellular compartment since newly produced QUIN is readily released and no reuptake process exists (Foster et al., 1984a; Speciale and Schwarcz, 1993).
As QUIN does not penetrate the blood-brain barrier under normal physiological conditions (Fukui et al., 1991), the comparatively higher concentrations of the metabolite in blood and peripheral tissues do not affect QUIN levels in the healthy brain. Intracellular QUIN is controlled by 3-HAO (see above) and its catabolic enzyme quinolinate phosphoribosyltransferase (QPRT), which converts the metabolite to nicotinic acid mononucleotide (NAMN). In turn, NAMN is further degraded to a series of metabolites, eventually producing nicotinamide adenine dinucleotide (NAD+) (Moroni, 1999), a pivotal cofactor in a multitude of biologically relevant redox-dependent and other processes (Ying, 2007).
Because it is saturated by relatively low QUIN concentrations (Km, 1 to 2 μM) (Foster et al., 1985), QPRT, which is also preferentially present in non-neuronal cells in the mammalian brain (Köhler et al., 1987), has received significant attention by neurobiologists for its regulation of brain QUIN levels and function in health and disease (Feldblum et al., 1988; Braidy et al., 2011a; Terakata et al., 2012).
5. Biosynthesis of Other Neuroactive Kynurenine Pathway Metabolites
In another side-arm of the KP (Fig. 1), 3-HANA is metabolized by 2-amino-3-carboxymuconate-semialdehyde decarboxylase (ACMSD) to 2-aminomuconic-6-semialdehyde, which is then nonenzymatically converted to picolinic acid (PIC). ACMSD is present in the mammalian brain, albeit at very low levels compared with peripheral organs (Pucci et al., 2007), and has been tentatively linked to brain pathology (Thirtamara-Rajamani et al., 2017). However, its localization in the mammalian brain has not been studied so far.
Although enzymatic formation has also been suggested to be responsible for the neosynthesis of cinnabarinic acid (Rao and Vaidyanahan, 1966), this metabolite is widely believed to be generated nonenzymatically by auto-oxidation of 3-HANA (Christen et al., 1992).
a. Picolinic acid
PIC has long been known as an effective but nonselective chelating agent of several biologically active metal ions, including zinc, iron, and copper (Suzuki et al., 1957; Aggett et al., 1989). Its related ability to potently control and regulate the physiological properties of these metals has led to the suggestion that PIC has substantive biological effects, including cellular growth control and antitumor activity (Ruffmann et al., 1987) as well as antifungal and antiviral properties (Fernandez-Pol et al., 1993; Abe et al., 2004). PIC has therefore been used as a tool to introduce bioactive metals in vivo and is used, for example, as a nutritional supplement to provide glycemic and lipidemic benefits (Broadhurst and Domenico, 2006).
PIC is present in mammalian blood, peripheral organs, and CSF (Brundin et al., 2016; Jacobs et al., 2019; Louvrou et al., 2024). Interestingly, in spite of the low levels of its biosynthetic enzyme (see above), the concentration of the metabolite in the brain is in line with other KP metabolites (approximately 1 μM) (Porter and O’Connor, 2021), though no functionally relevant receptor of PIC has been identified, and the neurophysiological function of endogenous PIC has not been clarified so far. Of note and possible biological significance, high concentrations of PIC can block QUIN-induced neurotoxicity without affecting neuronal excitation (Beninger et al., 1994). PIC-mediated neuroprotection may therefore not involve direct interference with glutamate receptor function but may be related to the ability of the metabolite to chelate endogenous zinc and/or to attenuate calcium-dependent glutamate release (Vrooman et al., 1993; Jhamandas et al., 1998).
b. Cinnabarinic acid
Cinnabarinic acid has recently received attention for its ability to function as a mGluR4 receptor and AhR agonist (see Fig. 2) (Fazio et al., 2012). Although it is present in the brain only in trace amounts (around 1 nM) (Ulivieri et al., 2020) and the localization and biochemical disposition of cinnabarinic acid in the normal and abnormal brain remain to be elucidated, its ability to affect the thresholds of inflammatory and neuropathic pain at low concentrations suggests a role of the metabolite as an endogenous regulator of pain transmission (Notartomaso et al., 2022).
III. Kynurenine Pathway in Brain Diseases/Disorders
As alluded to earlier, KP metabolites have garnered significant attention for their possible role(s) in the etiology of a number of brain diseases, and new insights could pave the way for novel therapeutic approaches targeting KP metabolism in the human brain (Pocivavsek and Erhardt, 2024). In light of the ever-expanding list of respective pathological events, the following summary places special emphasis on major psychiatric and neurologic disorders. Introductory comments will briefly summarize the current status of knowledge regarding the role of age, blood-brain barrier function, and inflammation in this context.
A. Pathology-Related Variables
1. Age
Studied most thoroughly in rodents, the continuous presence of most KP enzymes and metabolites has been demonstrated in the mammalian brain from the prenatal period into old age, with metabolite levels frequently shown to increase with advancing age (Gramsbergen et al., 1997; Kepplinger et al., 2005; Braidy et al., 2011b; Wennström et al., 2014; Sorgdrager et al., 2019; Solvang et al., 2022).
KP metabolism in the prenatal period and during adolescence has received most attention in this regard, but relatively little is still known about the synthesis and transfer of circulating kynurenines in the developing brain, their passage from mother to fetus, or the placenta's role in this process. Tryptophan is supplied to the fetus via the placenta (Nicholls et al., 2001), which then provides serotonin and other neuroactive metabolites to the fetal brain (Bonnin et al., 2011) (see Badawy, 2015 and Silvano et al., 2021 for comprehensive reviews of the dynamics of tryptophan metabolite in utero). Of note, there is a striking difference between concentrations of kynurenine and its metabolites in the prenatal and postnatal brain. For example, high fetal brain KYNA levels, which have been reported in nonhuman primates (Beal et al., 1992), sheep (Walker et al., 1999), rats (Ceresoli-Borroni and Schwarcz, 2000; Cannazza et al., 2001; Pocivavsek et al., 2014; Pershing et al., 2015), and mice (Notarangelo and Schwarcz, 2014; Beggiato et al., 2018), may provide neuroprotection during gestation and parturition (Beal et al., 1992; Walker et al., 1999; Ceresoli-Borroni and Schwarcz, 2000) by blunting excessive NMDAR signaling (Bagasrawala et al., 2016). Notably, the levels of other KP metabolites, including kynurenine and 3-HK, are also far higher in the fetal brain than in the postnatal brain (Ceresoli-Borroni and Schwarcz, 2000). After birth, the brain levels of all KP metabolites measured so far decrease rapidly and then stabilize into adulthood (Beal et al., 1992; Walker et al., 1999; Ceresoli-Borroni and Schwarcz, 2000; Notarangelo and Pocivavsek, 2017). This phenomenon is hypothesized to disinhibit NMDAR function and, together with effects on other mechanisms involved, enable brain development during the postnatal period (Balázs et al., 1988; Simon et al., 1992; Komuro and Rakic, 1993).
The production of KP metabolites is regulated differently during the neurodevelopmental period than in the adult brain. This has been most carefully studied with regard to the features of KYNA formation. In vitro studies show that although adult brain KYNA production is highly dependent on the availability of glucose and cellular energy metabolism, the developing brain is less susceptible to glucose deprivation (Gramsbergen et al., 1997). This is likely related to the fact that the developing brain is less dependent on glucose as the main energy source (Nehlig, 1997). Notably, pyruvate affects KYNA formation in the absence of glucose normally during early brain development, indicating that cosubstrate regulation of KYNA neosynthesis is fully functional in the immature brain (Schwarcz et al., 1998).
In alignment with emerging epidemiological findings linking insults during pregnancy to health risks, including neurodevelopmental and psychiatric disorders in offspring (Pearce, 2001; Susser and Bresnahan, 2002; Brown and Derkits, 2010; Pires et al., 2020), preclinical models of prenatal insults (stress, maternal immune activation, obstetric complications) have demonstrated increased KP metabolism in maternal and fetal compartments (Zavitsanou et al., 2014; Notarangelo and Schwarcz, 2016; Notarangelo and Pocivavsek, 2017; Keaton et al., 2019; Baratta et al., 2020). Lasting effects of elevated KP metabolism during neurodevelopment are of clinical significance as an excess of KYNA is found in CSF and post mortem brain tissue from patients with neurodevelopmental and psychiatric disorders, suggesting that KYNA dysregulation causatively impacts these illnesses (see below).
2. Blood-Brain Barrier Function
For decades, characterization of the mammalian KP focused exclusively on extracerebral observations, leading to a reasonably comprehensive understanding of the complex biochemical processes that are responsible for the vast majority of tryptophan degradation. Once the neuroactive properties and presence of KP metabolites in the brain became evident, the relationship between peripheral and central KP metabolism needed to be carefully investigated. Under normal physiological conditions, most acidic metabolites, including KYNA and QUIN, do not cross the blood-brain barrier to a significant extent, whereas others, including kynurenine, readily enter the brain from the circulation and then affect local KP metabolism and function (Gál and Sherman, 1978; Fukui et al., 1991). However, brain access of KP metabolites changes both qualitatively and quantitatively under pathological conditions that are associated with an impaired brain vasculature. The implications of an abnormal blood-brain barrier for both the function and dysfunction of KP metabolites in the brain are not likely to be consistent between brain disorders and require additional detailed scrutiny. These considerations are particularly relevant for studies in humans where changes in blood or CSF levels of KP metabolites are frequently—and at times probably inappropriately—used as indicators of altered KP metabolism and function in the brain (Skorobogatov et al., 2021).
3. Inflammation
As mentioned earlier, KP metabolism is stimulated by inflammatory cytokines and chemokines, specifically through the up-regulation of IDO in the initial step of the enzymatic cascade. Inflammatory processes therefore affect both peripheral and central KP dynamics and often result in an imbalance in the formation and levels of neuroactive KP metabolites in the brain. This effect has been shown consistently in both experimental animals and humans (Heyes et al., 1992a; Atlas et al., 2007, 2013; Holtze et al., 2012; Wickström et al., 2021). Although these neurochemical changes may play a substantive role in excitotoxic and other neuropathological events seen after infection, they also often appear to be causally associated with short- and long-term cognitive abnormalities in a variety of brain disorders (Schwarcz et al., 2012; Erhardt et al., 2017b; Stone et al., 2024). Notably, immune system–related changes in cerebral KP metabolism are not only seen, as expected, as a consequence of an infection with HIV or in individuals with tick-borne encephalitis and herpes encephalitis (Atlas et al., 2007, 2013; Holtze et al., 2012; Wickström et al., 2021) but also, for example, in patients with cerebral malaria (Medana et al., 2002, 2003; Miu et al., 2009; Holmberg et al., 2017; Hunt et al., 2017). Interestingly, circulating cytokines and chemokines are now increasingly measured in a large number of human brain diseases as part of routine clinical assessments or in the context of treatment trials. In most cases, these studies are not designed to test specific pathophysiologic mechanisms linking inflammatory processes with KP abnormalities. More often, they are an attempt to stratify complex (mainly psychiatric) diseases with the hope to generate novel, testable hypotheses regarding pathogenicity and treatment.
B. Neurologic Disorders
Dysfunction of KP metabolism has been investigated in a number of neurodegenerative disorders, including Huntington's disease (HD), Parkinson's disease (PD), and Alzheimer's disease (AD).
1. Huntington's Disease
HD is a neurodegenerative disorder characterized by progressive loss of motor function and cognitive decline. Although the genetic basis of HD, i.e., the expansion of CAG repeats in the huntingtin gene that leads to the production of mutant huntingtin protein, has been known since the 1990s (Mangiarini et al., 1996), the molecular mechanisms underlying neurodegeneration remain unclear. KP dysregulation may be a significant contributor to the neurodegenerative process in HD since changes in KP metabolism have been observed in the brain of HD patients. Normalization of the KP in individuals with HD may provide special therapeutic benefits in early stages of the disease, where 3-HK and QUIN levels are elevated—and may be causally involved in neurodegeneration—in neostriatum and cortex (Guidetti et al., 2004). Conceptually aligned with a role of the KP in neuropathology of the disease, KYNA levels are reduced in the striatum (Jauch et al., 1995) and in the CSF of HD patients (Heyes et al., 1992b). Notably, brain levels of 3-HK and QUIN are also increased and related to the onset of HD phenotypes in the R6/2 mouse model of HD (Guidetti et al., 2006). Increased KMO activity may be causally related to the elevation in brain 3-HK in R6/2 mice (Sathyasaikumar et al., 2010), but other KP abnormalities may play a role as well. Thus, IDO1 transcription is increased in the YAC128 HD mouse model (Mazarei et al., 2010) and could contribute to the enhanced kynurenine/tryptophan ratio seen in the blood of HD patients (Stoy et al., 2005; Forrest et al., 2010). Elevated levels of kynurenine stimulate the production of 3-HK/QUIN but at the same time, and for unknown reasons, reduce the formation of KYNA. The resulting imbalance of neurotoxic and neuroprotective KP metabolites may then play a causative role in the pathogenesis of HD.
Individuals with HD present with increased inflammatory cytokines, including TNF-α and interleukins, both in the periphery and in the brain (Björkqvist et al., 2008; Silvestroni et al., 2009), and elevations in immune signaling molecules occur prior to the onset of illness and correlate with disease severity and enhanced KP metabolism (Forrest et al., 2010). Immune activation therefore likely contributes to the activation of the KP during HD progression.
Attempts to target KMO pharmacologically or genetically in efforts to restore the balance of cerebral KP metabolism in R6/2 mice have yielded convincing results regarding biochemical consequences. Thus, chronic administration of the systemically active KMO inhibitor JM6 (Zwilling et al., 2011) as well as crossing with Kmo knockout mice (Bondulich et al., 2021) reduced 3-HK levels and increased KYNA in the striatum and cortex, and decreased peripheral inflammation, in R6/2 mice. JM6 treatment also had neuroprotective effects, though neither approach was associated with significant changes in behavioral impairments in the HD model mice.
2. Parkinson's Disease
PD is a neurodegenerative disease characterized by loss of dopaminergic neurons in the substantia nigra pars compacta and symptomatology related mostly to motor impairments. Classic biomarkers of PD include α-synuclein and dopamine metabolites in the CSF (Havelund et al., 2017). KP metabolite levels are altered in the brain of individuals with PD, however. Thus, post mortem analysis of PD brains shows elevated kynurenine/tryptophan ratios and 3-HK levels in the putamen, frontal cortex, and hippocampus (Ogawa et al., 1992; Widner et al., 2002). Related to KP abnormalities, the pathophysiology of PD may therefore involve excessive activation of NMDA receptors, production of reactive oxygen species and lipid peroxidation, and elevation of nitric oxide synthase levels.
3. Alzheimer's Disease
AD is the most common neurodegenerative disease, affecting nearly 60 million people across the world. Its pathology is related to a combination of genetic and environmental factors, and perturbations of the KP have been implicated in disease progression. In line with higher levels of brain IDO1 (Bonda et al., 2010), the kynurenine/tryptophan ratio is elevated in the CSF of AD patients (Gulaj et al., 2010). At the site of amyloid plaques, accumulated microglia, astrocytes, and neurons present with increased immunostaining for IDO1 and QUIN (Guillemin et al., 2005a; Bonda et al., 2010; Minhas et al., 2024). Similarly, IDO and QUIN are present in neurofibrillary tangles, and QUIN has been found in intracellular granular deposits within cortical neurons (Guillemin et al., 2005a).
Mechanistic studies demonstrate that amyloid peptide Aβ1-42 induces IDO1 expression and enhances QUIN production (Guillemin et al., 2003b). Aβ1-42 peptide drastically elevates a proinflammatory cytokine response, thereby inducing IDO1, TDO2, and KMO activity (Lue et al., 2001; Yamada et al., 2009). Taken together, a misbalance in the KP in AD patients points toward increased formation of excitotoxic metabolites that may contribute to AD pathology.
4. Traumatic Brain Injury
Traumatic brain injury (TBI) is a major public health concern that can result from events such as a fall, vehicle accidents, sports injuries, or violent assaults. TBI ranges in severity from mild concussions to severe brain damage, leading to a spectrum of neurologic and cognitive impairments. The pathophysiology of TBI is complex and involves primary injury from the initial impact and secondary injury processes that include inflammation, oxidative stress, and excitotoxicity. These injuries collectively contribute to neuronal damage and neurologic dysfunction. The KP has been implicated in the secondary injury mechanisms of TBI, though only a limited number of studies have directly investigated the relationship of TBI to KP metabolism (Meier and Savitz, 2022). Generally, research indicates increased initiation of the KP, noted as increased expression of IDO and increased kynurenine in the brain, and enhanced metabolism toward QUIN production. Significant elevations in QUIN (5- to 50-fold) as well as increases in kynurenine and KYNA have been reported in the CSF of individuals after severe TBI (Sinz et al., 1998; Yan et al., 2015). Along with enhanced IDO1 and kynureninase activity, these studies point to a preferred production of QUIN and related increased neurotoxicity (Yan et al., 2015). In preclinical studies, juvenile rabbits receiving controlled cortical impact showed upregulated IDO expression and protein levels, along with enhanced kynurenine concentrations, at the site of injury within hours. These effects lasted up to 3 weeks postinjury and also increased expression of the proinflammatory cytokines TNF-α, IL-1β, and IL-6 (Zhang et al., 2018). In rodent studies, increased expression of QUIN occurs within 24 hours at the focal site of cortical injury, subsequently spreading to adjacent sites (Chung et al., 2009). Rats exposed to blast-related TBI show increased KMO and kynureninase expression. Of note in this context, the KMO inhibitor Ro 61-8048 improves the survival of hippocampal neurons in vitro and has beneficial behavioral effects in vivo (Zakhary et al., 2020; Zhang et al., 2022). Although the kynureninase inhibitor benserazide is protective in vitro, it is unable to mitigate the impacts of TBI in vivo (Zhang et al., 2022). In mice, KMO inhibition protects against retinal ganglion cell dysfunction and structural abnormalities, which are observed over a month after high-pressure blast injury (Harper et al., 2019).
5. Epilepsy
Epilepsy is a neurologic disorder characterized by recurrent, unprovoked seizures resulting from abnormal electrical activity in the brain. Seizures can manifest in various forms, ranging from brief lapses of attention and muscle jerks to severe and prolonged convulsions. The underlying causes of epilepsy are diverse, including genetic predispositions, brain injuries, infections, and developmental disorders. Understanding the role of the KP and its metabolites in epilepsy has piqued research interests for decades, particularly with regard to understanding the balance between neuroprotection and neurotoxicity. Elevated levels of QUIN have been associated with increased neuronal excitability and seizure activity, and an increase in KYNA is postulated to occur as a counteractive response that maintains neuronal stability. Direct infusion of QUIN into the brain induces convulsion in animals (Lapin, 1978), whereas KYNA reduces spontaneous seizure activity (Foster et al., 1984b). The impact of manipulating KP metabolites to regulate seizure activity has been studied in several validated models (Vécsei et al., 1992). Yet although animal studies clearly support a misbalance in the KP when neuronal excitability is induced, findings from clinical studies in individuals with epilepsy are more variable. The first study to evaluate CSF from epileptic patients found decreased kynurenine (Young et al., 1983). CSF levels of KYNA were also lower in individuals with infantile spasms (Yamamoto et al., 1994) or with West syndrome, a rare condition that causes seizures in infants (Yamamoto et al., 1995). However, no changes in KYNA have been found in the CSF of individuals who experience complex partial seizures (Heyes et al., 1994). Recent metabolomic analysis of CSF from individuals who were hospitalized for status epilepticus, a life-threatening prolonged epileptic seizure, revealed an increase in kynurenine and QUIN (Dey et al., 2021; Hanin et al., 2024).
C. Psychiatric Disorders
During the past 20+ years, the KP has received increasing attention for its potential participation in the pathophysiology of various psychiatric disorders, and several individual KP metabolites have emerged as key players in this context.
Because of its ability to serve as an endogenous antagonist of both NMDARs and α7nAChRs, i.e., two receptors with key—and reasonably well understood—roles in brain function and dysfunction, KYNA has become a molecule of particular interest in the study of clinical conditions believed to involve glutamatergic and/or nicotinergic neurotransmission.
The excitotoxin QUIN, in contrast, is associated with neuroinflammatory and neurodegenerative processes that are prominent in major depressive disorder (MDD) and suicidality. PIC, although less studied, may modulate immune responses and neurochemical balance, influencing psychiatric symptoms and disease progression.
The following summary addresses ideas and hypotheses regarding the possible roles of these KP metabolites in the context of selected psychiatric disorders.
1. Schizophrenia and Bipolar Disorder
Induction of the KP in the brain is consistently found in psychotic disorders. Originally reported more than two decades ago (Erhardt et al., 2001a; Schwarcz et al., 2001), elevated levels of KYNA have been repeatedly observed in the CSF and in post mortem brain tissue of individuals with schizophrenia or bipolar disorder (BD). Notably, increased CSF KYNA levels are seen in both drug-treated and drug-naive patients with schizophrenia (Nilsson et al., 2005; Linderholm et al., 2012; Kegel et al., 2014; Plitman et al., 2017; Wang and Miller, 2018; Cao et al., 2021; Almulla et al., 2022; Inam et al., 2023; Rømer et al., 2023), and KYNA increases in post mortem brain have been confirmed several times in the disease (Sathyasaikumar et al., 2011; Wonodi et al., 2011; Antenucci et al., 2024). Although the levels of kynurenine, the precursor of KYNA, are elevated in both the CSF and cortical brain regions of people with schizophrenia (Miller et al., 2006; Linderholm et al., 2012), the neurotoxic branch of the KP appears to be unaffected as QUIN is found at normal levels in the CSF (Schwarcz et al., 1988; Kegel et al., 2014), and 3-HK levels remain unchanged in the post mortem brain (Schwarcz et al., 2001; Sathyasaikumar et al., 2011). Emerging evidence suggests that alterations in the KP, particularly involving KYNA, may also play a role in the pathophysiology of BD, a psychiatric condition characterized by dramatic mood swings, including episodes of mania and depression. Thus, increased levels of both kynurenine and KYNA are found post mortem in the anterior cingulate cortex of BD patients with a history of psychosis (Miller et al., 2006), and patients with BD exhibit elevated levels of KYNA in the CSF (Olsson et al., 2010; Wang and Miller, 2018; Trepci et al., 2021). This elevation is particularly pronounced in BD patients with a history of psychosis (Olsson et al., 2012; Lavebratt et al., 2014; Sellgren et al., 2016). The increased CSF KYNA levels in BD patients have been linked to cognitive impairments, particularly in tasks requiring executive function, such as set shifting (Sellgren et al., 2016). A small study investigating CSF KYNA in twin pairs discordant for BD or schizophrenia showed that KYNA associates with psychotic symptoms and cluster A personality traits, which include paranoid, schizoid, and schizotypal traits (Kegel et al., 2017). In contrast, no changes in CSF QUIN levels have been observed in patients with BD (Trepci et al., 2021). Further indication of a pathophysiological role of KYNA in psychosis comes from research showing that patients infected with HIV type 1 who exhibit psychotic symptoms have higher concentrations of CSF KYNA compared with HIV type 1 patients without such symptoms (Atlas et al., 2007).
Genetic studies have begun to shed light on the potential mechanisms underlying KYNA elevation in psychotic disorder. A genome-wide association study identified a significant association between CSF KYNA levels and the single-nucleotide polymorphism (SNP) rs10158645 within the 1p21.3 locus. This SNP is associated with decreased expression of sorting nexin 7 (SNX7), which has been linked to increased KYNA concentration through a caspase-8–driven activation of the proinflammatory cytokine IL-1β (Sellgren et al., 2016). Indeed, increased CSF levels of IL-1β are seen in BD patients with a history of psychosis as well as in first-episode psychosis patients (Söderlund et al., 2009, 2011). Consistent with these data, post mortem studies show increased mRNA levels of IL-1β, IL-6, IL-8, and TNF-α in the brains of individuals with both schizophrenia and BD (Fillman et al., 2013; Trépanier et al., 2016). Additional genetic evidence includes the discovery that a genetic variation in the GPCR kinases 3 (GRK3) gene correlates with levels of CSF KYNA and psychotic symptoms in individuals with BD (Sellgren et al., 2021). GRK3, also known as β-adrenergic receptor kinase 2 (ADRBK2), is a member of the GRK family involved in the desensitization of various receptors in the brain, including those for neurotransmitters, like dopamine and serotonin. Decreased GRK3 RNA expression and protein levels have also been observed in post mortem brain tissue (Bychkov et al., 2011) obtained from schizophrenia patients, and polymorphisms in the GRK3 promoter may increase the risk of BD (Barrett et al., 2003; Zhou et al., 2008; McCarthy et al., 2010).
Since the formation of KYNA indirectly relies on the activity of KMO (see above), polymorphisms in the Kmo gene might contribute to the elevated central levels of KYNA observed in patients with schizophrenia. Supportive evidence for this pathophysiologically significant hypothesis has been provided by a number of independent investigators over the past two decades. Thus, the Kmo gene, located on chromosome 1q42, has been linked to schizophrenia and schizoaffective disorder in families heavily affected by these conditions. Initial studies found associations between schizophrenia and specific SNPs in the Kmo gene [rs2275163 in a Japanese cohort (Aoyama et al., 2006) and rs2065799 in a Norwegian cohort (Holtze et al., 2012)], and post mortem studies showed reduced Kmo gene expression and KMO enzyme activity in the prefrontal cortex and frontal eye field of schizophrenia patients (Sathyasaikumar et al., 2011; Wonodi et al., 2011). Wonodi et al. (2011) explored the association between Kmo SNPs and schizophrenia-related oculomotor endophenotypes in a clinical sample and showed that the Kmo rs2275163 variant had modest effects on predictive pursuit and visuospatial working memory endophenotypes. This was confirmed in a separate follow-up study (Wonodi et al., 2014).
Interestingly, the nonsynonymous Kmo SNP rs1053230 affects CSF KYNA concentrations (Holtze et al., 2012), the C allele of this SNP is linked to reduced Kmo expression in lymphoblastoid cell lines (Lavebratt et al., 2014), and hippocampal biopsies obtained from epilepsy patients with at least one C allele showed lower Kmo expression than those without the C allele (Lavebratt et al., 2014). Notably, that study also revealed that the C allele is more prevalent among Swedish BD type 1 patients with psychotic features and is associated with higher CSF KYNA levels in these patients. Using the SMRI On-Line Database (www.stanleygenomics.org), reduced KMO mRNA levels were found in the prefrontal cortex of patients with schizophrenia or BD with lifetime psychotic features.
KYNA is the only known endogenous NMDAR antagonist present in the brain, leading to the hypothesis that increased production of KYNA might be causally related to schizophrenia and other psychotic disorders. Supporting this hypothesis, experimentally induced increases in brain KYNA levels result in a schizophrenia-like phenotype in animal models (discussed above). In experimental studies, KYNA is also shown to interact in various ways with dopaminergic neurotransmission (Erhardt et al., 2001b, 2009; Erhardt and Engberg, 2002; Wu et al., 2007), and CSF studies show a strong correlation between KYNA and the levels of the dopamine metabolite homovanillic acid (Nilsson et al., 2006, 2007). Possibly related, CSF KYNA is not only associated with a polymorphism in the gene encoding for SNX7 in patients with BD (see above) but also with the CSF concentrations of homovanillic acid (Sellgren et al., 2016). Decreased SNX7 expression was linked to activation of IL-1β, which can, in turn, increase CSF KYNA levels and enhance dopaminergic activity. This signaling pathway may therefore be one of several avenues resulting in the marx increased synthesis of KYNA and associated impairments in psychotic disorders.
Post mortem studies have also demonstrated an increase in kynurenine levels and TDO2 expression in the prefrontal cortex and anterior cingulate cortex of individuals affected by schizophrenia (Schwarcz et al., 2001; Miller et al., 2004, 2006). More recently, Kindler et al. (2020) found that KYNA levels, the kynurenine/tryptophan ratio, and mRNAs for the enzymes TDO and KATI/II were increased in the prefrontal cortex in a “high cytokine schizophrenia subgroup” identified by elevated proinflammatory cytokine mRNAs. Furthermore, peripheral changes in KP metabolites were linked to cognitive deficits and structural human brain volumetric abnormalities. These results further support the hypothesis that central neuroinflammation may be critically involved in the activation of the KP in psychosis, at least in a subgroup of patients (Antenucci et al., 2024).
Peripheral measures of KP metabolites and metabolism in schizophrenia and BD are less consistent. Although an early publication reported increased plasma levels of KYNA in individuals with schizophrenia (Ravikumar et al., 2000), this finding was not confirmed in a number of follow-up studies of patients with either schizophrenia or BD (Myint et al., 2007, 2011; Plitman et al., 2017; Morrens et al., 2020; Cao et al., 2021; Sapienza et al., 2023, 2024). A recent meta-analysis based on 30 studies concluded that peripheral levels of tryptophan are lower, and levels of KYNA are not altered, in schizophrenia. In 14 studies of patients with BD, tryptophan and KYNA were significantly lower in patients compared with healthy controls (Marx et al., 2021). Notably, two recent studies examined KYNA concentrations in the CSF and plasma of the same individuals—both healthy and diseased—without finding any correlations between the two compartments (Trepci et al., 2021; Orhan et al., 2024). While central levels of KYNA may shed light on underlying pathophysiological mechanisms, peripheral levels may therefore, at best, serve as biomarkers that provide insights into disease progression or treatment response. Notably, however, plasma KP metabolites have been evaluated as potential biomarkers of brain microstructure and function (Poletti et al., 2019; Comai et al., 2022; Hare et al., 2023).
Additional studies of translational interest regarding a role of the KP in schizophrenia and BD have been conducted and have revealed, for example, that cultured dermal fibroblasts derived from patients demonstrate a markedly higher ability to release KYNA compared with cells obtained from healthy controls (Johansson et al., 2013). Another interesting study reported that although there were no baseline differences between controls and people with schizophrenia, the latter exhibited higher distress intolerance levels, and patients who struggled with stressful tasks showed a greater increase in salivary KYNA levels. Moreover, the rise in KYNA correlated with more severe psychiatric symptoms in these individuals (Chiappelli et al., 2014). These and conceptually similar studies can be expected to provide critical new information with regard to the role of KP (dys)function in various aspects of the complex pathophysiology of the disease.
2. Major Depressive Disorders
MDD is a debilitating psychiatric condition characterized by persistent feelings of sadness, loss of interest, and various cognitive and physical symptoms. Since several kynurenines have been implicated in neuroinflammation, oxidative stress, and glutamate neurotransmission, all of which are processes believed to contribute to the development and persistence of depressive symptoms, the KP has been suggested to be actively involved in MDD. Kynurenines have been repeatedly measured in the CSF and brain of MDD patients (Coppen et al., 1972; Ashcroft et al., 1973; Bech et al., 1978; Curzon et al., 1980; Banki et al., 1981; Kaddurah-Daouk et al., 2012; Clark et al., 2016; Paul et al., 2022; Brown et al., 2024), but the results are inconsistent, and a recent systematic review found no significant differences in tryptophan, kynurenine, QUIN, or KYNA levels between MDD patients and healthy controls (Inam et al., 2023).
Recent evidence also suggests a role of KP metabolites in the neurobiology of suicidal behavior. Thus, QUIN, but not KYNA, levels are elevated in the CSF of individuals who attempted suicide, suggesting an overactivation of NMDARs (Erhardt et al., 2013). Interestingly, higher QUIN levels were significantly associated with the degree of suicidal intent and correlated with increased levels of the proinflammatory cytokine IL-6, in line with studies showing that inflammation triggers QUIN generation (Heyes et al., 1992b; Achim et al., 1993). This provides a neurobiological rationale for the observed efficacy of the NMDAR antagonist ketamine in alleviating symptoms of suicidality (Price et al., 2009; DiazGranados et al., 2010; Zarate et al., 2012). In a separate longitudinal study, QUIN and KYNA levels in the CSF were determined along with depressive and suicidal symptoms over time in psychiatric patients (Bay-Richter et al., 2015). Although QUIN levels were highest at the time of the suicide attempt and decreased during the first year, they remained significantly elevated for up to 2 years. The study also showed that CSF KYNA levels were decreased in these patients compared with healthy individuals and were negatively associated with suicidal ideation and depressive symptoms. Low CSF KYNA had been previously reported in schizophrenia patients with a history of suicidality (Carlborg et al., 2013).
Based on measurements in CSF and blood in conjunction with a suicide attempt across two independent cohorts, there is also evidence that reduced levels of PIC may play a role in suicidality. Importantly, these changes were not related to medication (Brundin et al., 2016). Possibly related (see above), a pilot genotyping study indicated that a single SNP of the ACMSD gene, rs2121337, was associated with increased QUIN in CSF and was more prevalent in suicide attempters than in healthy controls (Brundin et al., 2016). This suggests that reduced ACMSD activity leads to excess QUIN production observed in patients exhibiting suicidal behavior, contributing to increased neuroinflammation and NMDAR activation in vulnerable individuals. Notably, CSF PIC levels were also found to be reduced in a 2-year follow-up study after an initial suicide attempt, suggesting that decreased ACMSD activity may be a trait-marker rather than a state-marker of susceptibility to suicidal behavior (Brundin et al., 2016). A diminished PIC/QUIN ratio in the CSF of individuals who attempted suicide was replicated in a separate cohort (Schwieler et al., 2020), and lower CSF PIC concentrations are also seen in BD patients with a history of suicidal behavior (Trepci et al., 2021). Low CSF PIC may therefore be a bona fide marker of vulnerability for suicidality. Together with evidence mentioned earlier, these data highlight the critical involvement of KP metabolism in the pathophysiology of suicidal behavior, suggesting potential targets for therapeutic intervention.
IV. Translationally Relevant Pharmacological Manipulation of Brain Kynurenine Pathway Metabolism
1. Tryptophan 2,3-Dioxygenase 2 and Indoleamine 2,3-Dioxygenase 1
TDO2 and IDO1 have been extensively studied regarding their involvement in several pathological conditions, including malignancies (D’Amato et al., 2015; Théate et al., 2015), CNS disorders (Miller et al., 2004, 2006; Smith et al., 2012), infections (Boasso and Shearer, 2007; Zelante et al., 2009; Pallotta et al., 2022), and autoimmune diseases (Lee et al., 2014; Pallotta et al., 2014; Mancuso et al., 2015). Notably, TDO2 is not as effective as IDO1 in this regard (Ball et al., 2007). Dysregulation of IDO2 expression has been observed in various types of cancer, including non–small cell carcinoma, pancreatic malignancies, and cervical cancer.
1-Methyltryptophan (1-MT) has been employed in preclinical studies as a selective IDO inhibitor capable of crossing the blood-brain barrier, thus directly impacting brain function. Its major purpose is to mitigate immunosuppressive and neuroinflammatory effects driven by KP activation (Kiank et al., 2010; O’Farrell et al., 2017; Marim et al., 2021). In models of depression, 1-MT administration has been associated with an increase in serotonin availability and a decrease in depressive-like behaviors (Liu et al., 2015). In the context of neurodegenerative diseases, inhibition of IDO1 by 1-MT reduces neuroinflammation and oxidative stress, thereby protecting neuronal integrity and function (Sodhi et al., 2021). IDO1 is therefore a promising therapeutic target for enhancing the effectiveness of immunotherapy in brain disorders (Zhai et al., 2015; Platten et al., 2021).
2. Kynurenine Aminotransferases
A causal link between an experimental reduction in brain KYNA synthesis and behavioral enhancement was first demonstrated using KAT II knockout mice (Yu et al., 2004). These genetically modified animals show improved performance in several behavioral paradigms, including object recognition, passive avoidance, and spatial discrimination. Compared with wild-type control animals, LTP, too, is significantly enhanced in hippocampal slices from KAT II–deficient mice (Potter et al., 2010).
More generally, pharmacological inhibition of KAT enzymes may therefore improve cognitive and behavioral function. Since the transamination of kynurenine to KYNA by KAT I is curbed by the high endogenous levels of the competitive substrate glutamine, KAT II has been considered a more effective target for efforts to reduce brain KYNA levels by pharmacological means. Notably, the crystal structure of KAT II has been resolved, revealing that the protein exists as a functional dimer and contains two active sites with coordinated pyridoxal phosphate (PLP) cofactors (Rossi et al., 2008).
Several potent KAT II inhibitors, including S-ESBA (Pellicciari et al., 2006), BFF-122 (Amori et al., 2009), BFF-816 (Wu et al., 2014), and PF-04859989 (Kozak et al., 2014), have been identified (see Fig. 3). In contrast to the competitive enzyme inhibitors S-ESBA and BFF-816, PF-04859989 and BFF-122 are irreversible inhibitors of KAT II, binding to the PLP cofactor site (Rossi et al., 2010; Dounay et al., 2012). Notably, since PLP participates actively as a cofactor in numerous enzymatic reactions, irreversible binding of a KAT II inhibitor to the PLP site may be associated with unwanted side effects. In fact, PLP was recently shown to play a central role in maintaining homeostatic host-microbiota crosstalk through tryptophan metabolism (Cellini et al., 2020).
Pharmacological targeting of two key kynurenine pathway enzymes in the brain and biological implications.
S-ESBA was the first selective inhibitor of KAT II synthesized (Pellicciari et al., 2006) and has been an effective tool in preclinical studies. When administered locally in the brain, S-ESBA quickly reduces extracellular KYNA levels (Pellicciari et al., 2006; Amori et al., 2009; Wu et al., 2010; Pocivavsek et al., 2011), and learning and memory are improved in rats that are treated daily with S-ESBA (Pocivavsek et al., 2011). Subsequently, two brain-penetrable KAT II inhibitors became available and have been successfully employed in animal studies. The brain-penetrable compounds PF-04859989 (IC50, 263 nM; Dounay et al., 2012), administered subcutaneously, and BFF-816 (IC50, 14 μM; Wu et al., 2014), administered by oral gavage, rapidly reduce KYNA in the brain. Even with 5 consecutive days of administration, no tolerance is found with BFF-816 application (Wu et al., 2014). Both compounds have shown promising procognitive impacts in behavioral studies (Kozak et al., 2014; Pocivavsek et al., 2019). Notably, PF-04859989 attenuates stimulant-induced deficits in auditory gating, working memory, and spatial memory, along with improving sustained attention in rodents (Kozak et al., 2014). PF-04859989 also effectively modulates cognitive processes associated with fear discrimination after stress exposure in rats (Klausing et al., 2020). In nonhuman primates, PF-04859989 antagonizes ketamine-induced working memory impairments (Kozak et al., 2014). In mice with a targeted deletion of KMO and basal elevation of KYNA (Kmo−/− mice; see above), PF-04859989 facilitates LTP, confirming that a pharmacological reduction of KYNA improves learning and memory (Imbeault et al., 2021). Acutely reducing KYNA with PF-0459989 attenuates sleep disruptions in rodents and improves overall sleep quality (Milosavljevic et al., 2023; Rentschler et al., 2024), suggesting that KAT II inhibition could serve as an efficacious strategy to combat homeostatic sleep impairments. PF-04859989 also effectively dampens basal midbrain dopaminergic firing (Linderholm et al., 2016). KAT II inhibition by BFF-816, too, improves performance in spatial and reference memory (Wu et al., 2014) and stress-induced fear discrimination (Klausing et al., 2024) in rats and attenuates contextual memory deficits in adult offspring exposed to elevated kynurenine during neurodevelopment, an etiologically relevant model for the study of schizophrenia endophenotypes (Pocivavsek et al., 2019).
These preclinical studies indicate that KAT II inhibitors hold promise for enhancing cognitive function in healthy individuals and for overcoming cognitive impairments associated with psychiatric illnesses and neurodegenerative disorders in humans. In fact, any pharmacological or other intervention that decreases KYNA levels, or interferes with its function in the brain, may improve cognitive function. Antioxidants provide interesting options in this context (Lugo-Huitrón et al., 2011; Blanco Ayala et al., 2015, 2021; Blanco-Ayala et al., 2020), and this approach is currently being employed in a clinical trial evaluating N-acetylcysteine as a KAT II inhibitor (clinicaltrials.gov NCT04013555). The development of KAT II inhibitors for use in humans continues, and the first human phase I trial with a selective drug (KYN-5356) is currently in progress (clinicaltrials.gov NCT06225115). Successful completion of clinical trials with KAT II inhibitors would provide critical insights into the mechanistic roles of the KP in disease progression and advance understanding of novel therapeutic strategies for the treatment of neurocognitive and psychiatric disorders.
In addition to KAT II, KAT III appears to play a critical role in the production of KYNA under conditions of immune activation. Thus, an upregulation of KAT III expression is observed in the mouse brain following repeated LPS injections, increasing brain KYNA levels in both wild-type and KAT II knockout mice. Increased KAT III expression is also seen in post mortem brain tissue from COVID-19–infected patients, and immune activation induces KAT III and increases KYNA production in vitro in human fibroblasts, epidermal cells, and monocytes (Erhardt et al., 2023). These findings identify KAT III as an exciting, novel pharmacological target for treating cognitive impairments associated with psychiatric and infectious diseases.
3. Kynurenine 3-Monooxygenase
As inhibition of KMO induces a decrease of 3-HK and QUIN and shifts KP metabolism toward increased production of KYNA, KMO is an attractive therapeutic target for treating various neurodegenerative disorders. Yet, KMO inhibitors are still in preclinical development.
Inhibitors of KMO include halogenated derivatives of L-kynurenine (UPF-648, Ro 61-8048, JM-6), chlorine derivatives [Cure Huntington’s Disease Initiative (CHDI)-340246], and chlorinated benzisoxazole (GSK-180) (see Fig. 3). UPF-648 [(1S,2S)-2-(3,4-dichlorobenzoyl)-cyclopropane-1-carboxylic acid] is a potent and selective inhibitor (IC50, 40 nM) but has poor brain penetrability, thereby limiting its therapeutic potential for CNS diseases (Amaral et al., 2013). Preclinical in vitro and in vivo studies with UPF648 demonstrate inhibition of QUIN neosynthesis and increased KYNA formation (Pellicciari et al., 2003; Amori et al., 2009). Notably, UPF-648 treatment increases KYNA 10-fold in KAT II−/− mice, indicating that KAT II is not singularly responsible for KYNA synthesis in the mouse brain. Mice treated with UPF-648 also show reduced QUIN-induced toxicity in the striatum, in line with the hypothesis that an increase in the KYNA:3-HK/QUIN ratio provides neuroprotection (Sapko et al., 2006).
The crystal structure of yeast KMO was successfully determined both in its free form and when co-crystalized with UPF-648 (Amaral et al., 2013). Although the sequences of yeast and human KMO are not 100% homologous, the residues in the ligand binding domain are conserved between species. Discovering the chemical similarities between UPF-648 and L-kynurenine has supported critical molecular modeling of L-kynurenine in the active site, thereby advancing drug discovery efforts.
3,4-Dimethoxy-N-[4-(3-nitrophenyl)thiazol-2-yl] benzenesulfonamide (Ro-61-8048) is another well known, high-affinity KMO inhibitor (IC50, 37 nM) (Röver et al., 1997). Treatment with Ro-61-8048 for an extended period reduces the onset of levodopa-induced dyskinesia in a nonhuman primate model of Parkinson's disease (Grégoire et al., 2008) and prevents postischemic neuronal death (Carpenedo et al., 2002). Interestingly, Ro-61-8048 treatment also reduces the rewarding aspects of tetrahydrocannabinol in rodents by modulating KYNA levels and dopamine release (Justinova et al., 2013).
Since Ro-61-8048 is metabolically unstable, the slow-release prodrug form, JM6, was developed. Prolonged treatment of mice with JM6 inhibits KMO in the periphery, increases brain KYNA levels, and effectively normalizes spatial memory deficits, anxiety phenotypes, and synapse loss in a transgenic mouse model of AD (Zwilling et al., 2011). Moreover, JM6 extends their lifespan and attenuates neuronal damage in the R6/2 mouse model of HD (Zwilling et al., 2011). Subsequent investigation questioned the pharmacokinetic properties of the prodrug, however (Beconi et al., 2012).
Another KMO inhibitor, GSK180 (IC50, 6 nM), is suitable for intravenous administration and prevents multiple organ dysfunction in a rodent model of acute pancreatitis (Mole et al., 2016). Although the drug is potent and specific, it shows low cell penetration, making it an unattractive candidate for the treatment of CNS disorders. Because of its interest in their therapeutic use for the treatment of HD, the CHDI has patented several KMO inhibitors with the goal of identifying compounds that are capable of crossing the blood-brain barrier. One of the first of these compounds, CHDI-340246 (IC50, 0.5 nM), showed only limited ability to enter the rat brain following systemic administration in vivo (Toledo-Sherman et al., 2015). As CHDI-340246 does not inhibit other KP enzymes, preclinical testing has continued, and the drug indeed increases brain KYNA levels in rodent HD models (Dominguez and Munoz-Sanjuan, 2014; Beaumont et al., 2016).
More recently, diclofenac, a potent nonsteroidal anti-inflammatory therapeutic, was discovered to inhibit KMO and holds promise for being repurposed for targeting KP metabolism (Hutchinson et al., 2017; Phillips et al., 2017; Shave et al., 2018). Indeed, experimental studies show that diclofenac increases KYNA levels in the rat brain (Schwieler et al., 2005).
Continuing to investigate KMO inhibitors is crucial for advancing therapeutic options for neurodegenerative and neuroinflammatory disorders. Although additional advancements are needed to improve brain permeability, ongoing clinical trials with the first KMO inhibitor, KNS366 (Kynos Therapeutics), show promising preliminary results (www.isrctn.com; ISRCTN10496020). Ongoing and future research is expected to provide valuable data on the efficacy and safety of this unique pharmacological approach.
4. Kynureninase
Although pharmacological and genetic manipulations of kynureninase are largely speculative at this stage, they represent exciting opportunities for treating a range of brain disorders, including neurodegenerative disease, psychiatric disorders, and conditions with neuroinflammation. However, no efficient kynureninase inhibitors have been developed so far.
5. 3-Hydroxyanthranilic Acid Dioxygenase
In light of the central role of 3-HAO in the production of QUIN, targeting this enzyme presents a promising therapeutic strategy. By reducing QUIN levels in the brain, inhibitors of 3-HAO may mitigate excitotoxic damage and provide neuroprotection. Of relevance in this context, mice with a targeted elimination of 3-HAO are protected from the negative effects of an immune challenge with LPS on depressive-like behavioral phenotypes, suggesting that reduced kynurenine metabolism toward QUIN is neuroprotective (Parrott et al., 2016). Research into specific inhibitors of 3-HAO is ongoing and shows promising results in reducing QUIN production in the brain (Vallerini et al., 2013), although no clinically approved drugs are currently available.
V. Major Open Questions and Opportunities
A rapidly expanding body of evidence implicates important roles of KP metabolites in normal brain function and in psychiatric, neurocognitive, and neurodegenerative disorders. Recent advancements in genetic and pharmacological approaches in preclinical models have supported clinical studies in patients, suggesting that normalizing the levels of KP metabolites may be an effective therapeutic approach to achieving brain health. In most neurodegenerative diseases, there is a shift in the KP toward increased production of QUIN and 3-HK and away from KYNA. This imbalance can be corrected with interventions designed to reverse this metabolic shift. Targeting KMO shows potential as a therapeutic target as inhibition of this enzyme increases KYNA levels, whereas decreasing its product 3-HK and the downstream metabolite QUIN. On the opposite spectrum of metabolic shift are neuropsychiatric disorders wherein accumulation of KYNA levels in the brain may influence behavioral endophenotypes related to arousal and cognition in illnesses such as schizophrenia and BD. Targeting KAT enzymes to decrease KYNA formation and function appears to be an attractive therapeutic intervention in this case. Although achieving a balance in KP metabolism in the brain presents itself as an ideal circumstance, several questions remain to be addressed. Some of them are listed below in bullet form.
Further clarification of the relationship between peripheral and central KP metabolism. Studies in animals have repeatedly challenged the notion that measurements of KP metabolite levels in the periphery predict the features of central KP metabolism under either physiological or pathological conditions (Agudelo et al., 2014; Larsson et al., 2016; Baratta et al., 2018; Wright et al., 2021). This has been verified in humans (Sellgren et al., 2019; Skorobogatov et al., 2021; Orhan et al., 2024). Improved study designs and, in particular, the development of noninvasive methods to monitor KP metabolites in the brain in vivo will have a major impact in the field.
Role of bacterial KP metabolites in host KP physiology and pathology. Research into the role of bacterial metabolites in the KP of the host is a burgeoning field with significant implications for understanding gut-brain interactions. Bacteria in the gut microbiome produce KP metabolites that can influence the host’s physiology and pathology by modulating the local and systemic levels of kynurenine and its derivatives (Gheorghe et al., 2019; Schwarcz et al., 2024). Further research in this area could lead to novel probiotic and antibiotic therapies aimed at manipulating gut microbiota to beneficially alter host KP metabolism (Rudzki et al., 2021).
Effects of acute and chronic stress on KP physiology and pathology. Stress is a well known modulator of the immune system and has profound effects on KP metabolism. Acute stress can induce a rapid upregulation of KP metabolites, potentially leading to transient changes in neurotransmitter levels and neuroinflammation. Chronic stress, on the other hand, may cause sustained alterations in KP enzyme activity, contributing to the pathogenesis of chronic inflammatory and neurodegenerative diseases (Agudelo et al., 2014; Porter and O’Connor, 2021; Ye et al., 2024). Further research is warranted to delineate the mechanisms by which different types of stress affect KP physiology and to develop interventions that can mitigate these effects. Understanding stress-induced changes in the KP could lead to targeted treatments for stress-related disorders (Klausing et al., 2020, 2024).
Positive and negative effects of chronic interventions with pharmacological agents targeting individual KP enzymes. Long-term interventions with drugs targeting specific KP enzymes present both opportunities and challenges. Although such interventions hold promise for treating diseases linked to KP dysregulation, they also pose a risk of off-target effects and metabolic imbalances. Chronic inhibition or activation of KP enzymes may lead to unintended consequences, such as accumulation of toxic intermediates or the depletion of essential metabolites. Notably, these interferences may also affect the degradative cascade downstream of QUIN, causing impairments in the formation and function of the essential coenzyme NAD+. Comprehensive studies are required to evaluate the long-term safety and efficacy of these pharmacological agents, understanding both their therapeutic potential and adverse effects. This research will be crucial to developing safe, effective treatments for chronic conditions involving KP dysregulation.
Clinically relevant strategies to affect specific processes in the fetal brain pharmacologically with selective “KP drugs.” The development of selective KP drugs that can safely target the fetal brain is an exciting area of research with the potential to prevent or mitigate neurodevelopmental disorders. These strategies must be meticulously designed to avoid disrupting critical developmental processes while effectively modulating KP metabolites. Prenatal exposure to altered KP metabolism can have lasting impacts on brain development and function (Pocivavsek et al., 2012, 2014; Alexander et al., 2013; Forrest et al., 2013a,b; Pershing et al., 2015; Notarangelo and Pocivavsek, 2017; Beggiato et al., 2018; Rentschler et al., 2021; Wright et al., 2021), highlighting the need for precise, controlled interventions. Research in this area could lead to breakthroughs in preventing conditions like schizophrenia by pharmacologically modulating the KP during critical periods of brain development.
Changes in the role(s) of KP metabolism and function in the aging brain. The role of KP metabolism in the aging brain is an important area of study (Gramsbergen et al., 1992; Comai et al., 2005), particularly given the pathway’s involvement in neurodegenerative disease. As the brain ages, changes in KP enzyme activity and metabolite levels can contribute to cognitive decline, exacerbate sleep impairments, and increase susceptibility to neurological conditions. Research should focus on how these age-related changes in KP function affect brain health and on developing interventions that can preserve cognitive function in the elderly. Understanding the mechanisms behind age-related KP alterations could lead to targeted therapies that address the metabolic changes contributing to neurodegeneration.
Selective manipulation of brain KP (dys)function(s) with new genetic tools. The advent of advanced genetic tools presents new opportunities for selectively manipulating KP function in the brain. These tools can be used to precisely edit genes encoding KP enzymes, allowing the investigation of causal relationships between specific KP dysfunctions and brain disorders. By selectively knocking out or overexpressing KP genes in animal models, we will gain deeper insights into the pathway’s role in brain function and disease. These genetic interventions could pave the way for novel treatments that specifically target KP dysregulation without affecting other metabolic pathways.
Causal role of brain KP in the effects of psychedelics. Psychedelic compounds have been shown to have profound effects on brain function, and recent research suggests that the KP may play a role in mediating these effects. Understanding the interaction between psychedelics and KP metabolism could reveal new mechanisms of action for these substances and identify potential therapeutic applications for mental health disorders (Campanale et al., 2024). Studies should focus on how psychedelics influence KP enzyme activity and metabolite levels and whether these changes contribute to the therapeutic and psychotropic effects of these drugs. Further research could lead to new insights into the neurobiological basis of psychedelic therapeutic advancements occurring in psychiatry.
KP and drugs of abuse. Increasing evidence points to a functionally significant role of KP metabolism in the context of drugs of abuse, for example tetrahydrocannabidiol and other Cannabis derivatives (Panlilio et al., 2016; Secci et al., 2019). These phenomena, which may have common features among a variety of addictive substances (Davidson et al., 2023), may be of special pathophysiological relevance at early stages of brain development (i.e. prenatally and during adolescence) (Beggiato et al., 2020, 2022).
Changing contributions of various brain cells to KP function and dysfunction during the lifespan. Different brain cell types, including neurons, astrocytes, and microglia, contribute to KP metabolism, and their roles may change over the lifespan. Understanding how these contributions shift with age and in response to disease is crucial for developing targeted therapies. Research should investigate how the activity of KP enzymes in different cell types affects overall brain metabolism and how these changes contribute to neurodegeneration and other age-related conditions. Advancements in this area could inform the development of cell-specific interventions to modulate KP function and maintain brain health throughout the lifespan.
Genetic links between KP and psychiatric conditions. There is growing evidence of genetic links between KP metabolism and psychiatric conditions, such as depression, schizophrenia, and drug addiction. Identifying genetic variants that influence KP enzyme activity and metabolite levels could provide new biomarkers for these conditions and reveal potential targets for pharmacological intervention. Elucidating the genetic basis of KP dysregulation in psychiatric disorders and exploring how these genetic factors interact with environmental influences such as stress and substance abuse presents promising avenues towards personalized treatment strategies based on an individual's genetic profile and KP metabolic state.
Data Availability
This review article contains no datasets generated or analyzed during the present study.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Pocivavsek, Schwarcz, Erhardt.
Footnotes
- Received July 7, 2024.
- Revision received September 8, 2024.
- Accepted September 10, 2024.
This work was supported by National Institutes of Health National Institute on Aging [Grant R21 AG080335] (to A.P.) and National Institute of Mental Health [Grant P50 MH103222] (to R.S.) and the Swedish Medical Research Council [Grant 2021-02251_VR] (to S.E.).
R.S. serves on the scientific advisory board of Kynexis BV. The remaining authors declare no other conflicts of interest.
Abbreviations
- 1-MT
- 1-methyltryptophan
- 3-HANA
- 3-hydroxyanthranilic acid
- 3-HAO
- 3-hydroxyanthranilic acid dioxygenase
- 3-HK
- 3-hydroxykynurenine
- α7nAChR
- α7 nicotinic acetylcholine receptor
- ACMSD
- 2-amino-3-carboxymuconate-semialdehyde decarboxylase
- AD
- Alzheimer disease
- AhR
- aryl hydrocarbon receptor
- BD
- bipolar disorder
- CHDI
- Cure Huntington’s Disease Initiative
- CNS
- central nervous system
- CSF
- cerebrospinal fluid
- GRK3
- GPCR kinases 3
- HD
- Huntington disease
- IDO
- indoleamine 2,3-dioxygenase
- IFN
- interferon
- IL
- interleukin
- KAT
- kynurenine aminotransferase
- KMO
- kynurenine 3-monooxygenase
- Kmo−/−
- Kmo knockout
- KP
- kynurenine pathway
- KYNA
- kynurenic acid
- LPS
- lipopolysaccharide
- LTP
- long-term potentiation
- MDD
- major depressive disorder
- NMDAR
- N-methyl-D-aspartate receptor
- OAT
- organic anion transporter
- PD
- Parkinson's disease
- PIC
- picolinic acid
- PLP
- pyridoxal phosphate
- QUIN
- quinolinic acid
- SNP
- single-nucleotide polymorphism
- SNX7
- sorting nexin 7
- TBI
- traumatic brain injury
- TDO
- tryptophan 2,3-dioxygenase
- TNF
- tumor necrosis factor
- XANA
- xanthurenic acid
- Copyright © 2024 by The Author(s)
This is an open access article distributed under the CC BY-NC Attribution 4.0 International license.
References
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵